Evaluation of the Soil Type Effect on the Volatile Compounds in the Habanero Pepper (Capsicum chinense Jacq.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growing Conditions
2.2. Soil Analysis
2.3. Sample Preparation
2.4. Extraction of Volatile Compounds
2.5. Analysis of Volatiles by Gas Chromatography
2.6. Statistical Analysis
3. Results
3.1. Soil Analysis
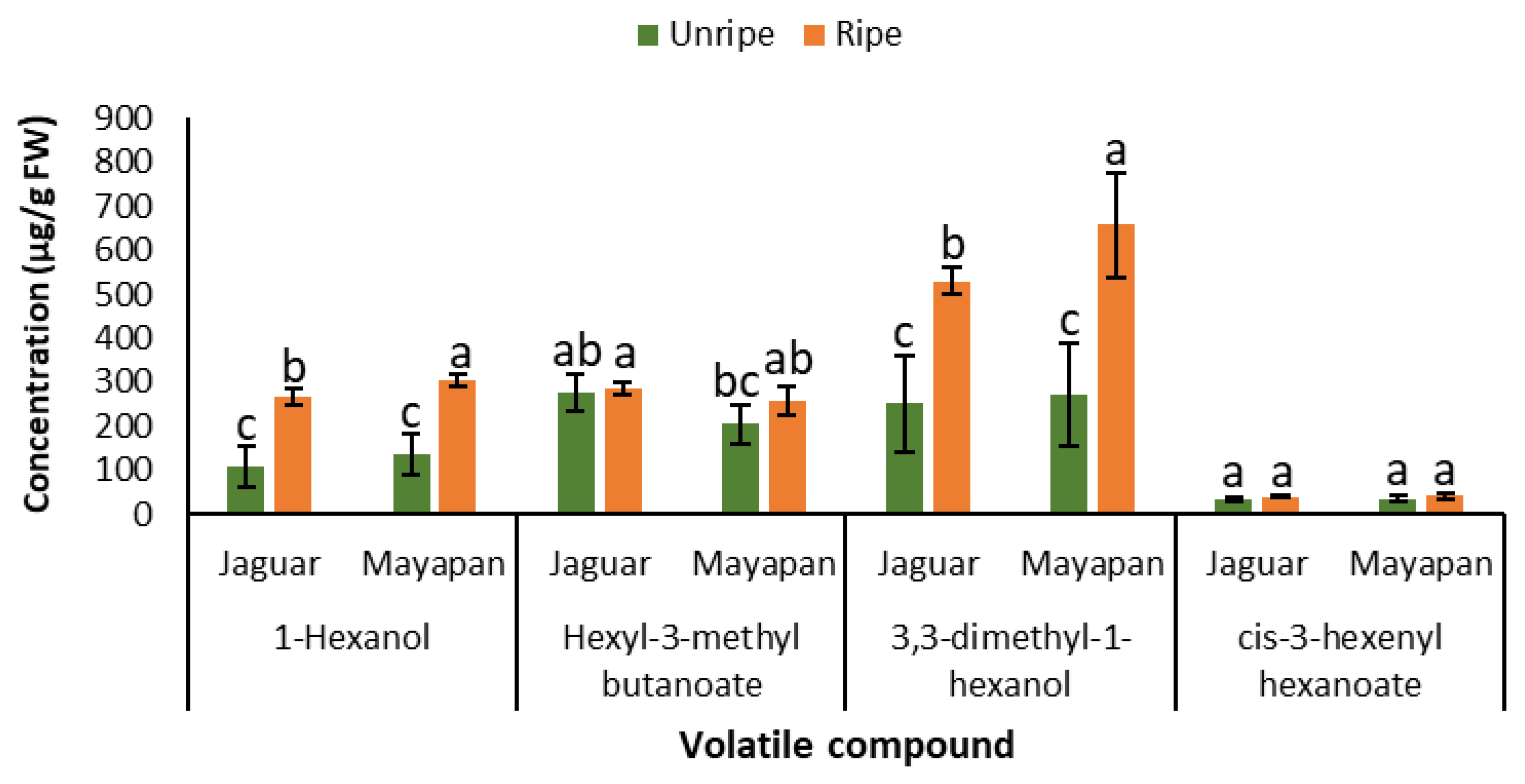
3.2. Quantification of Volatile Compounds by Gas Chromatography
3.3. Interaction of Factors Influencing the Composition of Volatiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
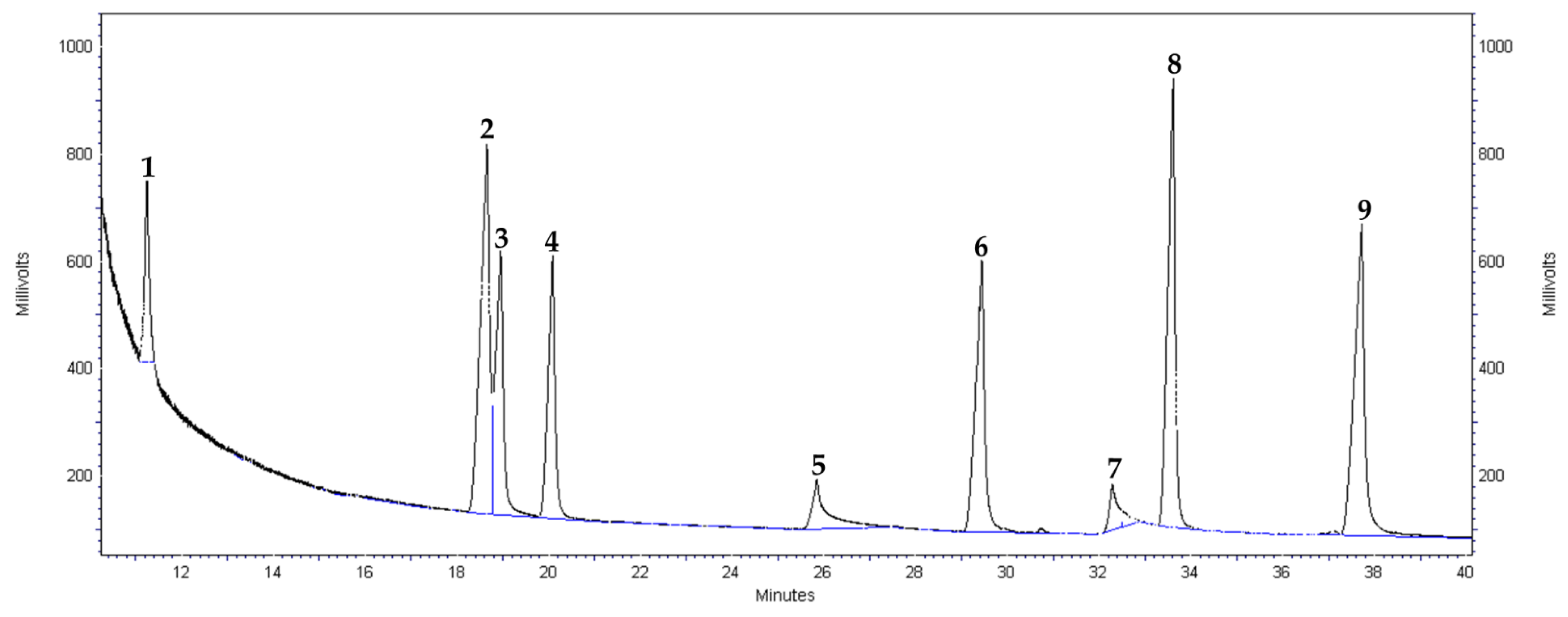
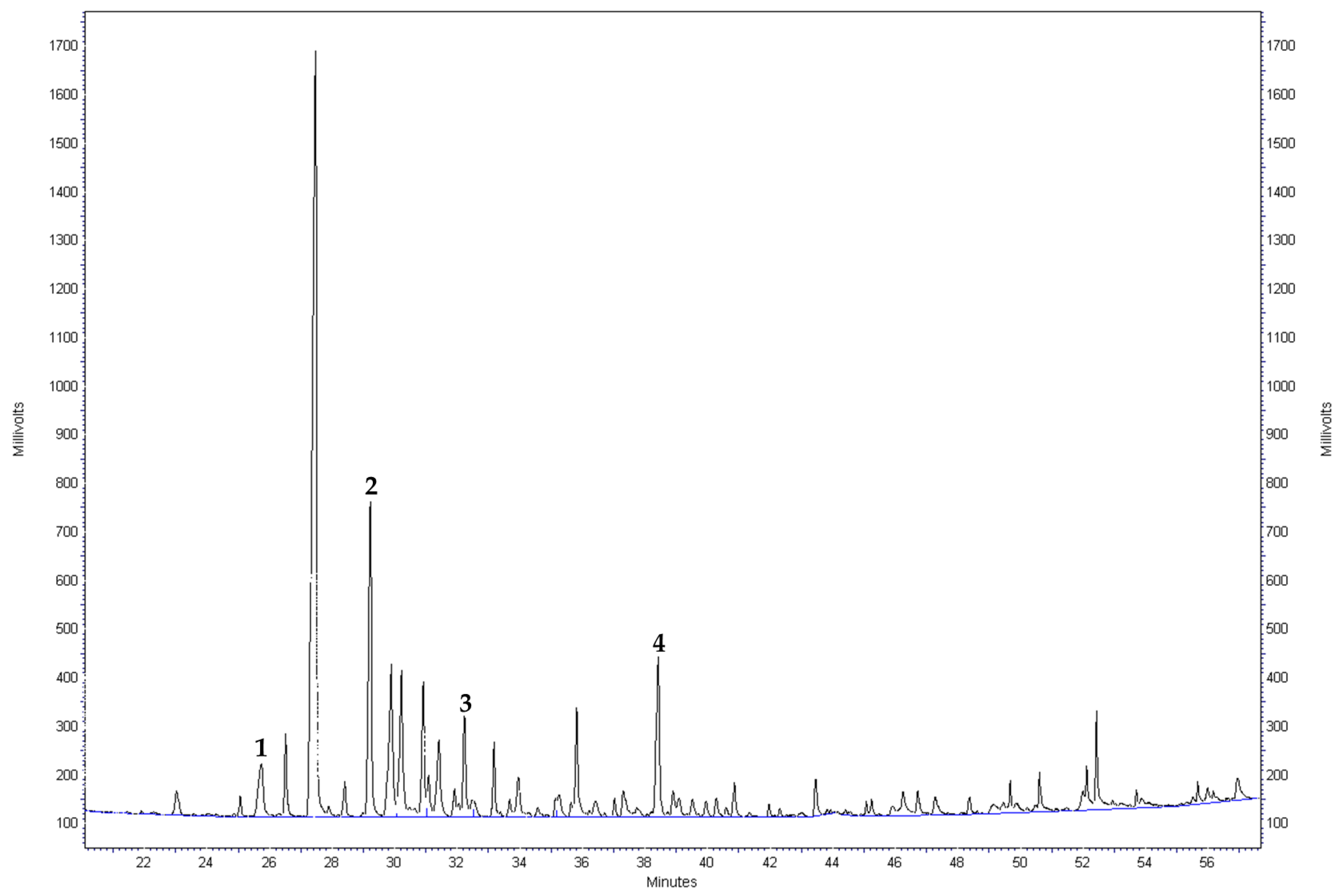

Appendix B
| Variety | Grade of Ripeness | Soil | L* | a* | b* | Chroma | |
|---|---|---|---|---|---|---|---|
| Jaguar | Unripe |  | Red | 44.8 ± 1.3 b | −12.7 ± 0.6 c | 27.8 ± 2.3 de | 30.6 ± 2.3 d |
| Brown | 43.4 ± 1.5 b | −11.8 ± 0.5 c | 26.6 ± 2.0 e | 29.1± 2.0 d | |||
| Black | 43.1 ± 1.5 b | −11.5 ± 0.8 c | 26.9 ± 4.2 e | 29.2 ± 4.2 d | |||
| Ripe |  | Red | 56.0 ± 0.4 a | 26.4 ± 1.4 a | 46.7 ± 1.3 a | 53.6 ± 1.6 a | |
| Brown | 54.7 ± 1.0 a | 19.3 ± 4.4 b | 42.4 ± 2.5 b | 46.7 ± 3.4 b | |||
| Black | 51.9 ± 1.0 a | 24.6 ± 2.0 a | 42.0 ± 1.2 b | 48.7 ± 1.6 b | |||
| Mayapan | Unripe |  | Red | 43.6 ± 3.2 b | −12.0 ± 0.4 c | 33.0 ± 0.3 d | 35.1 ± 0.4 c |
| Brown | 40.6 ± 4.1 b | −11.3 ± 0.9 c | 24.9 ± 2.4 e | 27.4 ± 2.5 d | |||
| Black | 44.0 ± 1.3 b | −11.8 ± 0.2 c | 31.1 ± 2.0 d | 33.3 ± 1.9 cd | |||
| Ripe |  | Red | 51.1 ± 3.6 a | 19.8 ± 1.5 b | 39.4 ± 2.6 bc | 44.1 ± 2.9 b | |
| Brown | 54.1 ± 1.3 a | 20.8 ± 1.0 b | 38.2 ± 0.8 c | 43.5 ± 1.2 b | |||
| Black | 54.4 ± 0.8 a | 24.3 ± 3.5 a | 39.7 ± 3.2 c | 46.6 ± 4.5 b | |||
