Abnormal Programmed Cell Death of Tapetum Leads to the Pollen Abortion of Lycium barbarum Linnaeus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sampling Methods
2.3. Paraffin Section Preparation for Histomorphological Observation
2.4. Ultramicroscopic Observation of Tapetal Cells
2.5. TUNEL Assay
2.6. Determination of H2O2, O2−, and Malondialdehyde (MDA) Contents and Antioxidative Enzyme Activities
2.7. Determination of the Expression Levels of Genes Regulating Tapetum Development
2.8. Statistical Analysis
3. Results
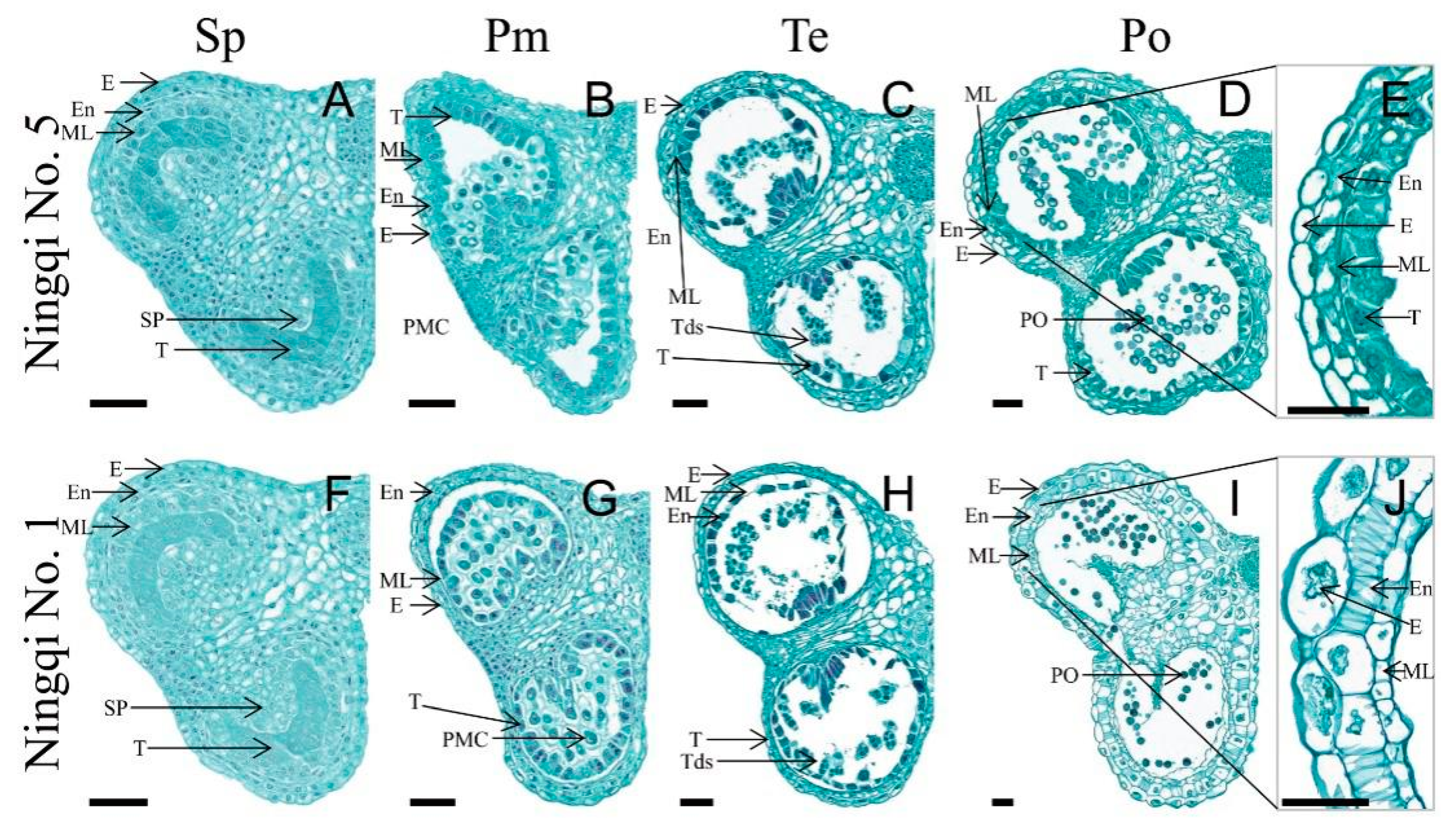
3.1. Histomorphological Characteristics of Ningqi No. 1 and Ningqi No. 5
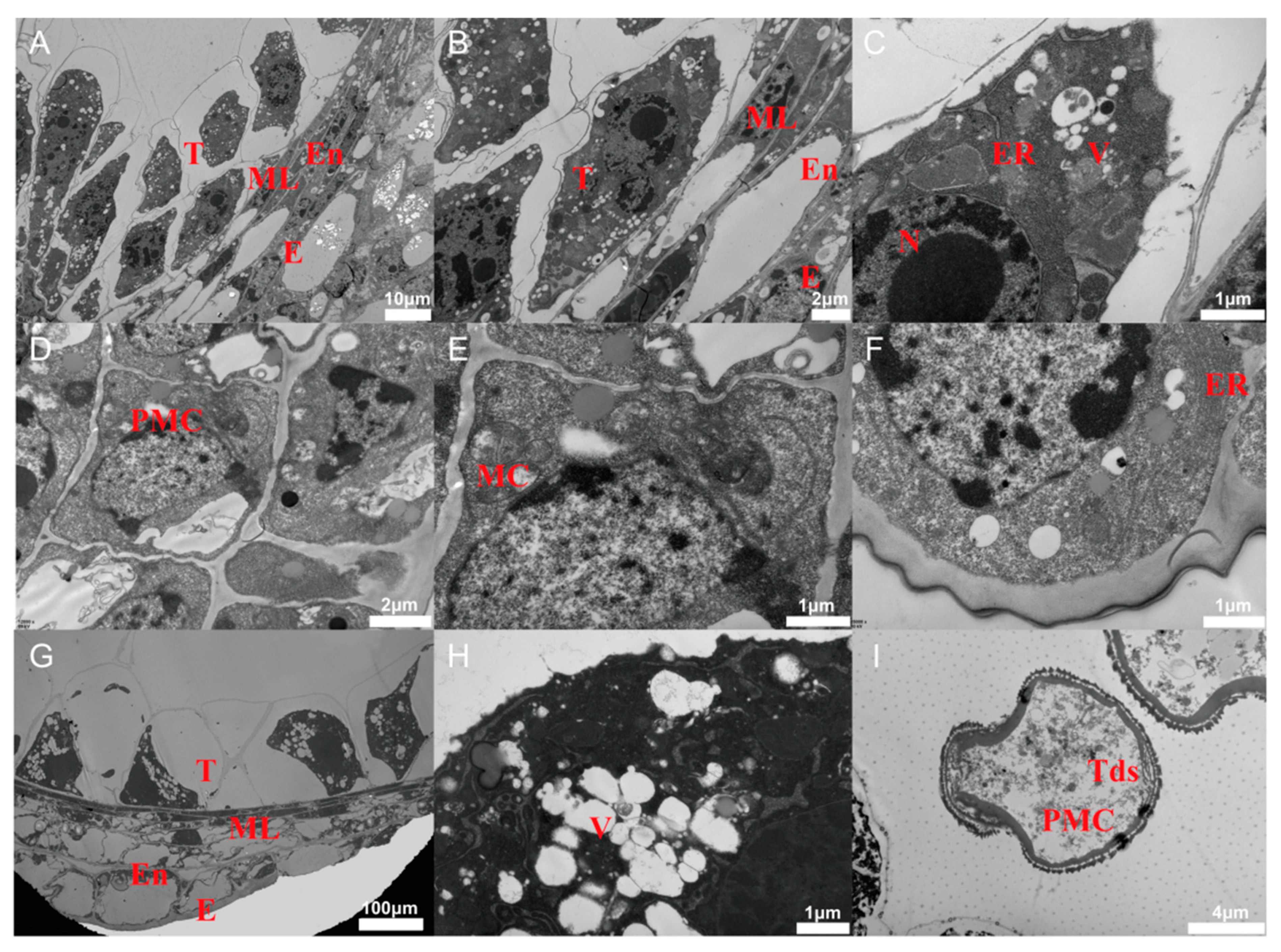
3.2. PCD Signals
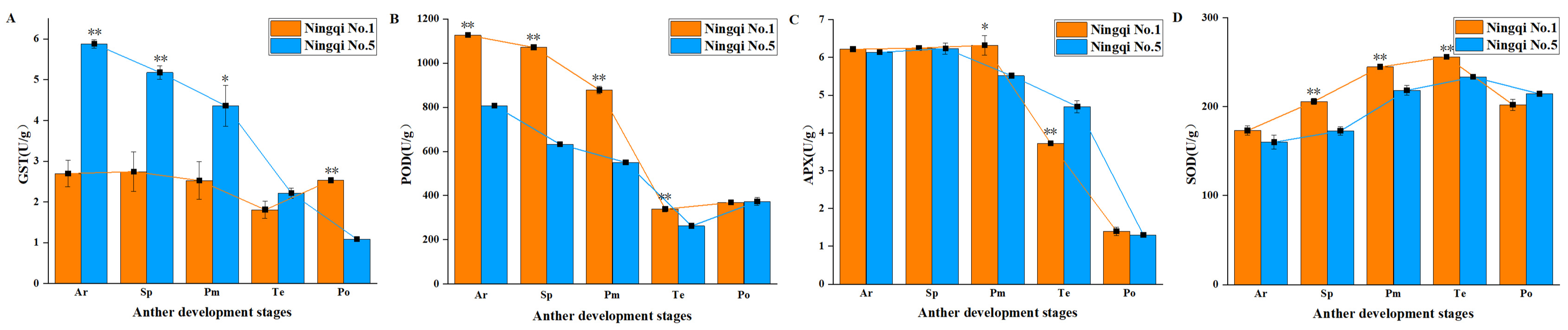
3.3. Contents of H2O2, O2−, and MDA in the Anthers of Ningqi No. 5 and Ningqi No. 1
3.4. Activities of Antioxidant Enzymes
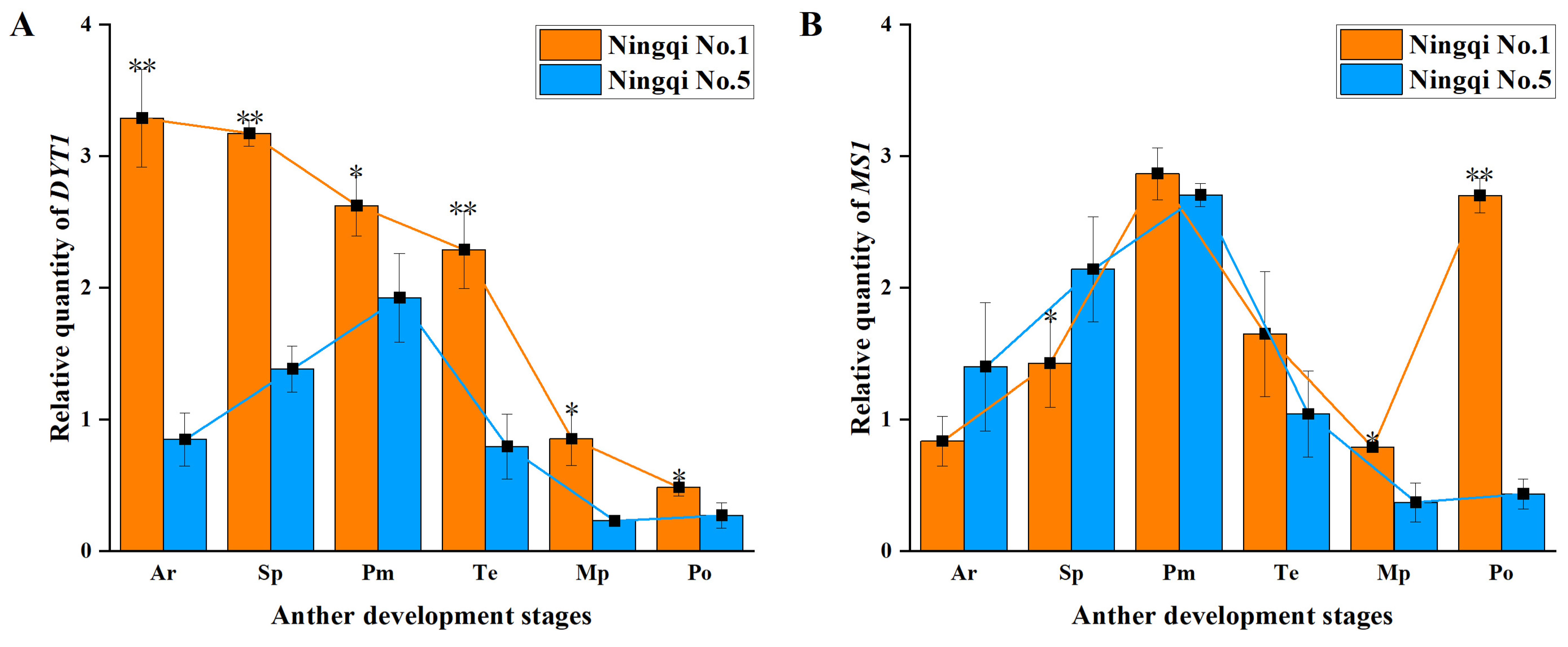
3.5. Expression of Genes Involved in Regulatory Pathways of Tapetum Development
4. Discussion
4.1. Tapetum and Pollen Development
4.2. ROS Level and PCD Progression
4.3. Expression of Genes Regulating Tapetum Development and PCD and ROS Levels
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kaul, M.L. Male Sterility in Higher Plants; Springer Science & Business Media: Berlin, Germany, 2012; Volume 10. [Google Scholar]
- Parish, R.W.; Li, S.F. Death of a tapetum: A programme of developmental altruism. Plant Sci. 2010, 178, 73–89. [Google Scholar] [CrossRef]
- Parish, R.W.; Phan, H.A.; Iacuone, S.; Li, S.F. Tapetal development and abiotic stress: A centre of vulnerability. Funct. Plant Biol. 2012, 39, 553–559. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Wei, Y.; Deng, L.; Ouyang, Y.; Chen, G.; Li, X.; Zhang, Q.; Wu, C. Rice APOPTOSIS INHIBITOR5 coupled with two DEAD-box adenosine 5′-triphosphate-dependent RNA helicases regulates tapetum degeneration. Plant Cell 2011, 23, 1416–1434. [Google Scholar] [CrossRef]
