The Seed Traits Associated with Dormancy and Germination of Herbaceous Peonies, Focusing on Species Native in Serbia and China
Abstract
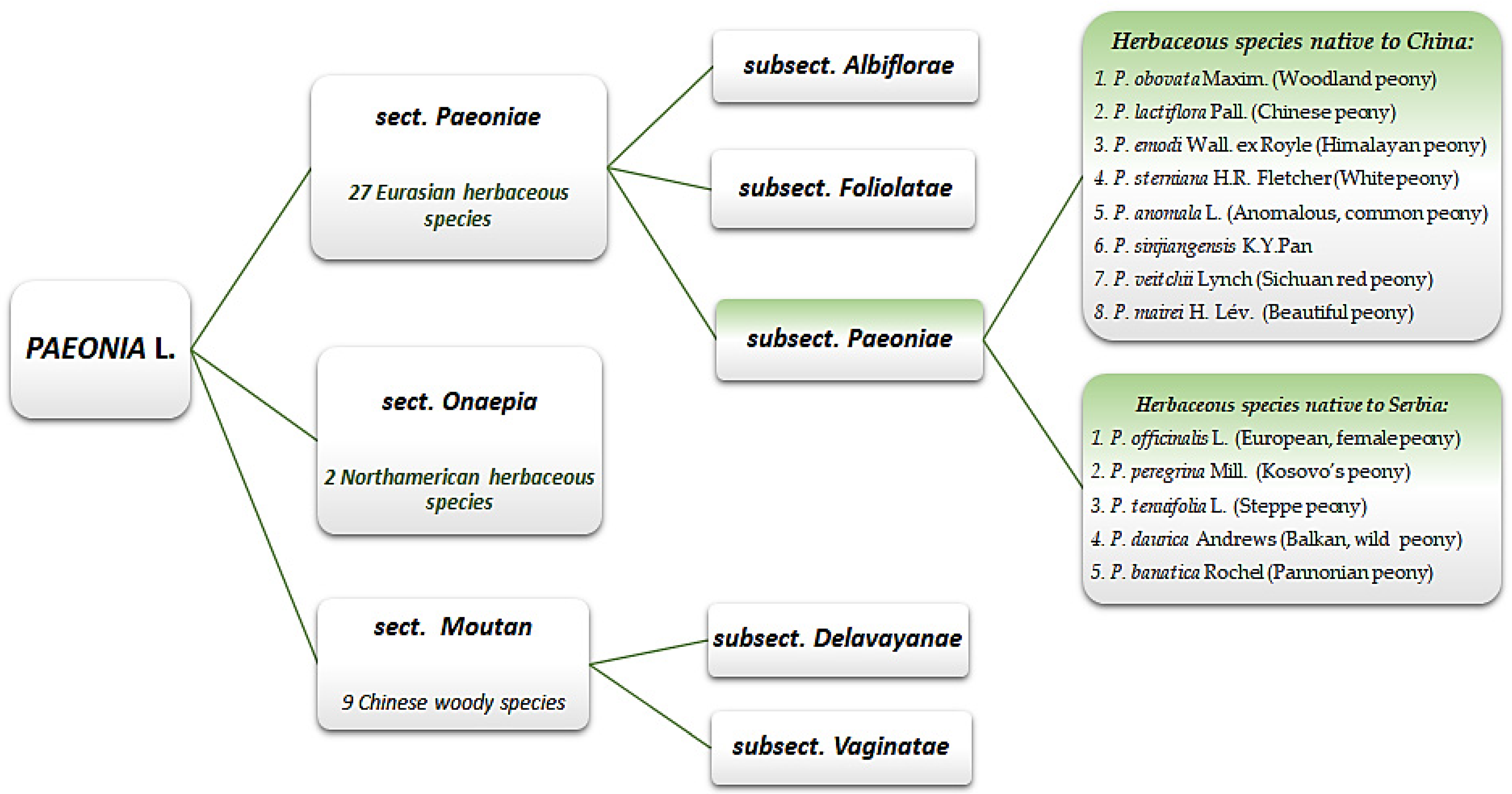
:1. Introduction
2. Seed Properties
2.1. Physical and Morphological Properties
2.2. Seed Collection Period
2.3. Seed Dormancy
3. Seed Germination
4. Pre-Treatments for Dormancy Release Process
4.1. Temperature
4.2. Light
4.3. Hormones, Enzymes, and Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Guo, H.; Tao, J. Effects of harvest stage, storage, and preservation technology on postharvest ornamental value of cut peony (Paeonia lactiflora) flowers. Agronomy 2022, 12, 230. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.; Wei, J.; Shi, X.; Ding, H.; Qiu, S.; Guo, J.; Li, D.; Zhu, K.; Horvath, D.P.; et al. Annual growth cycle observation, hybridization and forcing culture for improving the ornamental application of Paeonia lactiflora Pall. in the low-latitude regions. PLoS ONE 2019, 14, e0218164. [Google Scholar] [CrossRef] [PubMed]
- Weixing, L.; Shunbo, Y.; Hui, C.; Yanmin, H.; Jun, T.; Chunhua, Z. Nutritional evaluation of herbaceous peony (Paeonia lactiflora Pall.) petals. Emir. J. Food Agric. 2017, 29, 518–531. [Google Scholar] [CrossRef]
- Batinić, P.; Milošević, M.; Lukić, M.; Prijić, Ž.; Gordanić, S.; Filipović, V.; Marinković, A.; Bugarski, B.; Marković, T. In vitro evaluation of antioxidative activities of extracts of Paeonia lactiflora and Calendula officinalis L. petals incorporated in the new forms of biobased carriers. Food Feed Res. 2022, 49, 23–35. [Google Scholar] [CrossRef]
- Jin, Y.S.; Xuan, Y.H.; Jin, Y.Z.; Chen, M.L.; Tao, J. Biological activities of herbaceous peony flower extracts. Asian J. Chem. 2013, 25, 3835–3838. [Google Scholar] [CrossRef]
- Qi, Q.; Li, Y.; Xing, G.; Guo, J.; Guo, X. Fertility variation among Paeonia lactiflora genotypes and fatty acid composition of seed oil. Ind. Crops Prod. 2020, 152, 112540. [Google Scholar] [CrossRef]
- He, D.Y.; Dai, S.M. Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora Pall., a traditional Chinese herbal medicine. Front. Pharmacol. 2011, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Rudaya, O.A.; Chesnokov, N.N.; Kirina, I.B.; Tarova, Z.N.; Bobrovich, L.V.; Kiriakova, O.I. The research of seed reproduction peculiarities of wild-growing Paeonia L. genus and perspectives of using peony seeds in food-processing industry. IOP Conf. Ser. Earth Environ. Sci. 2021, 845, 012002. [Google Scholar]