References
- Medina-Lara, F.A.; Echevarría-Machado, I.; Pacheco-Arjona, R.; Ruiz-Lau, N.; Guzmán-Antonio, A.; Martinez-Estevez, M. Influence of Nitrogen and Potassium Fertilization on Fruiting and Capsaicin Content in Habanero Pepper (Capsicum chinense Jacq.). HortScience 2008, 43, 1549–1554. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Ramírez, L.S.; Peña-Yam, L.P.; Avilóes-Viñas, S.A.; Canto-Flick, A.; Guzmóan-Antonio, A.A.; Santana-Buzzy, N. Behavior of the hottest chili peppers in the world cultivated in Yucatan, Mexico. HortScience 2018, 53, 1772–1775. [Google Scholar] [CrossRef] [Green Version]
- Peña-Yam, L.P.; Muñoz-Ramírez, L.S.; Avilés-Viñas, S.A.; Canto-Flick, A.; Pérez-Pastrana, J.; Guzmán-Antonio, A.; Santana-Buzzy, N.; Aguilera-Cauich, E.A.; Mijangos-Cortés, J.O. Analysis of genetic parameters of Habanero pepper (Capsicum chinense Jacq.) in the yucatan, Mexico. HortScience 2019, 54, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Zapata-Aguilar, J.A.; Pérez-Akaki, P.; Moo-Novelo, C.A. Análisis de la cadena de comercialización del chile Habanero de Yucatán y su denominación de origen. Rev. CEA 2020, 6, 109–125. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Cuevas-Glory, L.F.; Sosa-Moguel, O.; Pino, J.; Sauri-Duch, E. GC–MS Characterization of Volatile Compounds in Habanero Pepper (Capsicum chinense Jacq.) by Optimization of Headspace Solid-Phase Microextraction Conditions. Food Anal. Methods 2015, 8, 1005–1013. [Google Scholar] [CrossRef]
- Pino, J.; Sauri-Duch, E.; Marbot, R. Changes in volatile compounds of Habanero chile pepper (Capsicum chinense Jack. cv. Habanero) at two ripening stages. Food Chem. 2006, 94, 394–398. [Google Scholar] [CrossRef]
