Phytonutrients and Metabolism Changes in Topped Radish Root and Its Detached Leaves during 1 °C Cold Postharvest Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Postharvest Storage
2.2. Glucosinolates Quantification
2.3. Glucosinolate Hydrolysis Product
2.4. Vitamins E and K Analyses
2.5. Primary Metabolite Extraction and Analysis
2.6. Amino Acid Quantification
2.7. Plant Hormone Analysis
2.8. Statistical Analysis
3. Results
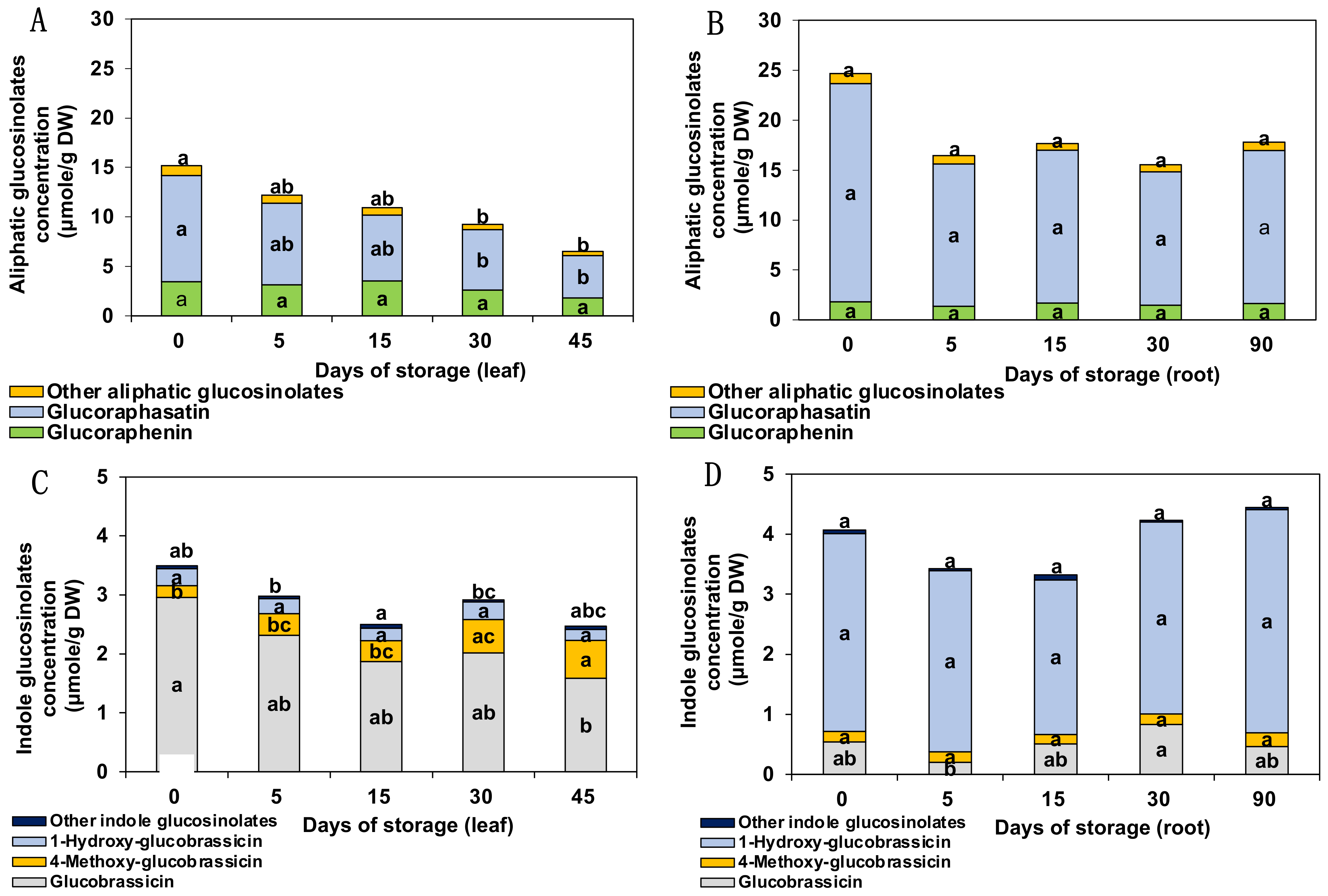
3.1. Glucosinolates
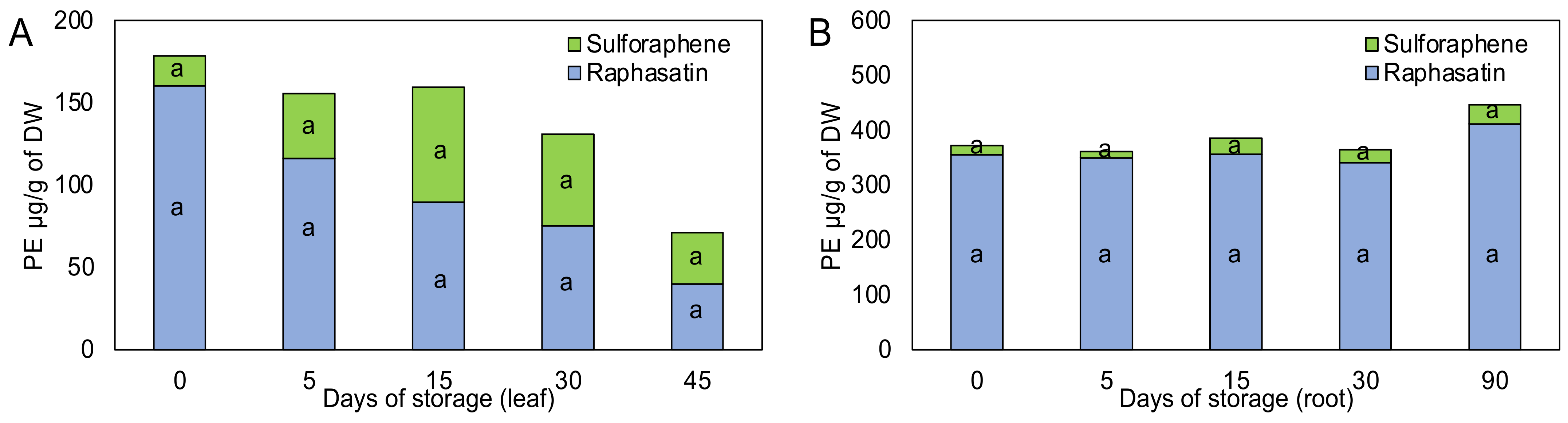
3.2. Glucosinolate Hydrolysis Products