- Demirboğa, Y.; Demirboğa, G.; Özbay, N. Types of Paeonia and Their Use in Phytotherapy. Karadeniz Bilim. Derg. 2021, 11, 318–327. [Google Scholar] [CrossRef]
- Ning, C.; Jiang, Y.; Meng, J.; Zhou, C.; Tao, J. Herbaceous peony seed oil: A rich source of unsaturated fatty acids and γ-tocopherol. Eur. J. Lipid Sci. Technol. 2015, 117, 532–542. [Google Scholar] [CrossRef]
- Ma, G.Y.; Shi, X.H.; Zou, Q.C.; Zhu, K.Y.; Liu, H.C.; Zhou, J.H.; Zhang, J.Q. Characters determination of herbaceous oil physicochemical property and comparative analysis of peony seed oil. Chin. Cereal Oil Assoc. 2017, 32, 130–1134. [Google Scholar]
- Andrieu, E.; Thompson, J.D.; Debussche, M. The impact of forest spread on a marginal population of a protected peony (Paeonia officinalis L.): The importance of conserving the habitat mosaic. Biodivers. Conserv. 2007, 16, 643–658. [Google Scholar] [CrossRef]
- Han, X.M.; Wu, S.X.; Wu, M.F.; Yang, X.M. Antioxidant effect of peony seed oil on aging mice. Food Sci. Biotechnol. 2017, 26, 1703–1708. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Gao, J.; Yi, J.; Liu, P. Could peony seeds oil become a high-quality edible vegetable oil? The nutritional and phytochemistry profiles, extraction, health benefits, safety and value-added-products. Food Res. Int. 2022, 156, 111200. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Deng, R.; Gao, J.; Yi, Y.; Liu, P.; Yang, X.; Zhang, Z.; Han, B.; Zhang, I. Comprehensive resource utilization of peony seeds shell: Extraction of active ingredients, preparation and application of activated carbon. Ind. Crops Prod. 2022, 180, 114764. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Wu, Y.; Yu, X.; Li, S.; Wang, L. Characterization of stilbenes, in vitro antioxidant and cellular anti-photoaging activities of seed coat extracts from 18 Paeonia species. Ind. Crops Prod. 2022, 177, 114530. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, P.; Gao, J.; Wang, X.; Yan, M.; Xue, N.; Qu, C.; Deng, R. Paeonia veitchii seeds as a promising high potential by-product: Proximate composition, phytochemical components, bioactivity evaluation and potential applications. Ind. Crops Prod. 2018, 125, 248–260. [Google Scholar] [CrossRef]