- Sosa-Moguel, O.; Pino, J.A.; Ayora-Talavera, G.; Sauri-Duch, E.; Cuevas-Glory, L. Biological activities of volatile extracts from two varieties of Habanero pepper (Capsicum chinense Jacq.). Int. J. Food Prop. 2018, 20, S3042–S3051. [Google Scholar] [CrossRef] [Green Version]
- Sosa-Moguel, O.; Cuevas-Glory, L.; Pino, J.A.; Sauri-Duch, E. Conocimientos actuales sobre el aroma de chile Habanero (Capsicum chinense Jacq.): Current knowledge about aroma of Habanero pepper (Capsicum chinense Jacq.). Cienc. Tecnol. Aliment. 2018, 28, 68–72. [Google Scholar]
- Oney, M.J.E.; Morozova, K.; Ferrentino, G.; Ramirez Sucre, M.O.; Rodríguez Buenfil, I.M.; Scampicchio, M. Effects of local environmental factors on the spiciness of Habanero chili peppers (Capsicum chinense Jacq.) by coulometric electronic tongue. Eur. Food Res. Technol. 2021, 247, 101–110. [Google Scholar] [CrossRef]
- Oney-Montalvo, J.; Uc-Varguez, A.; Ramírez-Rivera, E.; Ramírez-Sucre, M.; Rodríguez-Buenfil, I. Influence of Soil Composition on the Profile and Content of Polyphenols in Habanero Peppers (Capsicum chinense Jacq.). Agronomy 2020, 10, 1234. [Google Scholar] [CrossRef]
- Oney-Montalvo, J.E.; Madrigal, A.C.D.S.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Effect of the soil and ripening stage in Capsicum chinense var. Jaguar on the content of carotenoids and vitamins. Horticulturae 2021, 7, 11. [Google Scholar] [CrossRef]
- Mazida, M.M.; Salleh, M.M.; Osman, H. Analysis of volatile aroma compounds of fresh chilli (Capsicum annuum) during stages of maturity using solid phase microextraction (SPME). J. Food Compos. Anal. 2005, 18, 427–437. [Google Scholar] [CrossRef]
