Effects of Cu Substitution for Sn on the Electrochemical Performance of La0.7Mg0.3Al0.3Mn0.4Sn0.5−xCuxNi3.8 (x = 0–0.5) Alloys for Ni-MH Batteries
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Discharge Capacity
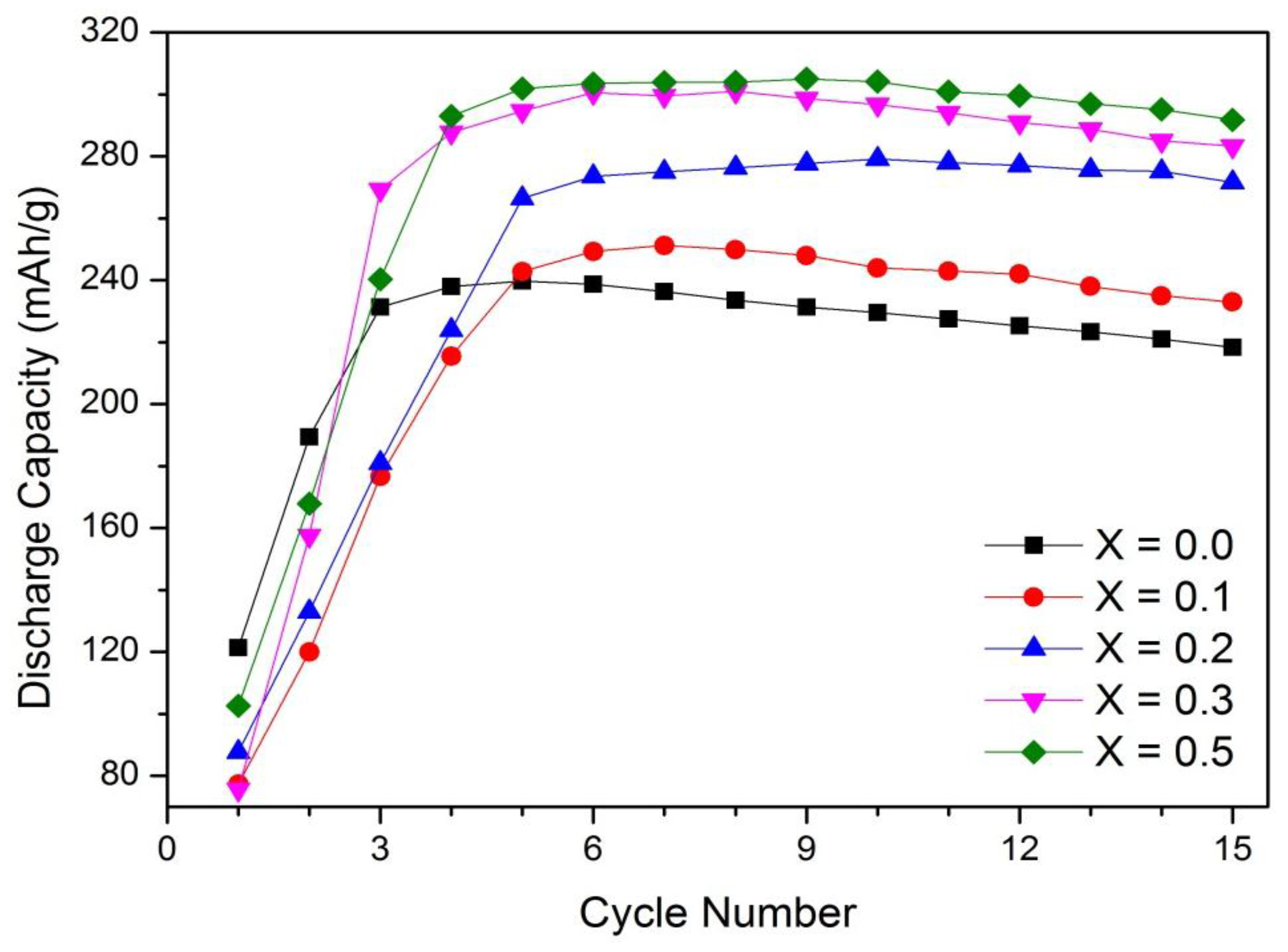

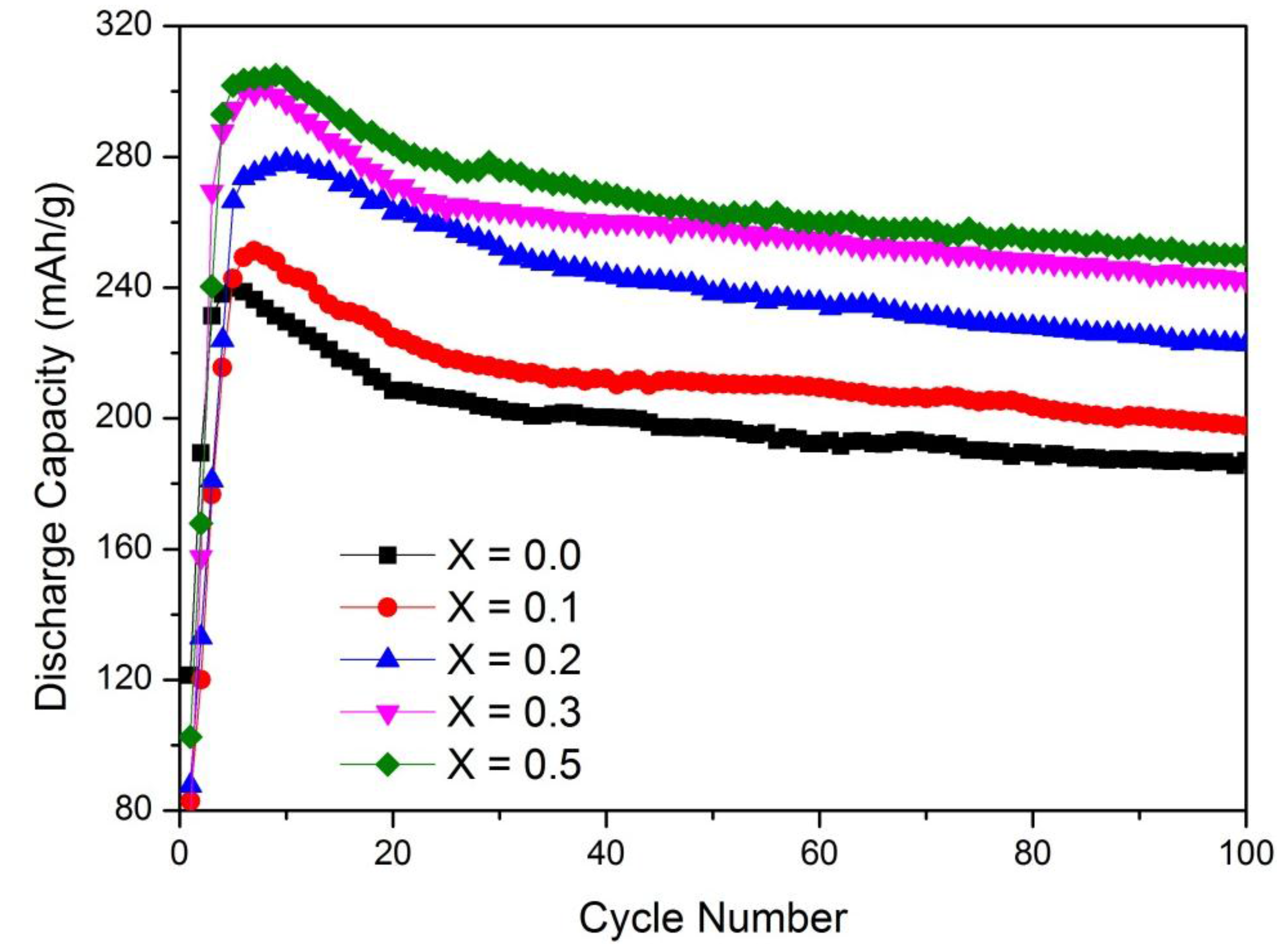
3.2. Capacity Retention
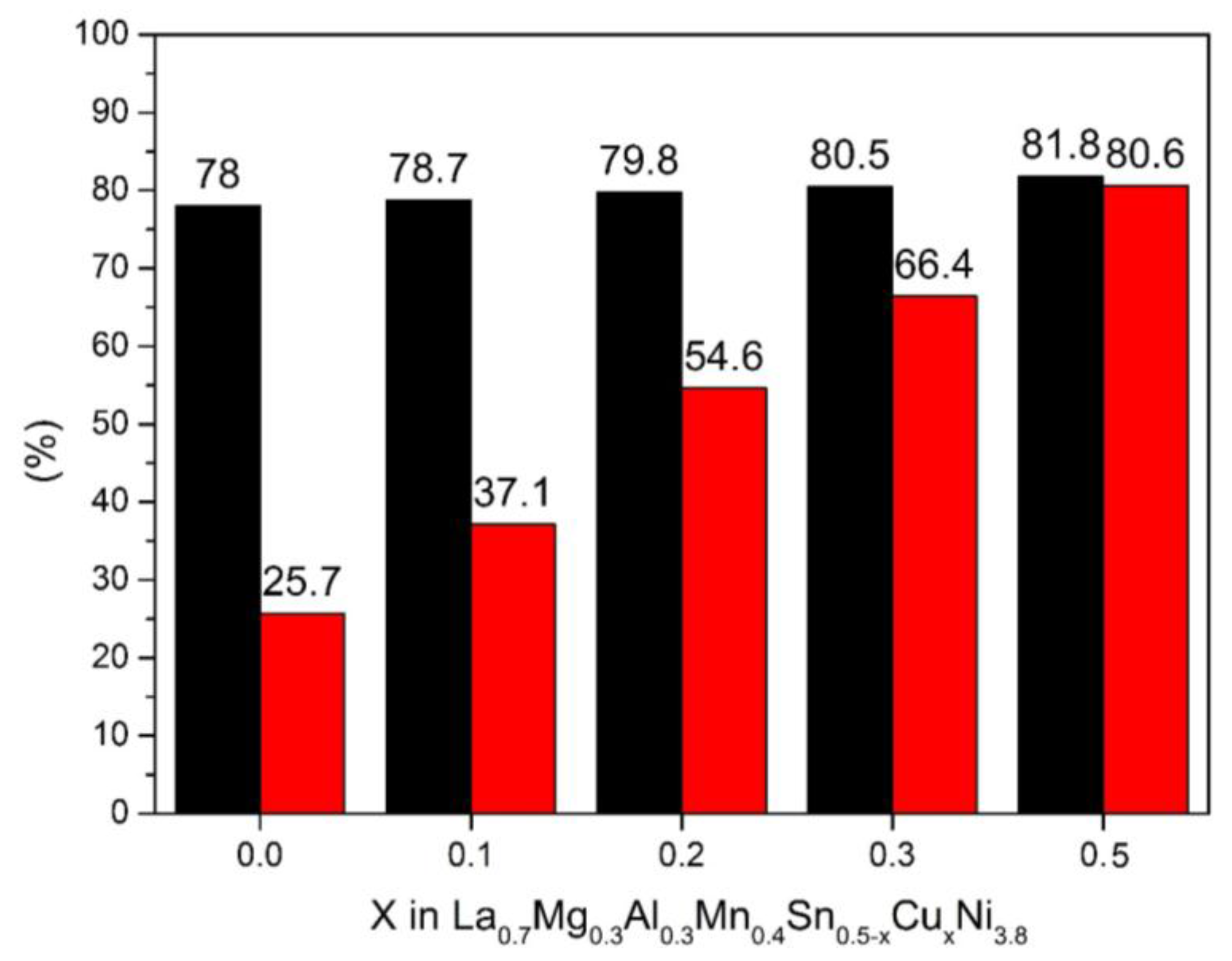
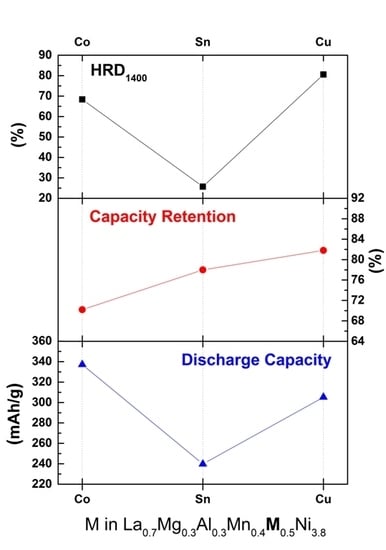
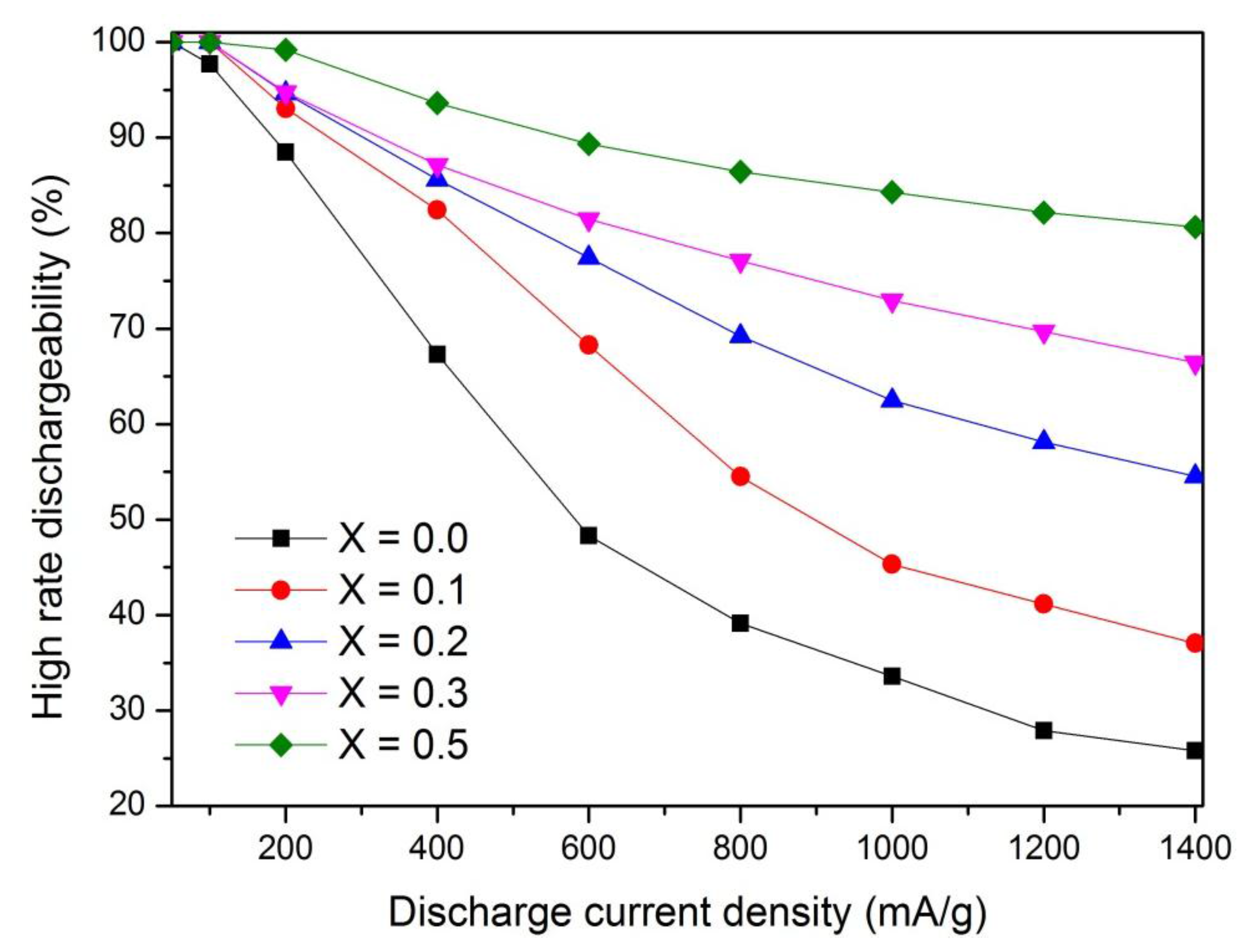
3.3. High Rate Dischargeability
| Alloy | Cmax (mA·h/g) | C100/Cmax (%) | HRD1400(%) a |
|---|---|---|---|
| La0.7Mg0.3Al0.3Mn0.4Sn0.5Ni3.8 | 239.8 | 78.0 | 25.7 |
| La0.7Mg0.3Al0.3Mn0.4Cu0.5Ni3.8 | 305.2 | 81.8 | 80.6 |
| La0.7Mg0.3Al0.3Mn0.4Co0.5Ni3.8 [9] | 337.1 | 70.2 | 68.4 |
| MmNi3.55Co0.75Mn0.4Al0.3 [17] | 283.0 | 92.2 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sakai, T.; Yoshinaga, H.; Miyamura, H.; Kuriyama, N.; Ishikawa, H. Rechargeable hydrogen batteries using rare-earth-based hydrogen storage alloys. J. Alloy. Compd. 1992, 180, 37–54. [Google Scholar] [CrossRef]