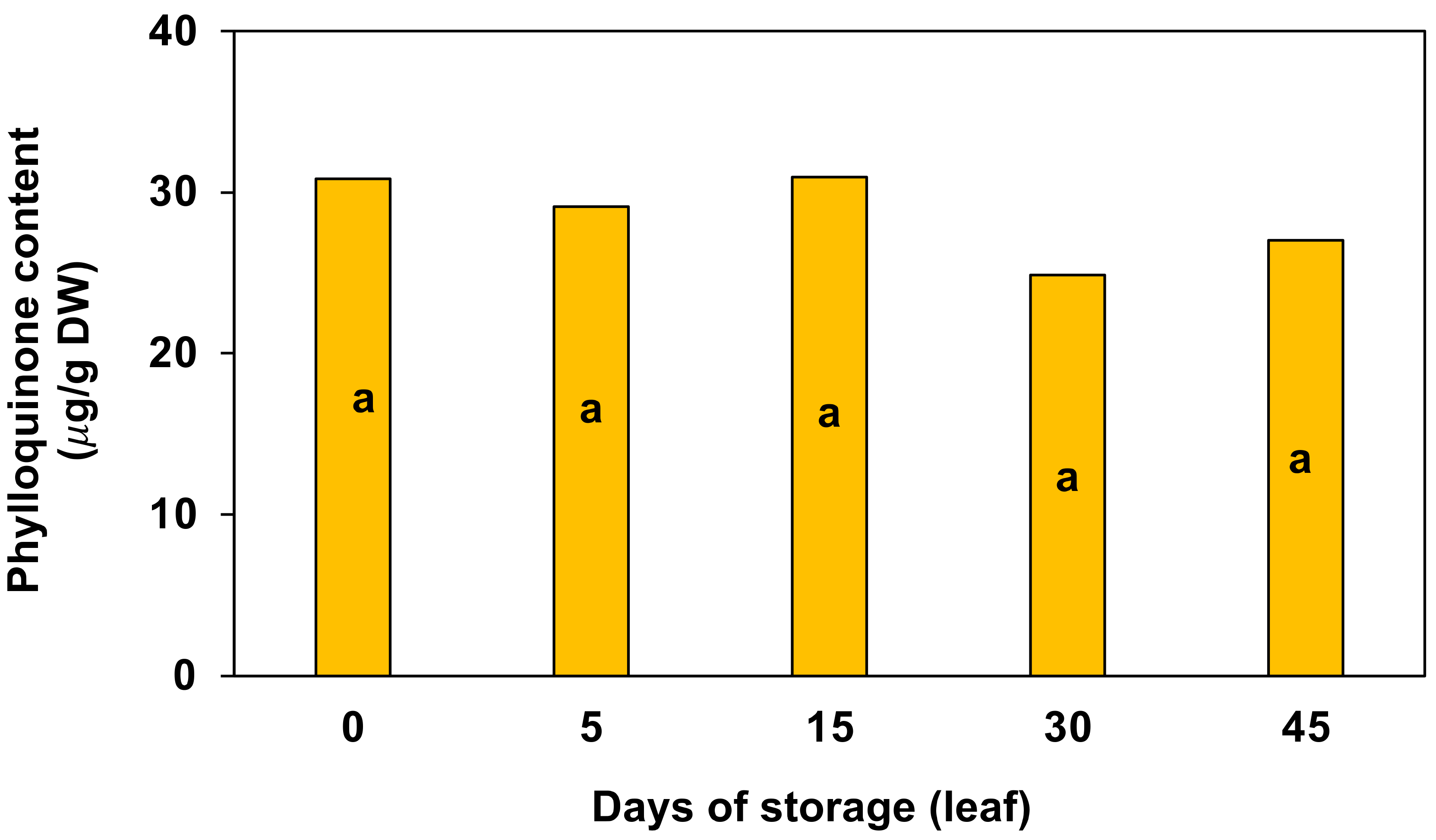
3.3. Vitamins E and K
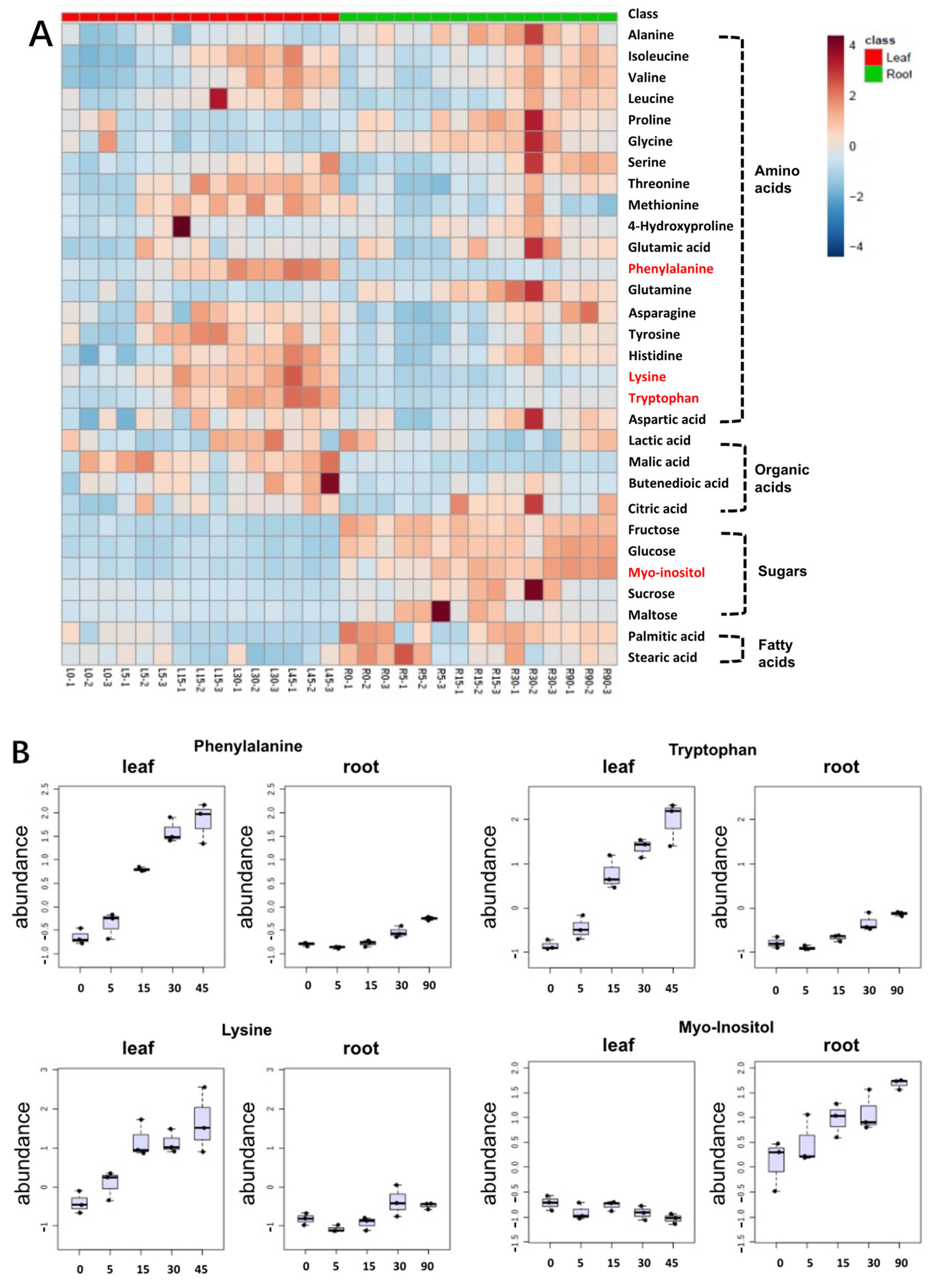
3.4. Primary Metabolite Extraction and Analysis
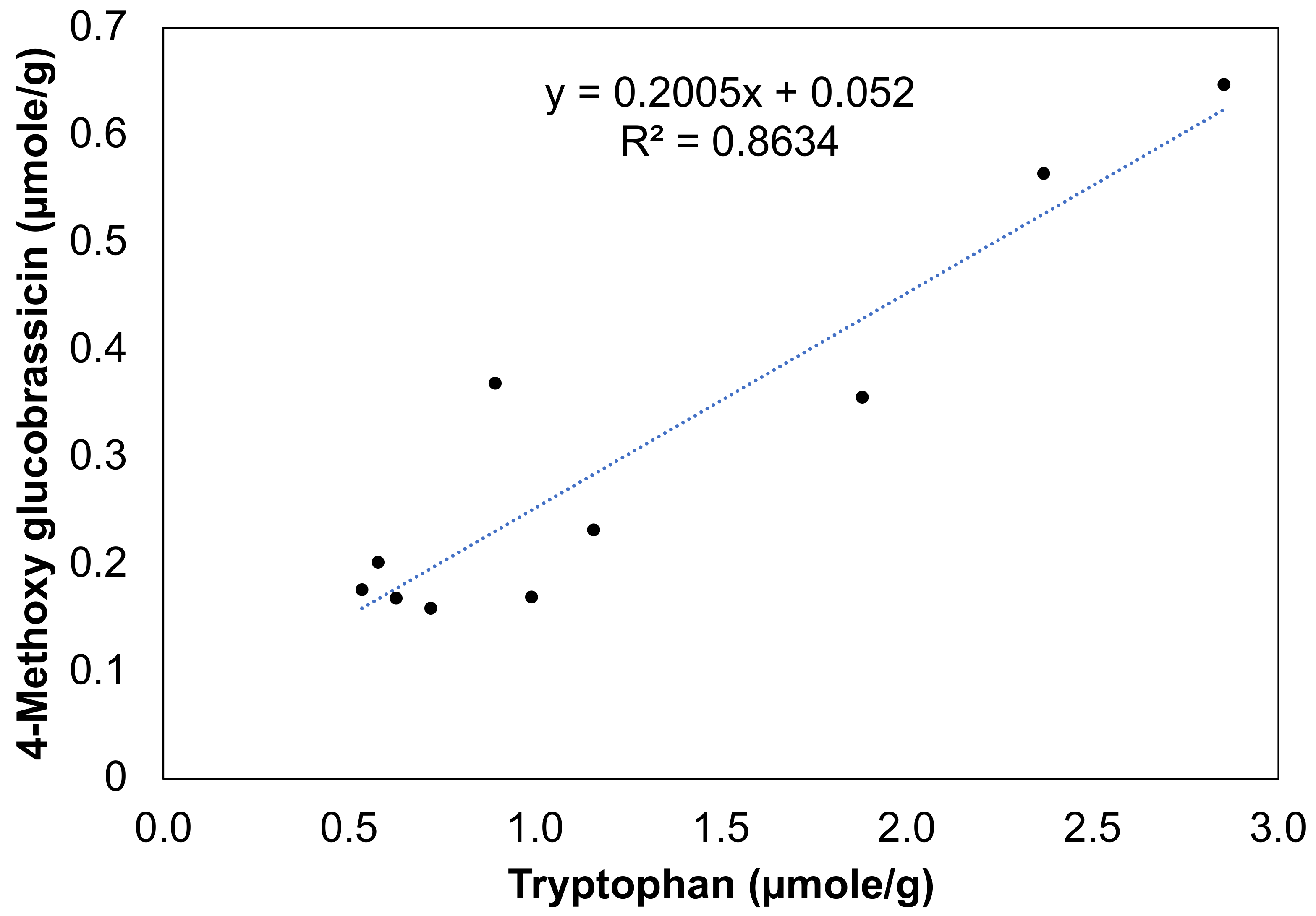
3.5. Correlation Analyses between Precursor Amino Acids and Glucosinolate during Storage
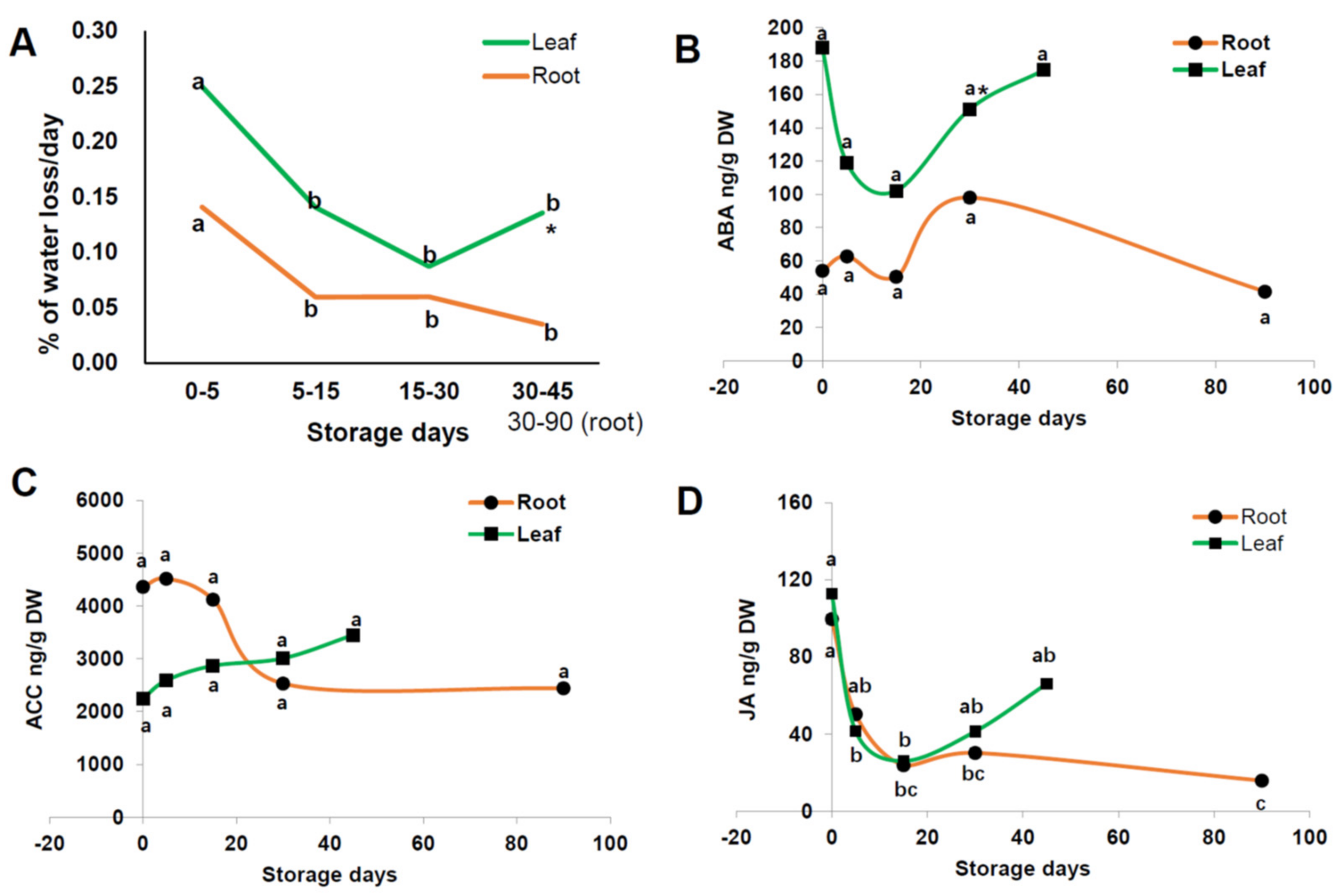
3.6. % Water Loss per Day and Plant Hormone Changes of Radish Root and Leaf during Cold Storage
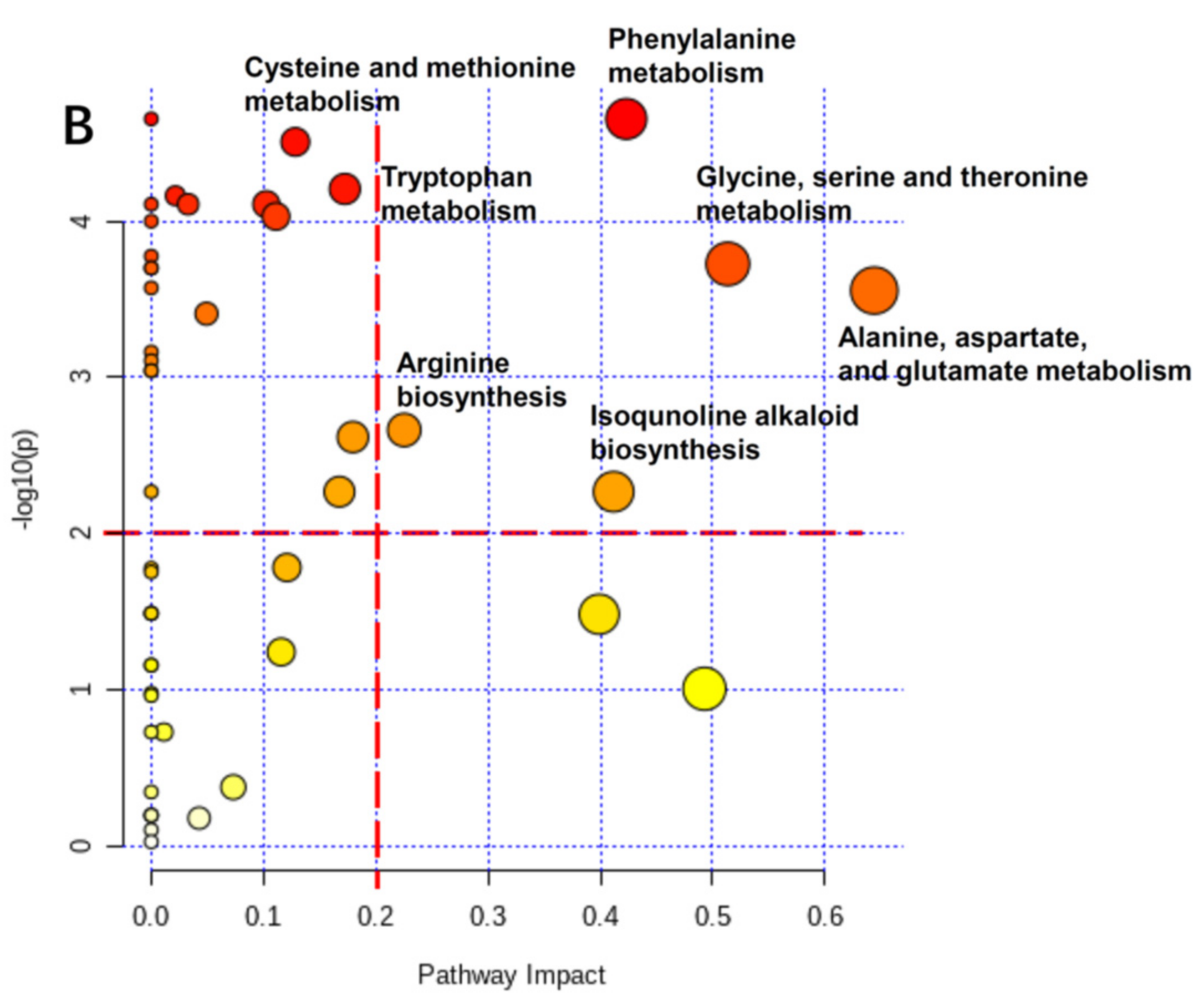
3.7. Physiological Changes of Radish Root and Leaf during Cold Storage Based on Pathway Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Han, N.; Na, H.; Kim, J. IIdentification