- Morozova, K.; Rodríguez-Buenfil, I.; López-Domínguez, C.; Ramírez-Sucre, M.; Ballabio, D.; Scampicchio, M. Capsaicinoids in Chili Habanero by Flow Injection with Coulometric Array Detection. Electroanalysis 2019, 31, 844–850. [Google Scholar] [CrossRef]
- Bautista-Zúñiga, F.; Jiménez-Osornio, J.; Navarro-Alberto, J.; Manu, A.; Lozano, R. Micro-Relief and Soil Color as Diagnostic Properties in Carstic Leptosols. Tierra Latinoam. 2003, 21, 1–11. [Google Scholar]
- Borges-Gómez, L.; Moo-Kauil, C.; Ruíz-Novelo, J.; Osalde-Balam, M.; González-Valencia, C.; Yam-Chimal, C.; Can-Puc, F. Suelos destinados a la producción de chile Habanero en Yucatán: Características físicas y químicas predominantes. Agrociencia 2014, 48, 347–359. [Google Scholar]
- Tolk, J.A. Soils, permanent wilting points. Encycl. Water Sci. 2003, 92, 927–929. [Google Scholar]
- Chapman, H.D. Cation-exchange capacity. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 2016, 9, 891–901. [Google Scholar] [CrossRef]
- Blake, G.R. Bulk density. In Methods of Soil Analysis, 1st ed.; The American Society of Agronomy, Inc.: California City, CA, USA, 1965; Volume 30, pp. 374–390. [Google Scholar] [CrossRef]
- Cassel, D.K.; Nielsen, D.R. Field capacity and available water capacity. Methods Soil Anal. Part 1 Phys. Mineral. Methods 2018, 9, 901–926. [Google Scholar] [CrossRef]
- Medina-Lara, F.; Souza-Perera, R.; Martínez-Estévez, M.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M.; MacHado, I.E. Red and brown soils increase the development and content of nutrients in Habanero pepper subjected to irrigation water with high electrical conductivity. HortScience 2019, 54, 2039–2049. [Google Scholar] [CrossRef] [Green Version]