- Tang, W.; Gai, Y.; Zheng, H. Deterioration of copper-containing mischmetal-nickel-based hydrogen absorption electrode materials. J. Alloy. Compd. 1995, 224, 292–298. [Google Scholar] [CrossRef]
- Yang, S.; Han, S.; Song, J.; Li, Y. Influences of molybdenum substitution for cobalt on the phase structure and electrochemical kinetic properties of AB5-type hydrogen storage alloys. J. Rare Earths 2011, 29, 692–697. [Google Scholar] [CrossRef]
- Liu, B.; Hu, M.; Li, A.; Zhang, B.; Zhu, X. Microstructure and electrochemical characteristics of La0.7Ce0.3Ni3.75Mn0.35Al0.15Cu0.75−x(V0.81Fe0.19)x hydrogen storage alloys. J. Rare Earths 2012, 30, 769–774. [Google Scholar] [CrossRef]
- Ren, J.; Zhanga, T.; Feng, M.; Wang, G.; Zhao, X.; Wang, X. Microstructures and electrochemical properties of cobalt-free LaNi4.0Al0.2Fe0.4Cu0.4−xSnx (x = 0–0.4) electrode alloys prepared by casting. J. Rare Earths 2006, 24, 574–578. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Dong, X.P.; Wang, G.Q.; Guo, S.H.; Wang, X.L. Effect of substituting Mm with La on the electrochemical performances of Co-free LaxMm1−x(NiMnSiAlFe)4.9 (x = 0–1) electrode alloys prepared by casting and rapid quenching. J. Power Sources 2005, 140, 381–387. [Google Scholar] [CrossRef]
- Cuscueta, D.J.; Melnichuka, M.; Perettia, H.A.; Salva, H.R.; Ghilarduccia, A.A. Magnesium influence in the electrochemical properties of La-Ni base alloy for Ni-MH batteries. Int. J. Hydrog. Energy 2008, 33, 3566–3570. [Google Scholar] [CrossRef]
- Ferreira, E.A.; Serra, J.M.; Casini, J.C.S.; Takiishi, H.; Faria, R.N. Microstructure and electrochemical properties of a LaMgAlMnCoNi based alloy for Ni/MH batteries. Mater. Sci. Forum 2012, 727–728, 80–84. [Google Scholar]
- Casini, J.C.S.; Ferreira, E.A.; Guo, Z.; Liu, H.K.; Faria, R.N.; Takiishi, H. Effect of Sn substitution for Co on microstructure and electrochemical