Dynamic Changes on Floral Aroma Composition of the Three Species from Tilia at Different Flowering Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Detection Methods
2.3. Identification and Quantification of Aroma Components
2.4. Calculation of Aroma Similarity Rates
2.5. Statistical Analysis
3. Results
3.1. Dynamic Changes of Floral Aroma at Different Stages
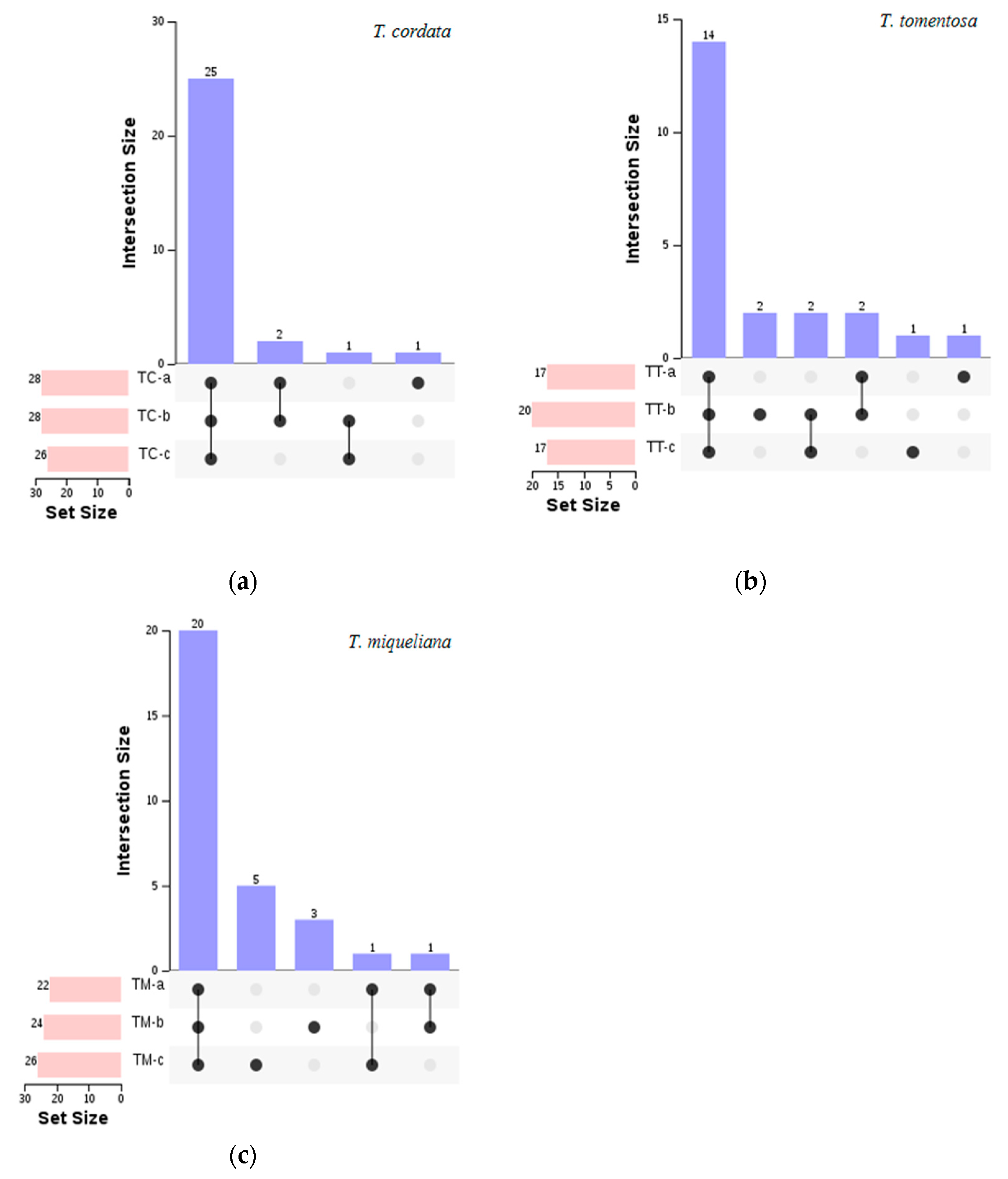
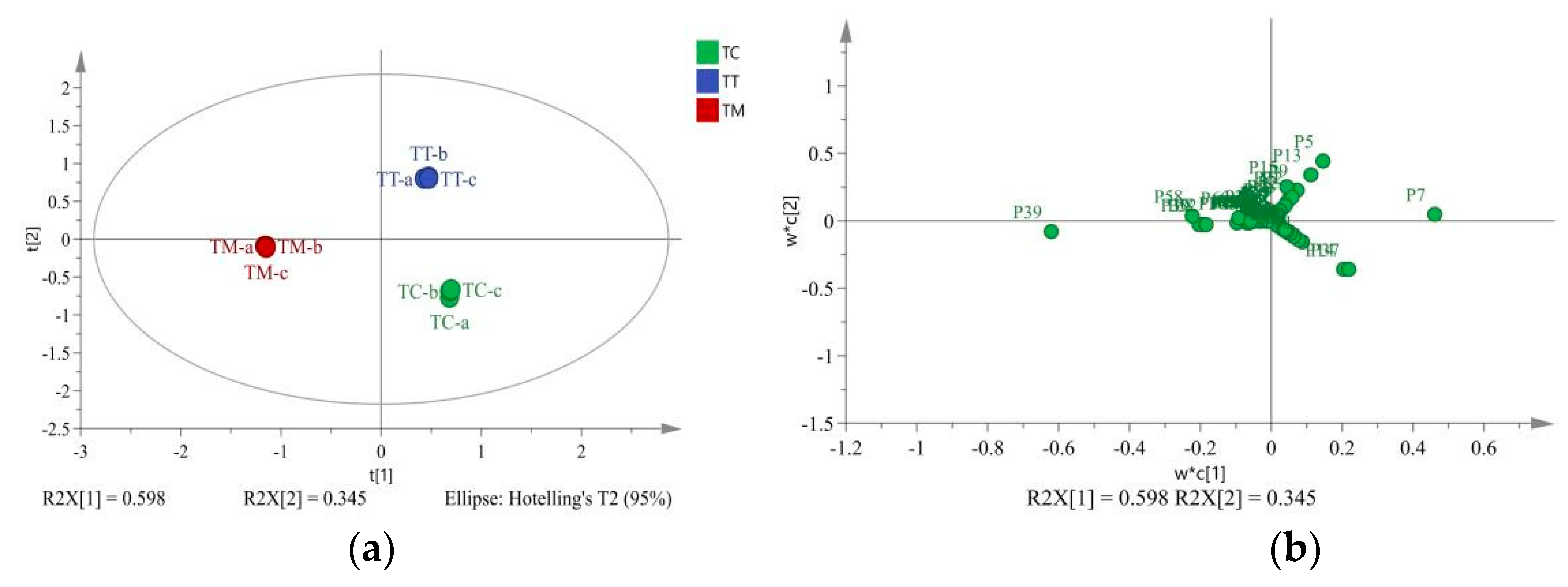
3.2. Chemometric Analysis of Floral Aroma Components
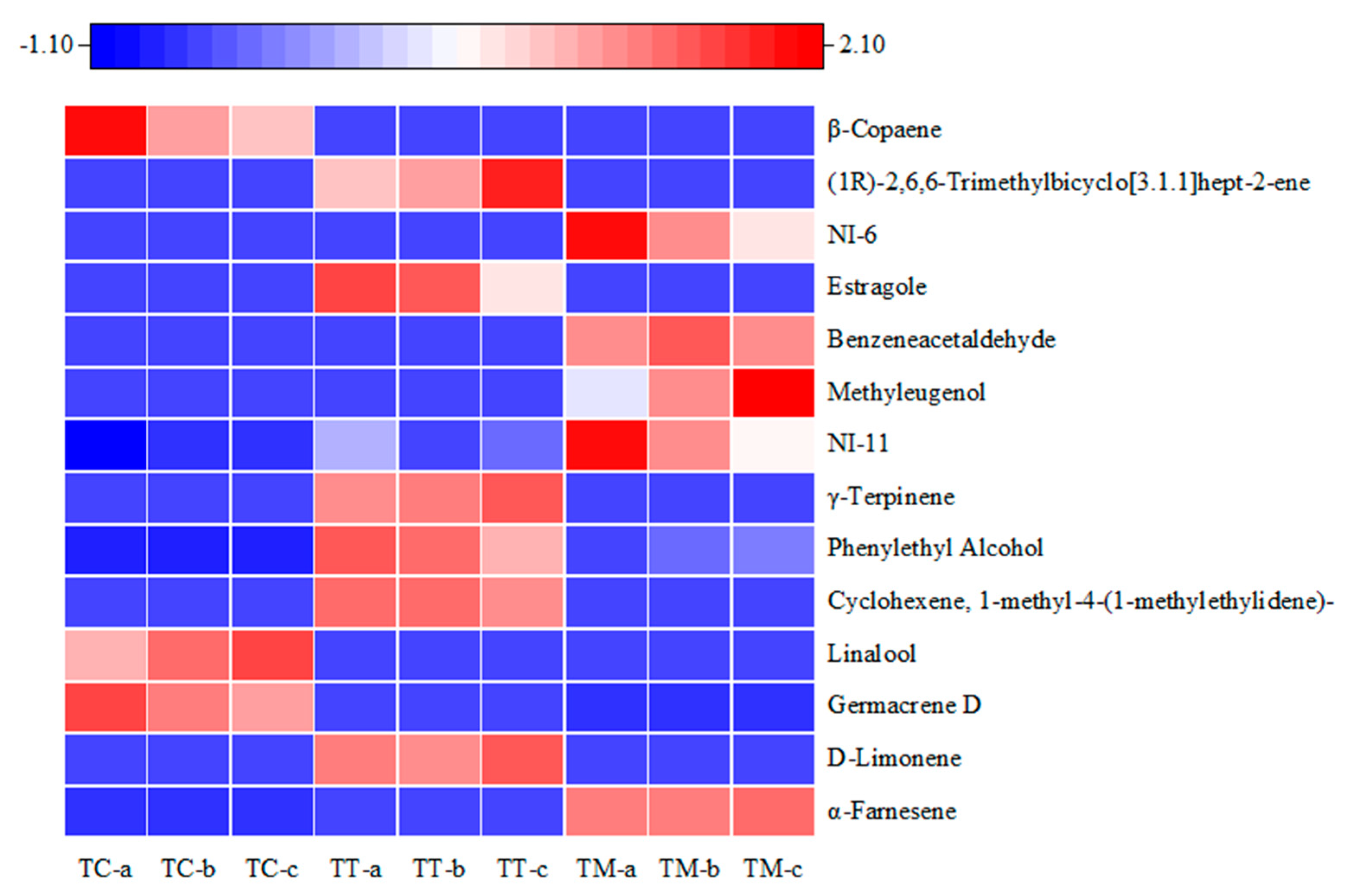
3.3. Determination of Crucial Components
3.4. Aroma Similarity Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pichersky, E.; Gang, D.R. Genetics and Biochemistry of Secondary Metabolites in Plants: An Evolutionary Perspective. Trends Plant Sci. 2000, 5, 439–445. [Google Scholar] [CrossRef]
- Jurgens, A.; Witt, T.; Gottsberger, G. Flower Aroma Composition in Dianthus and Saponaria Species (Caryophyllaceae) and Its Relevance for Pollination Biology and Taxonomy. Biochem. Syst. Ecol. 2003, 31, 345–357. [Google Scholar] [CrossRef]
- Klahre, U.; Gurba, A.; Hermann, K.; Saxenhofer, M.; Bossolini, E.; Guerin, P.M.; Kuhlemeier, C. Pollinator Choice in Petunia Depends on Two Major Genetic Loci for Floral Aroma Production. Curr. Biol. 2011, 21, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Raguso, R.A.; Levin, R.A.; Foose, S.E.; Holmberg, M.W.; McDade, L.A. Fragrance Chemistry, Nocturnal Rhythms and Pollination “Syndromes” in Nicotiana. Phytochemistry 2003, 63, 265–284. [Google Scholar] [CrossRef]
- Jin, L.; Yang, M.L.; Wang, J.Z. Research Progress of Floral Metabolism in Plants. Mod. Gard. 2019, 5, 8–10. [Google Scholar] [CrossRef]
- Piechulla, B.; Pott, M.B. Plant Scents-Mediators of Inter- and Intraorganismic Communication. Planta 2003, 217, 687–689. [Google Scholar] [CrossRef]
- Burger, H.; Dotterl, S.; Ayasse, M. Host-Plant Finding and Recognition by Visual and Olfactory Floral Cues in an Oligolectic Bee. Funct. Ecol. 2010, 24, 1234–1240. [Google Scholar] [CrossRef]
- Hoballah, M.E.; Stuurman, J.; Turlings, T.C.J.; Guerin, P.M.; Connetable, S.; Kuhlemeier, C. The Composition and Timing of Flower Odour Emission by Wild Petunia axillaris Coincide with the Antennal Perception and Nocturnal Activity of the Pollinator Manduca sexta. Planta 2005, 222, 141–150. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The Formation and Function of Plant Volatiles: Perfumes for Pollinator Attraction and Defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Schiestl, F.P. On the Success of a Swindle: Pollination by Deception in Orchids. Naturwissenschaften 2005, 92, 255–264. [Google Scholar] [CrossRef]