- Norton, E.R.; Silvertooth, J.C. Field Determination of Permanent Wilting Point Field Determination of Permanent Wilting Point. A Coll. Agric. Rep. 1997, 108, 185–191. [Google Scholar]
- Bogusz-Junior, S.; Tavares, A.M.; Filho, J.T.; Zini, C.A.; Godoy, H.T. Analysis of the volatile compounds of Brazilian chilli peppers (Capsicum spp.) at two stages of maturity by solid phase micro-extraction and gas chromatography-mass spectrometry. Food Res. Int. 2012, 48, 98–107. [Google Scholar] [CrossRef]
- Fellman, J.K.; Miller, T.W.; Mattinson, D.S.; Mattheis, J.P. Factors that influence biosynthesis of volatile flavor compounds in apple fruits. HortScience 2000, 35, 1026–1033. [Google Scholar] [CrossRef]
- Dimita, R.; Min Allah, S.; Luvisi, A.; Greco, D.; De Bellis, L.; Accogli, R.; Mininni, C.; Negro, C. Volatile Compounds and Total Phenolic Content of Perilla frutescens at Microgreens and Mature Stages. Horticulturae 2022, 8, 71. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Chen, J.; Lü, J.; He, Z.; Zhang, F.; Zhang, S.; Zhang, H. Investigations into the production of volatile compounds in Korla fragrant pears (Pyrus sinkiangensis Yu). Food Chem. 2020, 302, 125337. [Google Scholar] [CrossRef]
- Holland, D.; Larkov, O.; Bar-Ya’akov, I.; Bar, E.; Zax, A.; Brandeis, E.; Lewinsohn, E. Developmental and varietal differences in volatile ester formation and acetyl-CoA: Alcohol acetyl transferase activities in apple (Malus domestica Borkh.) fruit. J. Agric. Food Chem. 2005, 53, 7198–7203. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Bononi, M.; Giorgi, A.; Cocucci, M.; Tateo, F. Evaluation of productivity and volatile compound quality of Artemisia absinthium L. planted in Valle Camonica (Italy). J. Sci. Food Agric. 2006, 86, 2592–2596. [Google Scholar] [CrossRef]