performance of AB5 type La0.7Mg0.3Al0.3Mn0.4Co0.5−xSnxNi3.8 (x = 0–0.5) alloys. Trans. Nonferrous Met. Soc. China 2015, 25, 520–526. [Google Scholar] [CrossRef]
- Huang, T.; Wu, Z.; Han, J.; Sun, G.; Yu, J.; Cao, X.; Xu, N.; Zhang, Y. Study on the structure and hydrogen storage characteristics of as-cast La0.7Mg0.3Ni3.2Co0.35−xCux alloys. Int. J. Hydrog. Energy 2010, 35, 8592–8596. [Google Scholar]
- Huang, T.; Yuan, X.; Yu, J.; Wu, Z.; Han, J.; Sun, G. Effects of annealing treatment and partial substitution of Cu for Co on phase composition and hydrogen storage performance of La0.7Mg0.3Ni3.2Co0.35 alloy. Int. J. Hydrog. Energy 2012, 37, 1074–1079. [Google Scholar] [CrossRef]
- Mungole, M.N.; Balasubramaniam, R.; Rai, K.N. Correlation between microstructure and hydrogen storage capacity in MmNi5 alloys with Al, Mn and Sn substitutions. Int. J. Hydrog. Energy 1999, 24, 467–471. [Google Scholar] [CrossRef]