and Variation of Major Aliphatic Glucosinolates in Doubled Haploid Lines of Radish (Raphanus sativus L.). Hortic. Sci. Technol. 2018, 36, 202–311. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kwon, K.-J.; Kim, M.-S.; Sohn, H.-Y. Antimicrobial, Antioxidant and Anticoagulation Activities of Korean Radish (Raphanus sativus L.) Leaves. Korean J. Microbiol. Biotechnol. 2013, 41, 228–235. [Google Scholar] [CrossRef]
- Chung, D.H.; Kim, S.H.; Myung, N.; Cho, K.J.; Chang, M.J. The antihypertensive effect of ethyl acetate extract of radish leaves in spontaneously hypertensive rats. Nutr. Res. Pract. 2012, 6, 308–314. [Google Scholar] [CrossRef]
- Lim, S.; Lee, E.J.; Kim, J. Decreased sulforaphene concentration and reduced myrosinase activity of radish (Raphanus sativus L.) root during cold storage. Postharvest Biol. Technol. 2015, 100, 219–225. [Google Scholar] [CrossRef]
- Yi, G.; Lim, S.; Chae, W.B.; Park, J.E.; Park, H.R.; Lee, E.J.; Huh, J.H. Root Glucosinolate Profiles for Screening of Radish (Raphanus sativus L.) Genetic Resources. J. Agric. Food Chem. 2016, 64, 61–70. [Google Scholar] [CrossRef]