- Mishra, B.K.; Rastogi, A.; Shukla, S. Regulatory Role of Mineral Elements in the Metabolism of Medicinal Plants. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 1–13. [Google Scholar]
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.; Håkansson, I. Estimation of reference bulk density from soil particle size distribution and soil organic matter content. Geoderma 2010, 154, 398–406. [Google Scholar] [CrossRef]
- Cuevas-Glory, L.; Pino, J.; Lopez-Sauri, D.; Novelo-Torres, B.; Sauri-Duch, E. Characterization of odor-contributing volatiles in two Habanero pepper varieties by gas chromatography–olfactometry. Chem. Pap. 2020, 74, 2239–2246. [Google Scholar] [CrossRef]
- Lasekan, O.; Buettner, A.; Christlbauer, M. Investigation of the retronasal perception of palm wine (Elaeis Guineensis) aroma by application of sensory analysis and exhaled odorant measurement (exom). Afr. J. Food Agric. Nutr. Dev. 2009, 9, 793–813. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarska, K.T.; Chandra-Hioe, M.V.; Frank, D.; Arcot, J. Aroma characteristics of lupin and soybean after germination and effect of fermentation on lupin aroma. LWT-Food Sci. Technol. 2018, 87, 225–233. [Google Scholar] [CrossRef]
| Chromatographic Conditions | |
|---|---|
| Time of analysis: | 65 min |
| Initial temperature: | 40 °C |
| Ramp of temperature: | 3 °C min−1 |
| Final temperature: | 240 °C |
| Type of injection: | Splitless |
| Temperature of the injector: | 250 °C |
| Detector: | Flame ionization |
| Temperature of the detector: | 250 °C |
| Flow of the carrier gas: | 1 mL min−1 |
| Red | Brown | Black | |
|---|---|---|---|
| Cation exchange capacity (cmol kg−1) | 27.00 ± 1.80 c | 31.17 ± 1.15 b | 38.50 ± 0.50 a |