- Zhu, J.; Lou, Y.; Xu, X.; Yang, Z.N. A genetic pathway for tapetum development and function in Arabidopsis. J. Integr. Plant Biol. 2011, 53, 892–900. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, H.; Li, R.; Hong, Q.; Li, Y.; Xia, Q.; Zhang, W. Function identification of the nucleotides in key cis-element of Dysfunctional Tapetum1 (DYT1) promoter. Front. Plant Sci. 2017, 8, 153. [Google Scholar] [CrossRef]
- Feng, B.; Lu, D.; Ma, X.; Peng, Y.; Sun, Y.; Ning, G.; Ma, H. Regulation of the Arabidopsis anther transcriptome by DYT1 for pollen development. Plant J. 2012, 72, 612–624. [Google Scholar] [CrossRef]
- Xu, J.; Yang, C.; Yuan, Z.; Zhang, D.; Gondwe, M.Y.; Ding, Z.; Liang, W.; Zhang, D.; Wilson, Z.A. The ABORTED MICROSPORES regulatory network is required for postmeiotic male reproductive development in Arabidopsis thaliana. Plant Cell 2010, 22, 91–107. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Zhu, J.; Gao, J.F.; Wang, C.; Li, H.; Li, H.; Zhang, H.Q.; Zhang, S.; Wang, D.M.; Wang, Q.X. Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J. 2007, 52, 528–538. [Google Scholar] [CrossRef]
- Ito, T.; Nagata, N.; Yoshiba, Y.; Ohme-Takagi, M.; Ma, H.; Shinozaki, K. Arabidopsis MALE STERILITY1 encodes a PHD-type transcription factor and regulates pollen and tapetum development. Plant Cell 2007, 19, 3549–3562. [Google Scholar] [CrossRef]
- Yang, C.; Vizcay-Barrena, G.; Conner, K.; Wilson, Z.A. MALE STERILITY1 is required for tapetal development and pollen wall biosynthesis. Plant Cell 2007, 19, 3530–3548. [Google Scholar] [CrossRef]