- Abbey, M. Paeonia spp. Production and Future Developments; Report; University of Minnesota Digital Conservancy: Minnesota, MN, USA, 2015; pp. 1–23. Available online: https://hdl.handle.net/11299/175839 (accessed on 23 March 2022).
- Hong, D.Y.; Pan, K.Y. Paeoniaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press and Missouri Botanic Garden Press: Beijing, China, 2001; Volume 6, pp. 127–132. [Google Scholar]
- Wu, S.H.; Wu, D.G.; Chen, Y.W. Chemical constituents and bioactivities of plants from the genus Paeonia. Chem. Biodivers. 2010, 7, 90–104. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, R.; Xue, J.; Wang, S.; Zhang, X. Genetic diversity and relatedness analysis of nine wild species of tree peony based on simple sequence repeats markers. Hortic. Plant. J. 2021, 7, 579–588. [Google Scholar] [CrossRef]
- Tank, D.C.; Sang, T. Phylogenetic utility of the glycerol-3-phosphate acyltransferase gene: Evolution and implications in Paeonia (Paeoniaceae). Mol. Phylogenet. Evol. 2001, 19, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.L.; Xu, C.; Liu, J.; Yu, Y.; Wu, P.; Cheng, T.; Hong, D.Y. Out of the Pan—Himalaya: Evolutionary history of the Paeoniaceae revealed by phylogenomics. J. Syst. Evol. 2020, 59, 1170–1182. [Google Scholar] [CrossRef]
- Blecic, V. Paeonia L. In Flora of Serbia; Josifovic, M., Ed.; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 1972; Volume 3, pp. 98–102. [Google Scholar]
- Lazarevic, P.; Stojanovic, V. Wild peonies (Paeonia L.) in Serbia—The distribution, state of populations, threats and protection. Nat. Conserv. 2012, 62, 19–44. [Google Scholar]
- Boza, P.; Stojsic, V. Paeonia officinalis L. subsp. banatica /Rochel/ Soó). In Red Book of Flora; Stevanović, V., Ed.; Serbia 1—Extinct and Extremely Endangered Taxa; Ministry of Environmental Protection of Republic of Serbia: Belgrade, Serbia, 1999; pp. 167–169. [Google Scholar]
- Djurdjevic, L.; Dinic, A.; Stojsic, V.; Mitrovic, M.; Pavlovic, P.; Oldja, M. Allelopathy of Paeonia officinalis L.1753 ssp. banatica (ROCHEL) s061945, a Pannonian endemic and relict species. Arch. Biol. Sci. 2000, 52, 195–201. [Google Scholar]
- The National Forestry and Grassland Administration of China. List of National Protected Wild Plants. 2021. Available online: http://www.forestry.gov.cn/ (accessed on 23 March 2022).
- American Peony Society. Available online: https://americanpeonysociety.org (accessed on 23 March 2022).