| Bulk density (Tm−3) | 0.97 ± 0.04 a | 0.91 ± 0.07 b | 0.80 ± 0.04 c |
| Field capacity (%) | 45.94 ± 2.19 b | 53.20 ± 1.55 a | 30.57 ± 1.02 c |
| Permanent wilting point (%) | 29.00 ± 1.62 b | 34.36 ± 1.15 a | 17.62 ± 0.75 c |
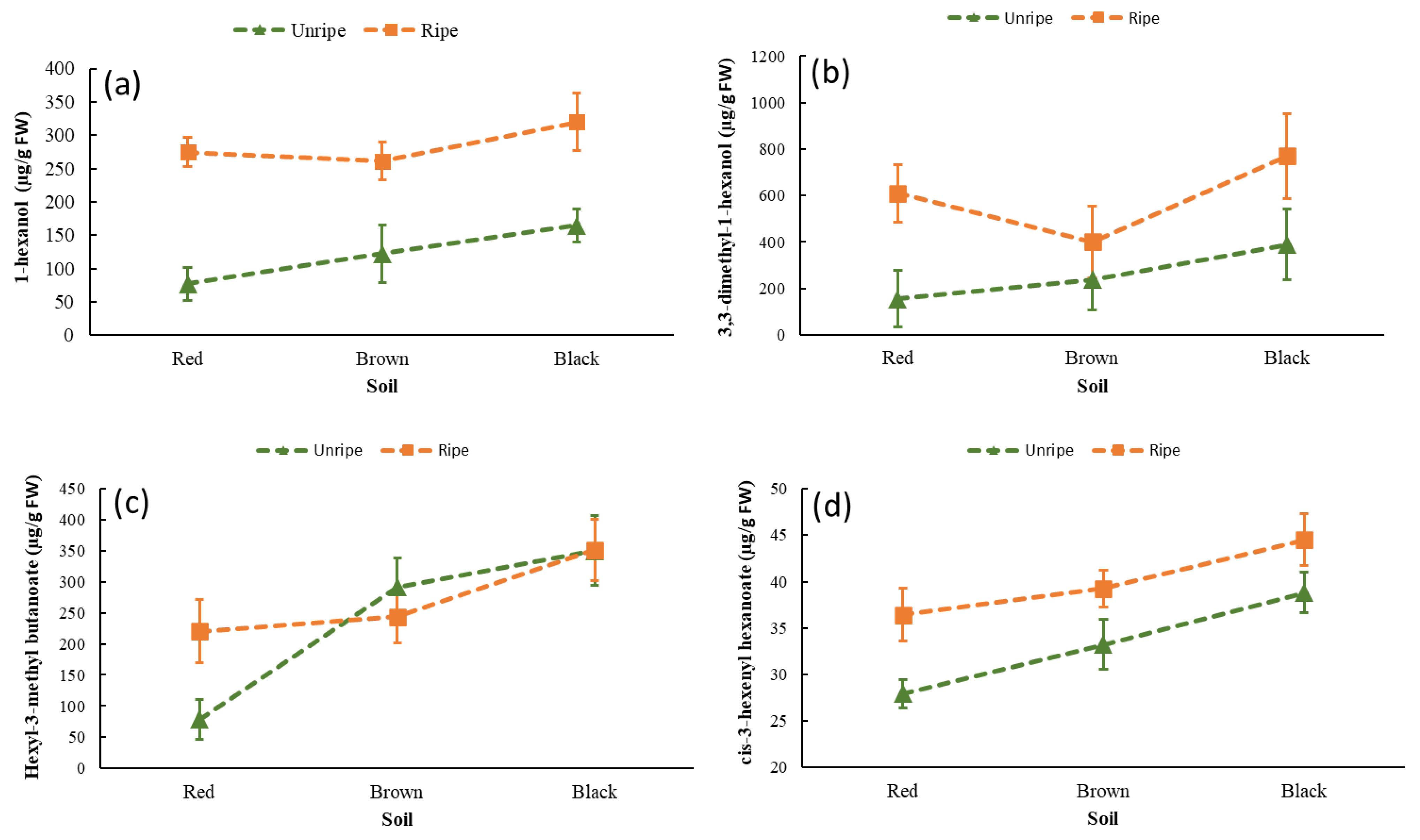
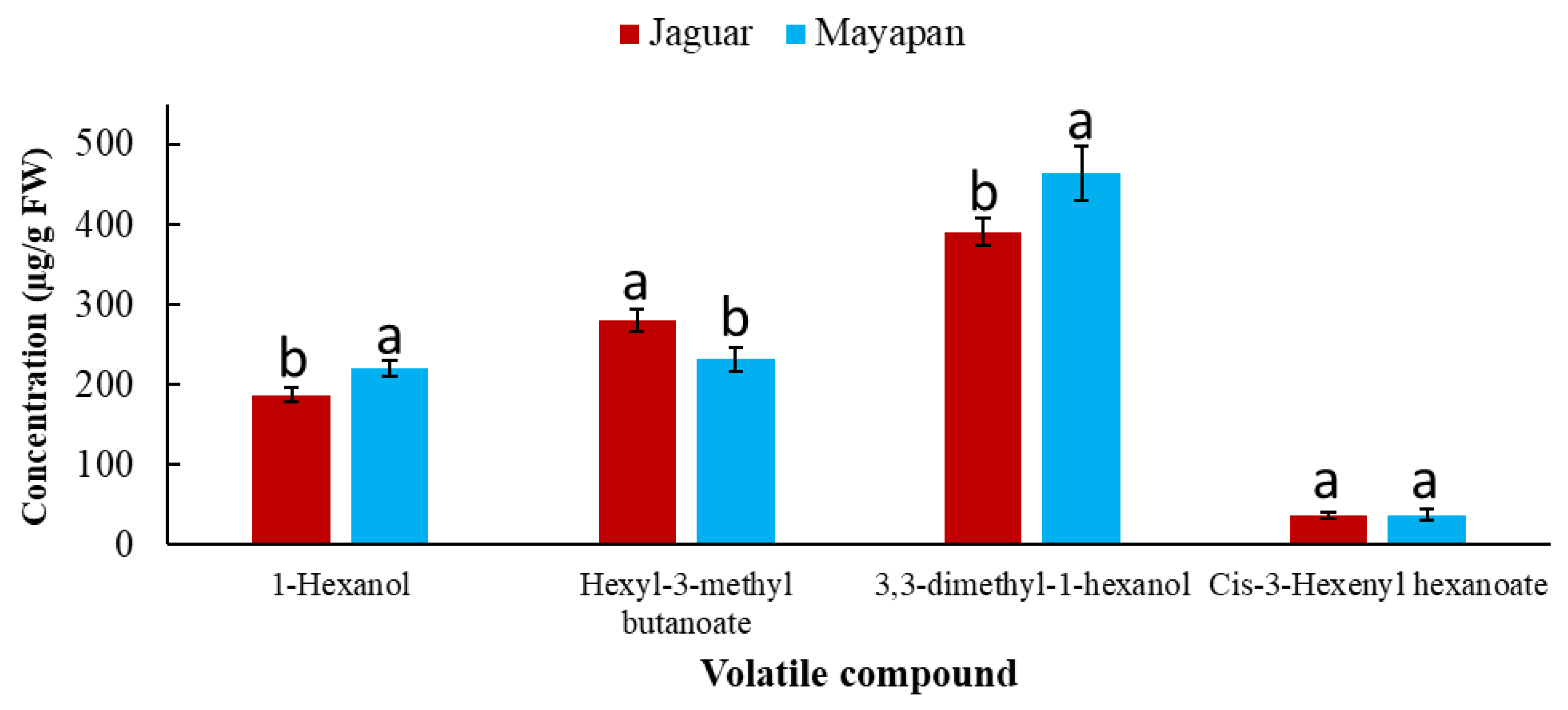
| Variety | Grade of Ripeness | Soil | 1-Hexanol (µg g−1 FW) | Hexyl-3-Methyl Butanoate (µg g−1 FW) | 3,3-Dimethyl-1-Hexanol (µg g−1 FW) | Cis-3-Hexenyl Hexanoate (µg g−1 FW) |
|---|---|---|---|---|---|---|
| Jaguar | Unripe | Red | 74.11 ± 3.53 d | 43.46 ± 3.24 h | 137.86 ± 4.79 g | 28.08 ± 2.09 f |
| Brown | 85.29 ± 6.50 d | 499.93 ± 5.78 a | 357.55 ± 6.40 d | 35.29 ± 1.19 e | ||
| Black | 163.08 ± 7.47 c | 284.06 ± 4.63 c | 256.25 ± 4.98 e | 33.89 ± 5.01 e | ||
| Ripe | Red | 273.29 ± 41.38 b | 280.12 ± 5.73 c | 549.02 ± 15.81 c | 37.57 ± 1.07 cd | |
| Brow | 243.27 ± 32.58 b | 277.89 ± 9.02 c | 515.51 ± 9.68 c | 39.57 ± 1.66 c | ||
| Black | 279.58 ± 5.40 b | 287.89 ± 9.02 c | 525.51 ± 9.68 c | 39.52 ± 1.45 c | ||
| Mayapan | Unripe | Red | 80.87 ± 3.06 d | 115.18 ± 3.79 f | 173.07 ± 4.74 f | 27.76 ± 1.67 f |
| Brow | 159.27 ± 22.55 c | 83.04 ± 13.61 g | 119.62 ± 20.11 g | 31.16 ± 1.85 e | ||
| Black | 166.53 ± 3.81 c | 417.11 ± 2.50 b | 521.85 ± 5.78 bc | 41.22 ± 2.25 b | ||
| Ripe | Red | 274.61 ± 14.34 b | 161.06 ± 29.76 e | 670.61 ± 177.72 b | 35.30 ± 4.27 de | |
| Brown | 279.58 ± 5.40 b | 199.49 ± 2.37 d | 279.47 ± 111.98 e | 38.96 ± 4.84 c | ||
| Black | 360.14 ± 8.57 a | 415.01 ± 4.68 b | 1020.61 ± 51.27 a | 49.49 ± 1.55 a |
| 1-Hexanol | Hexyl-3-Methyl Butanoate | 3,3-Dimethyl-1-Hexanol | Cis-3-Hexenyl Hexanoate | |