- Gómez, J.F.; Talle, B.; Wilson, Z.A. Anther and pollen development: A conserved developmental pathway. J. Integr. Plant Biol. 2015, 57, 876–891. [Google Scholar] [CrossRef]
- Lou, Y.; Zhou, H.S.; Han, Y.; Zeng, Q.Y.; Zhu, J.; Yang, Z.N. Positive regulation of AMS by TDF1 and the formation of a TDF1–AMS complex are required for anther development in Arabidopsis thaliana. New Phytol. 2018, 217, 378–391. [Google Scholar] [CrossRef]
- Li, D.-D.; Xue, J.-S.; Zhu, J.; Yang, Z.-N. Gene regulatory network for tapetum development in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1559. [Google Scholar] [CrossRef]
- Hu, P.; Tirelli, N. Scavenging ROS: Superoxide dismutase/catalase mimetics by the use of an oxidation-sensitive nanocarrier/enzyme conjugate. Bioconjugate Chem. 2012, 23, 438–449. [Google Scholar] [CrossRef]
- Li, J.; Dai, X.; Li, L.; Jiao, Z.; Huang, Q. Metabolism of reactive oxygen species in cytoplasmic male sterility of rice by marking upmost pulvinus interval. Appl. Biochem. Biotechnol. 2015, 175, 1263–1269. [Google Scholar] [CrossRef]
- Yi, J.; Moon, S.; Lee, Y.-S.; Zhu, L.; Liang, W.; Zhang, D.; Jung, K.-H.; An, G. Defective tapetum cell death 1 (DTC1) regulates ROS levels by binding to metallothionein during tapetum degeneration. Plant Physiol. 2016, 170, 1611–1623. [Google Scholar] [CrossRef]
- Hu, L.; Liang, W.; Yin, C.; Cui, X.; Zong, J.; Wang, X.; Hu, J.; Zhang, D. Rice MADS3 regulates ROS homeostasis during late anther development. Plant Cell 2011, 23, 515–533. [Google Scholar] [CrossRef]
- Naifu, Z.; Junpei, Z.; Hao, L.; Weiwei, Z.; Dong, P. New protocols for paraffin sections of heterogeneous tissues of woody plants. Chin. Bull. Bot. 2018, 53, 653. [Google Scholar]