- Rockström, J.; Steffen, W.; Noone, K.; Persson, A.; Chapin, F.S.; Lambin, E.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.; et al. Planetary boundaries: Exploring the safe operating space for humanity. Ecol. Soc. 2009, 14, 32. Available online: http://www.ecologyandsociety.org/vol14/iss2/art32/ (accessed on 23 March 2022). [CrossRef]
- Zhang, K.; Zhang, Y.; Tao, J. Predicting the potential distribution of Paeonia veitchii (Paeoniaceae) in China by incorpo-rating climate change into a maxent model. Forests 2019, 10, 190. [Google Scholar] [CrossRef] [Green Version]
- Ne’eman, G. To be or not to be—the effect of nature conservation management on flowering of Paeonia mascula (L.) Miller in Israel. Biol. Conserv. 2003, 109, 103–109. [Google Scholar] [CrossRef]
- Nikos, K.; Menteli, V.; Vokou, D. The Electronic Trade in Greek Endemic Plants: Biodiversity, Commercial and Legal Aspects. Econ. Bot. 2014, 68, 85–95. [Google Scholar] [CrossRef]
- Glick, P.; Stein, B.A.; Edelson, N.A. Scanning the Conservation Horizon: A Guide to Climate Change Vulnerability Assessment; National Wildlife Federation: Washington, DC, USA, 2011; 168p. [Google Scholar]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2014; ISBN 978012416683. [Google Scholar]
- Yu, X.; Zhao, R.; Cheng, F. Seed Germination of Tree and Herbaceous Peonies: A Mini-Review Seed. Sci. Biotech. 2007, 1, 11–14. [Google Scholar]
- Barga, S.C. Seed Dispersal of Wild Peony (Paeonia brownii): A Seed in the Pouch Is Worth Two in the Pod. Master’s Thesis, University of Nevada, Reno, Reno, NV, USA, 2011; p. 53. Available online: http://hdl.handle.net/11714/3838 (accessed on 23 March 2022).
- Zhang, K.; Yao, L.; Zhang, Y.; Baskin, J.M.; Baskin, C.C.; Xiong, Z.; Tao, J. A review of the seed biology of Paeonia species (Paeoniaceae), with particular reference to dormancy and germination. Planta 2018, 249, 291–303. [Google Scholar] [CrossRef]
- Andrieu, E.; Debussche, M.; Galloni, M.; Thomson, J.D. The interplay of pollination, costs of reproduction and plant size in maternal fertility limitation in perennial Paeonia officinalis. Oecologia 2007, 152, 515–524. [Google Scholar] [CrossRef]
- Gao, J.; Fu, Z.; Dong, X.; Wang, L.; Yuan, X.; Zhang, J.; Wang, H.; Li, Y.; Feng, N.; Wang, Y.; et al. Studies on Pollination Characteristics and Breeding System of Paeonia lactiflora Pall. Bot. Res. 2018, 7, 536–542. [Google Scholar] [CrossRef]
- Porceddu, M.; Mattana, E.; Pritchard, H.W.; Bacchetta, G. Sequential temperature control of multi-phasic dormancy release and germination of Paeonia corsica seeds. J. Plant. Ecol. 2016, 9, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Nanjidsuren, O.; Narantsetseg, A. Seed productivity of two species of Paeonia (Paeoniaceae) in Mongolia. Agric. Sci. Res. J. 2016, 6, 1–5. [Google Scholar]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Joshi, P.; Prakash, P.; Purohit, V.K. Seed germination and growth performance of Paeonia emodi Wall. ex Royle: Conservation and cultivation strategies. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100338. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Dole, J. Herbaceous peony (Paeonia): Genetics, physiology and cut flower production. Floric. Ornam. Biotechnol. 2012, 6, 62–77. [Google Scholar]
- Yang, Y.; Sun, M.; Li, S.; Chen, Q.; da Silva, T.J.A.; Wang, A.; Yu, X.; Wang, L. Germplasm resources and genetic breeding of Paeonia: A systematic review. Hortic. Res. 2020, 7, 107. [Google Scholar] [CrossRef]