|---|---|---|---|---|
| A: Variety | 0.0001 * | <0.0001 * | 0.0141 * | 0.0865 |
| B: Grade of ripeness | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * |
| C: Soil | <0.0001 * | <0.0001 * | <0.0001 * | <0.0001 * |
| A × B | 0.1300 | 0.0001 * | 0.0620 | 0.2161 |
| A × C | 0.2089 | <0.0001 * | <0.0001 * | 0.0009 * |
| B × C | 0.1216 | <0.0001 * | 0.0014 * | 0.3641 |
| A × B × C | 0.0217 * | <0.0001 * | 0.2299 | 0.2721 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oney-Montalvo, J.E.; López-Salas, D.; Ramírez-Rivera, E.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Evaluation of the Soil Type Effect on the Volatile Compounds in the Habanero Pepper (Capsicum chinense Jacq.). Horticulturae 2022, 8, 428. https://doi.org/10.3390/horticulturae8050428
Oney-Montalvo JE, López-Salas D, Ramírez-Rivera E, Ramírez-Sucre MO, Rodríguez-Buenfil IM. Evaluation of the Soil Type Effect on the Volatile Compounds in the Habanero Pepper (Capsicum chinense Jacq.). Horticulturae. 2022; 8(5):428. https://doi.org/10.3390/horticulturae8050428
Chicago/Turabian StyleOney-Montalvo, Julio Enrique, Diego López-Salas, Emmanuel Ramírez-Rivera, Manuel Octavio Ramírez-Sucre, and Ingrid Mayanin Rodríguez-Buenfil. 2022. "Evaluation of the Soil Type Effect on the Volatile Compounds in the Habanero Pepper (Capsicum chinense Jacq.)" Horticulturae 8, no. 5: 428. https://doi.org/10.3390/horticulturae8050428
APA StyleOney-Montalvo, J. E., López-Salas, D., Ramírez-Rivera, E., Ramírez-Sucre, M. O., & Rodríguez-Buenfil, I. M. (2022). Evaluation of the Soil Type Effect on the Volatile Compounds in the Habanero Pepper (Capsicum chinense Jacq.). Horticulturae, 8(5), 428. https://doi.org/10.3390/horticulturae8050428