- Wang, S.; Zhang, G.; Song, Q.; Zhang, Y.; Li, Z.; Guo, J.; Niu, N.; Ma, S.; Wang, J. Abnormal development of tapetum and microspores induced by chemical hybridization agent SQ-1 in wheat. PLoS ONE 2015, 10, e0119557. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Li, H.; Niu, H.; Xu, Q.; Jiao, Z.; An, J.; Jiang, Y.; Li, Q.; Niu, J. The major factors causing the microspore abortion of genic male sterile mutant NWMS1 in wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2019, 20, 6252. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Q.; Zhang, L.; Li, Z.; Wang, C.; Zhang, G. Comparative proteomic analysis of developmental changes in P-type cytoplasmic male sterile and maintainer anthers in wheat. Int. J. Mol. Sci. 2021, 22, 2012. [Google Scholar] [CrossRef] [PubMed]
- Irene, S.; Salvatore, P.; Adela, O. Programmed-cell-death hallmarks in incompatible pollen and papillar stigma cells of Olea europaea L. under free pollination. Plant Cell Rep. 2010, 29, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Y.; Gai, W.-X.; Li, C.; Gong, Z.-H. The CaCIPK3 gene positively regulates drought tolerance in pepper. Hortic. Res. 2021, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Kang, Y.; Liu, Y.; Shi, M.; Zhang, W.; Fan, Y.; Yao, Y.; Li, H.; Qin, S. Integrated analysis of miRNA-mRNA regulatory networks of potato (Solanum tuberosum L.) in response to cadmium stress. Ecotoxicol. Environ. Saf. 2021, 224, 112682. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Zhang, B.-L.; Li, F.; Li, Y.-R.; Li, J.-H.; Lu, Y.-T. General control non-repressible 20 functions in the salt stress response of Arabidopsis seedling by modulating ABA accumulation. Environ. Exp. Bot. 2022, 198, 104856. [Google Scholar] [CrossRef]