- Hong, D.Y. Peonies of the World: Taxonomy and Phytogeography; Missouri Botanical Garden: St. Louis, MI, USA, 2010; p. 302. [Google Scholar]
- Hudson, A.R.; Ayre, D.J.; Ooi, M.K.J. Physical dormancy in a changing climate. Seed. Sci. Res. 2015, 25, 66–81. [Google Scholar] [CrossRef] [Green Version]
- Bojnanský, V.; Fargašová, A. Taxonomy and Morphology of Seeds. In Atlas of Seeds and Fruits of Central and East-European Flora; Springer: Dordrecht, The Netherlands, 2007; pp. 1–954. [Google Scholar] [CrossRef]
- Nazir, S.; Yaqoob, U.; Nawchoo, I.A.; Lone, F.A.; Rather, A.A.; Hassan, A.; Ashraf, A. Paeonia emodi: An Ethnopharmacological and Phytochemical Review. Res. Rev. J. Herbal Sci. 2017, 6, 11–20. [Google Scholar]
- Meng, J.; Li, M.; Zhang, K.; Zhao, D.; Tao, J. Kinetics of seed reserve compounds during the maturation of herbaceous peony (Paeonia lactiflora Pall.) seeds. J. Seed Sci. 2021, 43, e202143041. [Google Scholar] [CrossRef]
- Pons, T.L. Seed responses to light. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CABI Publishing: Wallingford, UK, 2000; pp. 237–260. [Google Scholar]
- Qi, Q.; Li, Y.; Meng, F.; Xing, G.; Zhou, J.; Guo, X. Dynamic changes of nutrient content in herbaceous peony seed. Oil Crop Sci. 2020, 5, 36–41. [Google Scholar] [CrossRef]
- Yang, H.C.; Per, D.L. Study on embryo culture of peony (Paeonia suffruticosa Andr. L.) seed. Guangxi Agric. Sci. 2006, 37, 108–119. [Google Scholar]
- Pausas, J.G.; Lamont, B.B. Fire-released seed dormancy—A global synthesis. Biol. Rev. 2022. [Google Scholar] [CrossRef]
- Dole, J.M. Research approaches for determining cold requirements for forcing and flowering of geophytes. Hort. Sci. 2003, 38, 341–346. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. The great diversity in kinds of seed dormancy: A revision of the Nikolaeva–Baskin classification system for primary seed dormancy. Seed. Sci. Res. 2021, 31, 249–277. [Google Scholar] [CrossRef]
- Nikolaeva, M.G. Factors controlling the seed dormancy pattern. In The Physiology and Biochemistry of Seed Dormancy and Germination; Khan, A.A., Ed.; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherlands, 1977; pp. 51–74. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed. Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.M.; Zhang, M.M.; Gao, H.D.; Yang, H.G. Study on characteristic for seed coat of Paeonia lactifora. North. Hort. 2012, 6, 55–57. [Google Scholar]
- Rodrigues-Junior, A.G.; Mello, A.C.M.P.; Baskin, C.C.; Baskin, J.M.; Oliveira, D.M.T.; Garcia, Q.S. Why large seeds with physical dormancy become nondormant earlier than small ones. PLoS ONE 2018, 13, e022038. [Google Scholar] [CrossRef]
- Corner, E.J.H. The Seeds of Dicotyledons; Cambridge University Press: Cambridge, UK, 1976; ISBN 13 9780521116053. [Google Scholar]
- Suleymanova, G.; Boldyrev, V.; Savinov, V. Post-fire restoration of plant communities with Paeonia tenuifolia in the Khvalynsky National Park (Russia). Nat. Conserv. Res. 2019, 4, 57–77. [Google Scholar] [CrossRef]
- Tao, X.Y.; Yang, H.L.; Li, L. The research of the germination characteristic of herbaceous peony. J. Chifeng Coll. 2005, 21, 13–19. [Google Scholar]
- Guo, L.P. Study on Dormancy and Dormancy Breaking of Tree Peony Seeds. Master’s Thesis, Northwest A&F University, Shaanxi, China, 2016. [Google Scholar]