- Miao, M.M.; Chen, J.C.; Zhang, Z.D. Physiology, Genetics and Manipulation of Flower Fragrance. Mol. Plant Breed. 2007, 5, 67–74. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and Distribution of Floral Aroma. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Qiu, Y.X.; Chen, J.Y.; Tian, M.J.; Xie, Z.H.; Chen, X.M.; Zhan, J.S. Study on the Volatile Components in Flowers of 12 Camellia Species. For. Res. 2015, 28, 358–364. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, J.J.; Feng, S.C. The Aroma Components Analysis of Rosa odorata var. Erubescens and Its Application in Urban Gardens. Chin. Agric. Sci. Bull. 2021, 37, 71–75. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Li, J.Y.; Li, X.L.; Yin, H.F. Analysis on the Aroma Components of Different Floral Organs of Aromatic Camellia‘Kramer’s Supreme’Based on HS-SPME/GC-MS. Bull. Bot. Res. 2014, 34, 136–142. [Google Scholar] [CrossRef]
- Yi, H.Z.; Chuanyuan, D. Study on Anatomical Characters and Garden Application of Some Species in Tilia L. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, April 2011. [Google Scholar]
- Fitsiou, I.; Tzakou, O.; Hancianu, M.; Poiata, A. Volatile Constituents and Antimicrobial Activity of Tilia tomentosa Moench and Tilia Cordata Miller Oils. J. Essent. Oil Res. 2007, 19, 183–185. [Google Scholar] [CrossRef]
- Zori, M.; Kosti, S.; Kebert, M.; Bozin, B.; Orlovi, S. Volatile organic compounds of Tilia cordata Mill. from Serbia, in terms of ecosystem services. Topola 2020, 206, 21–28. [Google Scholar] [CrossRef]
- Toker, G.; Baser, K.H.C.; Kürkçüoglu, M. and Özek, T. The composition of essential oils from Tilia L. species growing in Turkey. J. Essent. Oil Res. 1999, 11, 369–374. [Google Scholar] [CrossRef]
- Kowalski, R.; Baj, T.; Kalwa, K.; Kowalska, G.; Sujka, M. Essential oil composition of Tilia cordata flowers. J. Essent. Oil Bear. Plants 2017, 20, 1137–1142. [Google Scholar] [CrossRef]
- Ji, X. Comparative Investigation of Volatile Components and Bioactive Compounds in Beers by Multivariate Analysis. Flavour Fragr. J. 2021, 36, 374–383. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Zang, M.W.; Zhang, K.H.; Wang, S.W.; Li, D.; Li, X.M. Effects of Phospholipids and Reheating Treatment on Volatile Compounds in Phospholipid-Xylose-Cysteine Reaction Systems. Food Res. Int. 2021, 139, 109918. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.C.; Nunes, C.E.P.; Brito, V.L.G.; Caetano, A.P.S. Floral Oil Production in a Family Dominated by Pollen Flowers: The Case of Macairea radula (Melastomataceae). Flora 2022, 288, 152008. [Google Scholar] [CrossRef]