- Chartouni, D.; Meli, F.; Züttel, A.; Gross, K.; Schlapbach, L. The influence of cobalt on the electrochemical cycling stability of LaNi5-based hydride forming alloys. J. Alloy. Compd. 1996, 241, 160–166. [Google Scholar] [CrossRef]
- Liu, B.; Hu, M.; Ji, J.; Fan, Y.; Wang, Y.; Zhang, Z.; Li, A. Phase structure and electrochemical properties of La0.7Ce0.3Ni3.75Mn0.35Al0.15Cu0.75−x(Fe0.43B0.57)x hydrogen storage alloys. J. Alloy. Compd. 2012, 516, 53–57. [Google Scholar] [CrossRef]
- Zhang, X.B.; Zi, D.; Yin, W.Y.; Chai, Y.J.; Zhao, M.S. Crystallographic and electrochemical characteristics of La0.7Mg0.3Ni5.0−x(Al0.5Mo0.5)x hydrogen-storage alloys. ChemPhysChem 2005, 6, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ma, J.; Wang, C.; Chen, S.; Wang, X.; Chen, C.; Wang, Q. Studies on the electrochemical properties of MlNi4.3−xCoxAl0.7 hydride alloy electrodes. J. Alloy. Compd. 1999, 293–295, 648–652. [Google Scholar] [CrossRef]