- Maeda, A.B.; Wells, L.W.; Sheehan, M.A.; Dever, J.K. Stories from the greenhouse—A brief on cotton seed germination. Plants 2021, 10, 2807. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Xue, J.; Wang, S.; Xue, Y.; Zhang, P.; Jiang, H.; Zhang, X. Proteomic analysis of tree peony (Paeonia ostii ‘Feng Dan’) seed germination affected by low temperature. J. Plant. Physiol. 2017, 224–225, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D. Seed Germination and Dormancy. Plant. Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2001; p. 666. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seed dormancy in wild flowers. In Flower Seeds: Biology and Technology; McDonald, M.B., Kwong, F.Y., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 163–185. [Google Scholar]
- Soltani, E.; Baskin, C.C.; Baskin, J.M. A graphical method for identifying the six types of nondeep physiological dormancy in seeds. Plant. Biol. 2017, 19, 673–682. [Google Scholar] [CrossRef]
- Yang, Y. Research on Biological Characteristics of Paeonia lactiflora Seed’s Germination and Dormancy Breaking Technique. Ph.D. Thesis, Northeast Forest University, Harbin, China, 2009. [Google Scholar]
- Yuan, Y.B.; Xiao, N.Y. Effects of exogenous GA3 and chilling treatments on seed germination and seedling growth of Paeonia lactiflora. J. Anhui Agric. Univ. 2014, 41, 273–277. [Google Scholar]
- Nikolaeva, M.G.; Rasumova, M.V.; Gladkova, V.N. Reference Book on Dormant Seed Germination; Nauka Publishers: Leningrad, Russia, 1985. [Google Scholar]
- Li, X.R.; Chen, Z.; Fan, C.; Sun, X.; Min, X.J. Plant hormonal changes and differential expression profiling reveal seed dormancy removal process in double dormant plant-herbaceous peony. PLoS ONE 2020, 15, e0231117. [Google Scholar] [CrossRef] [Green Version]
- Bentsink, L.; Koornneef, M. Seed Dormancy and Germination. In The Arabidopsis Book; BioOne: Washington, DC, USA, 2008; Volume 6, p. e0119. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Liu, Y.; Jeong, B.R. A Two-Stage Culture Method for Zygotic Embryos Effectively Overcomes Constraints Imposed by Hypocotyl and Epicotyl Seed Dormancy in Paeonia ostii ‘Fengdan’. Plants 2019, 8, 356. [Google Scholar] [CrossRef] [Green Version]
- Ren, R.; Zhou, H.; Zhang, L.; Jiang, X.; Zhang, M.; Liu, Y. ROS-induced PCD affects the viability of seeds with different moisture content after cryopreservation. Plant. Cell Tiss. Organ. Cult. 2022, 148, 623–633. [Google Scholar] [CrossRef]
- Fei, R.; Sun, X.; Yang, P.; Chen, Z.; Ma, Y. Anatomical observation of Paeonia lactiflora seeds during stratification process. J. Shenyang Agric. Univ. 2017, 48, 354–359. [Google Scholar]
- European Peony Society. Available online: https://www.peonysociety.eu (accessed on 23 March 2022).
- Halevy, A.H. Evaluation of Methods for Flowering Advancement of Herbaceous Peonies. Hort. Sci. 2002, 37, 885–889. [Google Scholar] [CrossRef] [Green Version]
- Vandelook, F.; Assche, J.A. Temperature requirements for seed germination and seedling development determine timing of seedling emergence of three monocotyledonous temperate forest spring geophytes. Ann. Bot. 2008, 102, 865–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deno, N.C. Seed Germination Theory and Practice, 2nd ed.; Norman C. Deno: State College, PA, USA, 1993; p. 242. [Google Scholar]
- Fei, R.; Duan, S.; Ge, J.; Sun, T.; Sun, X. Functional Identification of 9-cis-epoxycarotenoid Dioxygenase Genes in Double Dormant Plant-herbaceous Peony. Res. Sq. 2021, 1–26. [Google Scholar] [CrossRef]