- Xu, W.; Qixiang, Z. Study of Emission Patterns of Characteristic Volatiles Identified in Lagerstroemia caudata Flowers and Key Generelated to MEP Pathway. Ph.D. Dissertation, Beijing Forestry University, Beijing, China, June 2019. [Google Scholar] [CrossRef]
- Wang, H.F.; Li, M.J.; Liu, Z.H.; Wang, Z.H.; Shi, Z.P. The Aroma Change of Fu Brick Tea during Its Blooming. J. Tea Sci. 1991, 81–86. [Google Scholar] [CrossRef]
- Allebone, J.; Hamilton, R.J.; Knights, B.A.; Middleditch, B.S.; Power, D.M. Cuticular Leaf Waxes Part II. Chenopodium album L. and Lolium perenne L. Chem. Phys. Lipids 1970, 4, 37–46. [Google Scholar] [CrossRef]
- Hirri, A.; Bassbasi, M.; Platikanov, S.; Tauler, R.; Oussama, A. FTIR Spectroscopy and PLS-DA Classification and Prediction of Four Commercial Grade Virgin Olive Oils from Morocco. Food Anal. Methods 2016, 9, 974–981. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, H.Q.; Jiang, Z.H. Study on Discriminant Analysis of Wood Bio-Decay by PLS-DA Method. Spectrosc. Spectr. Anal. 2008, 4, 793–796. [Google Scholar] [CrossRef]
- Liu, B.Q.; Chen, X.Q.; Wu, X.G.; Zhang, W.; Wang, Z. Study of Pu’er Raw Materials Grade Classification by PCA and PLS-DA. J. Tea Sci. 2015, 35, 179–184. [Google Scholar] [CrossRef]
- Effmert, U.; Große, J.; Röse, U.S.R.; Ehrig, F.; Kägi, R.; Piechulla, B. Volatile composition, emission pattern, and localization of floral scent emission in Mirabilis jalapa (Nyctaginaceae). Am. J. Bot. 2005, 92, 2–12. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.Y.; Zheng, C.S.; Wang, C.; Yoo, Y.K. Studies of Aroma Compounds in Chrysanthemum in Different Florescence and Inflorescence Parts and Aroma Releasing. Acta Bot. Boreali-Occident. Sin. 2012, 32, 722–730. [Google Scholar] [CrossRef]
- Goodwin, S.M.; Kolosova, N.; Kish, C.M.; Wood, K.V.; Jenks, M.A. Cuticle Characteristics and Volatile Emissions of Petals in Antirrhinum majus. Physiol. Plant. 2010, 117, 435–443. [Google Scholar] [CrossRef]
- Li, Y.Y.; Xu, H.Y.; Ma, H.; Li, Z.H. Temporal Variation of Flora Aroma Emission of a Hybrid Variety of Luculia. J. Shanxi Agric. Univ. Nat. Sci. Ed. 2020, 40, 8–18. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, G.F.; Zhang, J.Z.; Li, X.D. Study on Floral Aroma of the Genus Hosta. Sci. Agric. Sin. 2015, 48, 4323–4334. [Google Scholar] [CrossRef]