- Ba, Q.S.; Zhang, G.S.; Wang, J.S.; Che, H.X.; Liu, H.Z.; Niu, N.; Ma, S.C.; Wang, J.W. Relationship between metabolism of reactive oxygen species and chemically induced male sterility in wheat (Triticum aestivum L.). Can. J. Plant Sci. 2013, 93, 675–681. [Google Scholar] [CrossRef]
- Gu, J.N.; Zhu, J.; Yu, Y.; Teng, X.D.; Lou, Y.; Xu, X.F.; Liu, J.L.; Yang, Z.N. DYT 1 directly regulates the expression of TDF 1 for tapetum development and pollen wall formation in Arabidopsis. Plant J. 2014, 80, 1005–1013. [Google Scholar] [CrossRef]
- Lu, J.-Y.; Xiong, S.-X.; Yin, W.; Teng, X.-D.; Lou, Y.; Zhu, J.; Zhang, C.; Gu, J.-N.; Wilson, Z.A.; Yang, Z.-N. MS1, a direct target of MS188, regulates the expression of key sporophytic pollen coat protein genes in Arabidopsis. J. Exp. Bot. 2020, 71, 4877–4889. [Google Scholar] [CrossRef]
- Shamrov, I.I.; Anisimova, G.M.; Babro, A.A. Tapetum Types and Forms in Angiosperms. Proc. Latv. Acad. Sci. 2021, 75, 167–179. [Google Scholar] [CrossRef]
- Flores-Rentería, L.; Orozco-Arroyo, G.; Cruz-García, F.; García-Campusano, F.; Alfaro, I.; Vázquez-Santana, S. Programmed cell death promotes male sterility in the functional dioecious Opuntia stenopetala (Cactaceae). Ann. Bot. 2013, 112, 789–800. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Zhang, G. Formation pattern and regulatory mechanisms of pollen wall in Arabidopsis. J. Plant Physiol. 2021, 260, 153388. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Guo, X.; Zhang, J.; Liu, Y.; Wang, B.; Li, H.; Lu, H. βVPE is involved in tapetal degradation and pollen development by activating proprotease maturation in Arabidopsis thaliana. J. Exp. Bot. 2020, 71, 1943–1955. [Google Scholar] [CrossRef]