- Sirotyuk, A.E.; Shadge, A.E.; Gunina, G.N. Paeonia caucasica (Schipcz.) Schipcz. in phytocenoses of the Republic of Adygea. Ecol. Montenegrina 2020, 37, 43–50. [Google Scholar] [CrossRef]

| Species | Seeds | Reference | |||
|---|---|---|---|---|---|
| Testa Colour | Size (mm) | Shape | Maturation | ||
| P. algeriensis | black | 9.0 × 7.5 | ovoid to oblong | August | [48] |
| P. anomala | black | 6.6–8.8 × 5.1–6.0 | ellipsoidal | August | [8,38,47] |
| P. banatica | black | 6.0–8.0 × 5.0 | ellipsoidal | late July to August | [49] |
| P. broteri | black | 7.0–8.0 | oblong | August to September | [49] |
| P. cambessdesii | black | 5.0 | globular | June to July | [49] |
| P. clusii | black | 8.0 × 5.0 | ovoid to ellipsoidal | August | [48] |
| P. coriacea | black | 7.0–8.0 × 5.0–6.0 | oblong | September | [48] |
| P. corsica | black | 7.0 × 5.0–6.0 | ovoid to globular | late July to September | [48] |
| P. daurica | black | 6.1–7.5 × 4.2–7.0 | globular | August to September | [38,50] |
| P. emodi | brownish black | 2.0–3.5 | globular | August to September | [51] |
| P. intermedia | black glossy | 5.0–5.5 × 3.0–3.5 | cylindrical to ovoid | August to September | [48] |
| P. lactiflora | brownish black | 5.5–10.0 × 4.1–6.8 | ellipsoidal or globular to rhomboid, flattish | late July to September | [8,38,48,50] |
| P. mairei | black with blue shine | 7.0–8.0 × 4.0–5.0 | irregular, round | July to August | [48] |
| P. mascula | first red than black | 7.0–8.5 × 5.0–7.0 | ellipsoidal to globular | late July to August | [50] |
| P. obovata | black | 6.0–7.0 × 5.0–6.0 | ovoid to globular | August to September | [48] |
| P. officinalis | black | 6.0–9.0 × 4.5–6.5 | obovate to ellipsoidal | late July to August | [50] |
| P. peregrina | black | 7.5–10.0 × 5.0–6.0 | ellipsoidal | late July to August | [48,50] |
| P. sterniana | indigo blue | 7.0–8.0 × 5.0 | ellipsoidal | August to September | [48] |
| P. tenuifolia | brownish black | 5.9–8.0 × 3.5–4.9 | cylindrical to ellipsoidal | July to August | [8,38,50] |
| P. veitchii | dark blue | 6.0 × 4.0 | ellipsoidal to oval | July | [8] |
| P. kesrouanensis | black | 6.0–10.0 × 6.0–8.0 | ovoid to globular | July to September | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, T.; Prijić, Ž.; Xue, J.; Zhang, X.; Radanović, D.; Ren, X.; Filipović, V.; Lukić, M.; Gordanić, S. The Seed Traits Associated with Dormancy and Germination of Herbaceous Peonies, Focusing on Species Native in Serbia and China. Horticulturae 2022, 8, 585. https://doi.org/10.3390/horticulturae8070585
Marković T, Prijić Ž, Xue J, Zhang X, Radanović D, Ren X, Filipović V, Lukić M, Gordanić S. The Seed Traits Associated with Dormancy and Germination of Herbaceous Peonies, Focusing on Species Native in Serbia and China. Horticulturae. 2022; 8(7):585. https://doi.org/10.3390/horticulturae8070585
Chicago/Turabian StyleMarković, Tatjana, Željana Prijić, Jingqi Xue, Xiuxin Zhang, Dragoja Radanović, Xiuxia Ren, Vladimir Filipović, Milan Lukić, and Stefan Gordanić. 2022. "The Seed Traits Associated with Dormancy and Germination of Herbaceous Peonies, Focusing on Species Native in Serbia and China" Horticulturae 8, no. 7: 585. https://doi.org/10.3390/horticulturae8070585
APA StyleMarković, T., Prijić, Ž., Xue, J., Zhang, X., Radanović, D., Ren, X., Filipović, V., Lukić, M., & Gordanić, S. (2022). The Seed Traits Associated with Dormancy and Germination of Herbaceous Peonies, Focusing on Species Native in Serbia and China. Horticulturae, 8(7), 585. https://doi.org/10.3390/horticulturae8070585