- Lin, R.Y.; Zhong, H.X.; Huang, M.L.; Luo, Y.H.; Lin, B. Study on the Temporal and Spatial Dynamics of Polianthes tuberosa. J. Nucl. Agric. Sci. 2017, 31, 2434–2442. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Pan, H.T.; Zhang, Q.X.; Sun, M.; Pan, C.B. Dynamics of Fragrant Compounds from Prunus mume Flowers. J. Beijing For. Univ. 2010, 18, 310–315. [Google Scholar] [CrossRef]
- Shi, T.T.; Yang, X.L.; Wang, L.G. Dynamic Characteristics of Floral Components and Anatomical Observation of Petals in Three Cultivars of Osmanthus fragrans. J. Nanjing For. Univ. Sci. Ed. 2020, 44, 12–20. [Google Scholar] [CrossRef]
- Dudareva, N.; Murfitt, L.M.; Mann, C.J.; Gorenstein, N.; Kolosova, N.; Kish, C.M.; Bonham, C.; Wood, K. Developmental Regulation of Methyl Benzoate Biosynthesis and Emission in Snapdragon Flowers. Plant Cell 2000, 12, 949–961. [Google Scholar] [CrossRef]
- Kotze, M.J.; Jürgens, A.; Johnson, S.D.; Hoffmann, J.H. Volatiles Associated with Different Flower Stages and Leaves of Acacia cyclops and Their Potential Role as Host Attractants for Dasineura dielsi (Diptera: Cecidomyiidae). S. Afr. J. Bot. 2010, 76, 701–709. [Google Scholar] [CrossRef]
- Shi, T.T.; Yang, X.L.; Wang, L.G. Study on the Aroma Component Emission Pattern of Osmanthus fragrans‘Boye Jingui’. J. Nanjing For. Univ. Sci. Ed. 2018, 42, 97–104. [Google Scholar] [CrossRef]
- Liu, C.; Zeng, X.L.; Zheng, R.R.; Luo, J.; Wang, C.Y. Cloning and Expression of the Alcohol Acyltransferase Gene from Osmanthus fragrans Flowers. J. Huazhong Agric. Univ. Nat. Sci. Ed. 2016, 35, 36–42. [Google Scholar] [CrossRef]
- Kong, Y.; Sun, M.; Pan, H.T.; Zhang, Q.X. Advances in Metabolism and Regulation of Floral Aroma. J. Beijing For. Univ. 2012, 34, 146–154. [Google Scholar] [CrossRef]
- Huang, X.L.; Zheng, B.Q.; Wang, Y. Study of Aroma Compounds in Flowers of Dendrobium chrysotoxum in Different Florescence Stages and Diurnal Variation of Full Blooming Stage. For. Res. 2018, 31, 142–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, M.; Wang, C.X. Components and Sensory Evaluation of Flower Aroma of Oncidium Sharry Baby‘Sweet Fragrance’under Different Light Conditions. J. Plant Resour. Environ. 2018, 27, 107–109. [Google Scholar] [CrossRef]
- Gao, L.P.; Wang, L.M.; Zhang, Y.Q.; Xia, T.; Wan, X.C. Studies on the Aroma Releasing Enzymes of Jasmine Flowers. J. Tea Sci. 2001, 2, 140–143. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.H. Transcriptional Regulation of α-Farnesene Synthesis by Md MYC2 and Md ERF3 in Apple Fruit. Master’s Thesis, Shandong Agricultural University, Taian, China, May 2019. [Google Scholar]