- Lei, T.; Zhang, L.; Feng, P.; Liu, Y.; Yin, W.; Shang, L.; He, G.; Wang, N. OsMYB103 is essential for tapetum degradation in rice. Theor. Appl. Genet. 2022, 135, 929–945. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, R.; Yue, Z.; Yan, X.; Cheng, D.; Li, J.; Li, H.; Zhang, Y.; Ma, J.; Yang, J. The impaired biosynthetic networks in defective tapetum lead to male sterility in watermelon. J. Proteom. 2021, 243, 104241. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Xia, X.; Hong, D.; Li, J.; Yang, G. Abnormal vacuolization of the tapetum during the tetrad stage is associated with male sterility in the recessive genic male sterile Brassica napus L. Line 9012A. J. Plant Biol. 2010, 53, 121–133. [Google Scholar] [CrossRef]
- Hales, K.G.; Fuller, M.T. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell 1997, 90, 121–129. [Google Scholar] [CrossRef]
- Yaffe, M.P. The machinery of mitochondrial inheritance and behavior. Science 1999, 283, 1493–1497. [Google Scholar] [CrossRef]
- Skulachev, V.P. Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem. Sci. 2001, 26, 23–29. [Google Scholar] [CrossRef]
- Frank, S.; Gaume, B.; Bergmann-Leitner, E.S.; Leitner, W.W.; Robert, E.G.; Catez, F.; Smith, C.L.; Youle, R.J. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 2001, 1, 515–525. [Google Scholar] [CrossRef]
- Xie, H.-T.; Wan, Z.-Y.; Li, S.; Zhang, Y. Spatiotemporal production of reactive oxygen species by NADPH oxidase is critical for tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell 2014, 26, 2007–2023. [Google Scholar] [CrossRef]
- Zheng, S.; Li, J.; Ma, L.; Wang, H.; Zhou, H.; Ni, E.; Jiang, D.; Liu, Z.; Zhuang, C. OsAGO2 controls ROS production and the initiation of tapetal PCD by epigenetically regulating OsHXK1 expression in rice anthers. Proc. Natl. Acad. Sci. USA 2019, 116, 7549–7558. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-X.; Feng, Q.-N.; Xie, H.