- Chandra, D.; Chien, W.M.; Talekar, A. Metal hydrides for NiMH battery applications. Mater. Matters 2011, 6, 48–53. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casini, J.C.S.; Guo, Z.; Liu, H.K.; Faria, R.N.; Takiishi, H. Effects of Cu Substitution for Sn on the Electrochemical Performance of La0.7Mg0.3Al0.3Mn0.4Sn0.5−xCuxNi3.8 (x = 0–0.5) Alloys for Ni-MH Batteries. Batteries 2015, 1, 3-10. https://doi.org/10.3390/batteries1010003
Casini JCS, Guo Z, Liu HK, Faria RN, Takiishi H. Effects of Cu Substitution for Sn on the Electrochemical Performance of La0.7Mg0.3Al0.3Mn0.4Sn0.5−xCuxNi3.8 (x = 0–0.5) Alloys for Ni-MH Batteries. Batteries. 2015; 1(1):3-10. https://doi.org/10.3390/batteries1010003
Chicago/Turabian StyleCasini, Julio Cesar Serafim, Zaiping Guo, Hua Kun Liu, Rubens Nunes Faria, and Hidetoshi Takiishi. 2015. "Effects of Cu Substitution for Sn on the Electrochemical Performance of La0.7Mg0.3Al0.3Mn0.4Sn0.5−xCuxNi3.8 (x = 0–0.5) Alloys for Ni-MH Batteries" Batteries 1, no. 1: 3-10. https://doi.org/10.3390/batteries1010003