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T.S. Identification and Microbial Production of a Terpene-Based Advanced Biofuel. Nat. Commun. 2011, 2, 483. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.L.; Dai, H. Application and challenges of plant essential oils in health pest control. Chin. J. Hyg. Insectic. Equip. 2019, 25, 284–288. [Google Scholar] [CrossRef]
- Elegbede, J.A.; Elson, C.E.; Qureshi, A.; Tanner, M.A.; Gould, M.N. Inhibition of DMBA-Induced Mammary Cancer by the Monoterpene d-Limonene. Carcinogenesis 1984, 5, 661–664. [Google Scholar] [CrossRef]
- Huo, M.; Cui, X.; Xue, J.; Chi, G.; Gao, R.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H.; et al. Anti-Inflammatory Effects of Linalool in RAW 264.7 Macrophages and Lipopolysaccharide-Induced Lung Injury Model. J. Surg. Res. 2013, 180, E47–E54. [Google Scholar] [CrossRef]
- Zhang, J.S.; Li, B.; Chen, J.K.; Zhou, T.S. Chemical Constituents and Antimicrobial Activity of Volatile Oil from Solidago canadensis L. J. Fudan Univ. Nat. Sci. 2006, 3, 412–416. [Google Scholar] [CrossRef]
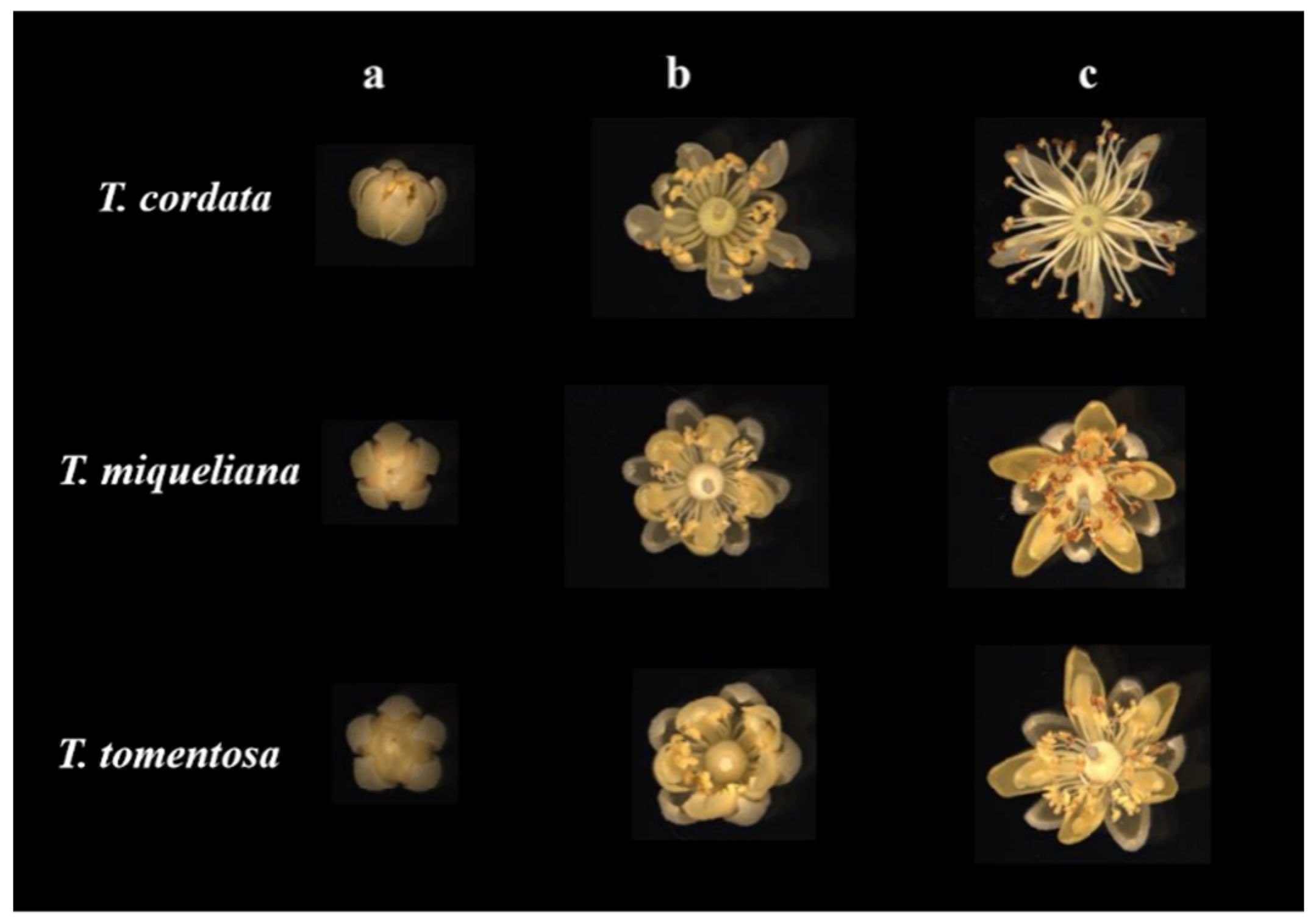
| Species | Abbreviation | Blooming Stages Flower Diameter (mm) | Collection Date |
|---|---|---|---|
| T. cordata | TC | 10–12 | 27 May 2021 |
| T. miqueliana | TM | 11–13 | 29 May 2021 |
| T. tomentosa | TT | 12–14 | 1 June 2021 |
| No. | Compounds Name | CAS Number | RI | Relative Content (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TC-a | TC-b | TC-c | TT-a | TT-b | TT-c | TM-a | TM-b | TM-c | ||||
| 1 | NI-1 | - | - | - | - | - | 3.30 | - | - | - | - | - |
| 2 | NI-2 | - | - | - | - | - | - | - | - | - | 0.06 | - |
| 3 | NI-3 | - | - | - | - | - | - | - | - | - | - | 0.65 |
| 4 | NI-4 | - | 731 | - | - | - | - | - | - | 0.17 | 0.20 | 0.19 |
| 5 | NI-5 | - | 749 | - | - | - | - | - | - | 0.43 | - | 0.19 |
| 6 | (1R)-2, 6, 6-Trimethylbicyclo [3.1.1]hept-2-ene | 7785-70-8 | 948 | - | - | - | 2.41 | 2.85 | 4.04 | - | - | - |
| 7 | β-Myrcene | 123-35-3 | 996 | - | - | - | - | 2.90 | 4.40 | - | - | - |
| 8 | NI-6 | - | 1004 | - | - | - | - | - | - | 7.33 | 5.21 | 3.62 |
| 9 | NI-7 | - | 1011 | 0.70 | 0.90 | 0.99 | - | - | - | - | - | - |
| 10 | NI-8 | - | 1013 | - | - | - | - | 0.83 | - | - | - | - |
| 11 | α-Phellandrene | 99-83-2 | 1014 | - | - | - | - | - | 0.32 | - | - | - |
| 12 | D-Limonene | 5989-27-5 | 1038 | - | - | - | 19.88 | 19.04 | 22.55 | 0.21 | 0.31 | - |
| 13 | trans-β-Ocimene | 3779-61-1 | 1040 | 0.88 | 1.03 | 0.96 | - | - | - | - | - | - |
| 14 | β-Ocimene | 13877-91-3 | 1052 | 29.47 | 34.44 | 36.90 | 32.62 | 32.44 | 32.68 | - | - | - |