-T.; Li, S.; Zhang, Y. Reactive oxygen species mediate tapetal programmed cell death in tobacco and tomato. BMC Plant Biol. 2017, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, L. Specification of tapetum and microsporocyte cells within the anther. Curr. Opin. Plant Biol. 2014, 17, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef]
- Ko, S.-S.; Li, M.-J.; Lin, Y.-J.; Hsing, H.-X.; Yang, T.-T.; Chen, T.-K.; Jhong, C.-M.; Ku, M.S.-B. Tightly controlled expression of bHLH142 is essential for timely tapetal programmed cell death and pollen development in rice. Front. Plant Sci. 2017, 8, 1258. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Bei, Z.; Ma, H.; Wei, Z.; Zhou, J.; Ren, Y.; Xu, W.; Nan, P.; Wang, Y.; Li, L.; et al. Abnormal Programmed Cell Death of Tapetum Leads to the Pollen Abortion of Lycium barbarum Linnaeus. Horticulturae 2022, 8, 1056. https://doi.org/10.3390/horticulturae8111056
Zhang X, Bei Z, Ma H, Wei Z, Zhou J, Ren Y, Xu W, Nan P, Wang Y, Li L, et al. Abnormal Programmed Cell Death of Tapetum Leads to the Pollen Abortion of Lycium barbarum Linnaeus. Horticulturae. 2022; 8(11):1056. https://doi.org/10.3390/horticulturae8111056
Chicago/Turabian StyleZhang, Xin, Zhanlin Bei, Haijun Ma, Zhaojun Wei, Jun Zhou, Yufeng Ren, Wendi Xu, Peng Nan, Yuguo Wang, Linfeng Li, and et al. 2022. "Abnormal Programmed Cell Death of Tapetum Leads to the Pollen Abortion of Lycium barbarum Linnaeus" Horticulturae 8, no. 11: 1056. https://doi.org/10.3390/horticulturae8111056
APA StyleZhang, X., Bei, Z., Ma, H., Wei, Z., Zhou, J., Ren, Y., Xu, W., Nan, P., Wang, Y., Li, L., Zhang, W., Yang, J., Zhong, Y., & Song, Z. (2022). Abnormal Programmed Cell Death of Tapetum Leads to the Pollen Abortion of Lycium barbarum Linnaeus. Horticulturae, 8(11), 1056. https://doi.org/10.3390/horticulturae8111056