| 15 | Benzene, 1-methoxy-4-methyl- | 104-93-8 | 1056 | - | - | - | - | - | - | - | - | 0.19 |
| 16 | γ-Terpinene | 99-85-4 | 1067 | - | - | - | 4.80 | 4.97 | 5.53 | - | - | - |
| 17 | Bicyclo [3.1.0]hexan-2-ol, 2-methyl-5-(1-methylethyl)-, (1α,2β,5α)- | 15537-55-0 | 1079 | - | - | - | - | 0.33 | 0.36 | - | - | - |
| 18 | Benzeneacetaldehyde | 122-78-1 | 1088 | - | - | - | - | - | - | 5.48 | 6.41 | 5.40 |
| 19 | NI-9 | - | 1090 | - | - | - | - | - | - | 0.27 | 0.15 | 0.15 |
| 20 | trans-Linalool oxide (furanoid) | 34995-77-2 | 1098 | 1.55 | 1.04 | 0.87 | - | - | - | - | - | - |
| 21 | Cyclohexene, 1-methyl-4-(1-methylethylidene)- | 586-62-9 | 1099 | - | - | - | 12.22 | 12.35 | 11.39 | - | - | - |
| 22 | Benzene, (2-methoxyethyl)- | 3558-60-9 | 1102 | - | - | - | - | - | - | 0.25 | 0.39 | 0.43 |
| 23 | Linalool | 78-70-6 | 1109 | 14.74 | 18.73 | 21.30 | - | - | - | - | - | - |
| 24 | NI-10 | - | 1113 | - | - | - | - | 1.79 | - | - | - | - |
| 25 | 2, 4, 6-Octatriene, 2, 6-dimethyl- | 673-84-7 | 1139 | 1.56 | 1.21 | 1.19 | - | - | - | - | - | - |
| 26 | NI-11 | - | 1149 | 2.22 | 3.45 | 3.40 | 6.79 | 4.21 | 5.00 | 15.17 | 12.03 | 9.12 |
| 27 | Lilac aldehyde (isomer II) | - | 1166 | - | 0.59 | 0.41 | - | - | - | - | - | 0.10 |
| 28 | Phenylethyl Alcohol | 60-12-8 | 1180 | - | - | - | 7.43 | 7.17 | 5.70 | 0.68 | 1.59 | 1.77 |
| 29 | (3R,6S)-2,2,6-Trimethyl-6-vinyltetrahydro-2H-pyran-3-ol | 39028-58-5 | 1196 | 0.51 | 0.34 | 0.29 | - | - | - | - | - | - |
| 30 | 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-, (R)- | 20126-76-5 | 1204 | - | - | - | 0.35 | 0.32 | 0.37 | - | - | - |
| 31 | Benzoic acid, ethyl ester | 93-89-0 | 1208 | - | - | - | 0.62 | 0.94 | 0.70 | - | - | - |
| 32 | Benzene, (2-methoxyethenyl)- | 4747-15-3 | 1216 | - | - | - | - | - | - | 0.13 | 0.19 | 0.22 |
| 33 | NI-12 | - | 1219 | 0.73 | - | - | - | - | - | - | - | - |
| 34 | Estragole | 140-67-0 | 1225 | - | - | - | 3.96 | 3.87 | 2.32 | - | - | - |
| 35 | Lilac alcohol (isomer III) | - | 1228 | 0.64 | 0.40 | - | - | - | - | - | - | - |
| 36 | Lilac alcohol (isomer IV) | - | 1241 | 0.88 | 0.46 | - | - | - | - | - | - | - |
| 37 | Lilac alcohol D | 33081-37-7 | 1259 | - | - | - | - | - | - | - | - | 0.10 |
| 38 | Acetic acid, 2-phenylethyl ester | 103-45-7 | 1295 | - | - | - | 0.44 | 0.50 | - | 0.16 | 0.29 | 0.61 |
| 39 | Tridecane | 629-50-5 | 1300 | - | - | - | - | - | - | - | 0.19 | - |
| 40 | NI-13 | - | 1301 | - | - | - | - | - | - | - | - | 0.09 |
| 41 | NI-14 | - | 1319 | 0.83 | 1.21 | 1.12 | 1.95 | 1.18 | 1.79 | 3.61 | 2.61 | 2.11 |
| 42 | NI-15 | - | 1359 | 0.84 | 0.53 | 0.46 | - | - | - | - | - | - |
| 43 | α-Cubebene | 17699-14-8 | 1370 | 0.43 | 0.34 | 0.34 | - | - | - | - | - | - |
| 44 | Aromadendrene | 109119-91-7 | 1409 | 1.03 | 0.82 | 0.85 | - | - | - | - | - | - |
| 45 | Phenol, 2-methoxy-4-(1-propenyl)- | 97-54-1 | 1434 | - | - | - | 1.14 | 2.20 | 1.00 | - | - | - |
| 46 | β-Copaene | 18252-44-3 | 1436 | 4.39 | 3.02 | 2.54 | - | - | - | - | - | - |
| 47 | Methyleugenol | 93-15-2 | 1443 | - | - | - | - | - | - | 3.71 | 7.00 | 10.26 |
| 48 | NI-16 | - | 1446 | 2.57 | 1.74 | 1.47 | - | - | - | - | - | - |
| 49 | (E)-β-Famesene | 18794-84-8 | 1459 | - | - | - | - | - | - | 0.09 | 0.11 | 0.16 |
| 50 | NI-17 | - | 1461 | 1.18 | 0.86 | 0.81 | - | - | - | - | - | - |
| 51 | NI-18 | - | 1468 | - | - | - | - | - | - | 0.12 | 0.11 | 0.13 |
| 52 | NI-19 | - | 1470 | 1.71 | 1.16 | 1.11 | - | - | - | - | - | - |
| 53 | cis-Muurola-4(15),5-diene | 157477-72-0 | 1479 | 0.63 | 0.56 | 0.50 | - | - | - | - | - | - |
| 54 | NI-20 | - | 1489 | - | - | - | - | - | - | 0.25 | 0.18 | 0.18 |
| 55 | γ-Muurolene | 30021-74-0 | 1491 | 1.52 | 1.25 | 1.11 | - | - | - | - | - | - |
| 56 | 1, 3, 6, 10-Dodecatetraene, 3, 7, 11-trimethyl-, (Z,E)- | 26560-14-5 | 1498 | - | - | - | - | - | - | 1.23 | 1.28 | 1.68 |
| 57 | Germacrene D | 23986-74-5 | 1501 | 22.30 | 18.67 | 16.83 | 0.48 | 0.66 | 0.90 | - | - | - |
| 58 | NI-21 | - | 1511 | 0.89 | 0.80 | 0.84 | - | - | - | - | - | - |
| 59 | α-Farnesene | 502-61-4 | 1514 | - | - | - | 0.75 | 0.93 | 0.95 | 58.63 | 59.35 | 60.37 |
| 60 | NI-22 | - | 1516 | 1.26 | 0.96 | 0.80 | - | - | - | - | - | - |
| 61 | NI-23 | - | 1519 | 1.00 | 0.62 | 0.48 | - | - | - | - | - | - |
| 62 | Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-7-methyl-4-methylene-1-(1-methylethyl)-, (1α,4aβ,8aα)- | 39029-41-9 | 1533 | 1.81 | 1.59 | 1.45 | - | - | - | - | - | - |
| 63 | NI-24 | - | 1535 | - | - | - | - | - | - | 0.34 | 0.35 | 0.36 |
| 64 | Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methyl ethyl)-, (1S-cis)- | 483-76-1 | 1539 | 3.07 | 2.69 | 2.44 | - | - | - | - | - | - |
| 65 | NI-25 | - | 1550 | - | - | - | - | - | - | - | 0.06 | - |
| 66 | Naphthalene, 1,2,4a,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, [1S-(1α,4aβ,8aα)]- | 24406-05-1 | 1556 | 0.66 | 0.59 | 0.54 | - | - | - | - | - | - |
| 67 | NI-26 | - | 1603 | - | - | - | - | - | - | 0.59 | 0.62 | 0.69 |
| 68 | Benzene, 1,2,3-trimethoxy-5-(2-propenyl)- | 487-11-6 | 1609 | - | - | - | - | - | - | 0.60 | 0.76 | 0.66 |
| 69 | NI-27 | - | 1623 | - | - | - | - | - | - | 0.55 | 0.55 | 0.57 |
| 70 | Benzyl Benzoate | 120-51-4 | 1744 | - | - | - | 0.86 | 0.52 | - | - | - | - |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | |||
| No. | Components Name | VIP | p-Value | Aroma Characteristics |
|---|---|---|---|---|
| 1 | α-Farnesene | 3.70 | 0.02 | Citrus, herbal, lavender |
| 2 | D-Limonene | 2.76 | 0.03 | Citrus, orange |
| 3 | Germacrene D | 2.50 | 0.02 | Woody, spice |
| 4 | Linalool | 2.47 | 0.02 | Citrus, woody, floral |
| 5 | Cyclohexene, 1-methyl-4-(1-methylethylidene)- | 2.11 | 0.02 | Citrus, woody |
| 6 | Phenylethyl Alcohol | 1.55 | 0.02 | floral, rose |
| 7 | γ-Terpinene | 1.38 | 0.02 | Oily, woody, lemon |
| 8 | NI-11 | 1.33 | 0.03 | - |
| 9 | Methyleugenol | 1.21 | 0.02 | Spicy, cinnamon, clove |
| 10 | Benzeneacetaldehyde | 1.16 | 0.02 | Honey, floral, cocoa |
| 11 | Estragole | 1.10 | 0.02 | Spice, herbal, anise |
| 12 | NI-6 | 1.09 | 0.02 | - |
| 13 | (1R)-2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene | 1.06 | 0.02 | Terpene, aromatic, minty |
| 14 | β-Copaene | 1.04 | 0.02 | - |
| TC-a | TC-b | TC-c | TT-a | TT-b | TT-c | TM-a | TM-b | TM-c | |
| TC-a | 1.000 | 0.986 | 0.972 | 0.578 | 0.585 | 0.572 | 0.015 | 0.012 | 0.009 |
| TC-b | 1.000 | 0.997 | 0.626 | 0.631 | 0.616 | 0.021 | 0.016 | 0.012 | |
| TC-c | 1.000 | 0.635 | 0.641 | 0.625 | 0.020 | 0.015 | 0.012 | ||
| TT-a | 1.000 | 0.991 | 0.986 | 0.063 | 0.058 | 0.048 | |||
| TT-b | 1.000 | 0.993 | 0.052 | 0.050 | 0.043 | ||||
| TT-c | 1.000 | 0.056 | 0.052 | 0.044 | |||||
| TM-a | 1.000 | 0.996 | 0.987 | ||||||
| TM-b | 1.000 | 0.997 | |||||||
| TM-c | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, W.; Shen, Y. Dynamic Changes on Floral Aroma Composition of the Three Species from Tilia at Different Flowering Stages. Horticulturae 2022, 8, 719. https://doi.org/10.3390/horticulturae8080719
Bao W, Shen Y. Dynamic Changes on Floral Aroma Composition of the Three Species from Tilia at Different Flowering Stages. Horticulturae. 2022; 8(8):719. https://doi.org/10.3390/horticulturae8080719
Chicago/Turabian StyleBao, Wenqin, and Yongbao Shen. 2022. "Dynamic Changes on Floral Aroma Composition of the Three Species from Tilia at Different Flowering Stages" Horticulturae 8, no. 8: 719. https://doi.org/10.3390/horticulturae8080719
APA StyleBao, W., & Shen, Y. (2022). Dynamic Changes on Floral Aroma Composition of the Three Species from Tilia at Different Flowering Stages. Horticulturae, 8(8), 719. https://doi.org/10.3390/horticulturae8080719