- Kim, M.J.; Chiu, Y.-C.; Ku, K.-M. Glucosinolates, Carotenoids, and Vitamins E and K Variation from Selected Kale and Collard Cultivars. J. Food Qual. 2017, 2017, 5123572. [Google Scholar] [CrossRef]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A.; Kushad, M.M. Correlation of quinone reductase activity and allyl isothiocyanate formation among different genotypes and grades of horseradish roots. J. Agric. Food Chem 2015, 63, 2947–2955. [Google Scholar] [CrossRef]
- Yasushi, N.; Kei, N.; Yumi, A.; Toyoaki, W.; Kiwamu, T.; Tomoaki, M.; Shigehisa, O.; Johan, M.; Yasuki, K.; Akiyoshi, N.; et al. Comparison of the Glucosinolate−Myrosinase Systems among Daikon (Raphanus sativus, Japanese White Radish) Varieties. J. Agric. Food Chem. 2008, 56, 2702–2707. [Google Scholar]
- Wang, J.; Qiu, Y.; Wang, X.; Yue, Z.; Yang, X.; Chen, X.; Zhang, X.; Shen, D.; Wang, H.; Song, J.; et al. Insights into the species-specific metabolic engineering of glucosinolates in radish (Raphanus sativus L.) based on comparative genomic analysis. Sci. Rep. 2017, 7, 16040. [Google Scholar] [CrossRef]
- Ruud, V.; Monika, S.; Angelika, K.; Ewa, C.; Birgit, H.; Ian, R.; Remi, D.S.; Magnor, H.; Clarissa, G.; Richard, M.; et al. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, S219. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; Institute of Medicine: Washington, DC, USA, 2000. [Google Scholar]
- Chun, J.; Lee, J.; Ye, L.; Exler, J.; Eitenmiller, R.R. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. J. Food Compos. Anal. 2006, 19, 196–204. [Google Scholar] [CrossRef]
- Knecht, K.; Sandfuchs, K.; Kulling, S.E.; Bunzel, D. Tocopherol and tocotrienol analysis in raw and cooked vegetables: A validated method with emphasis on sample preparation. Food Chem. 2015, 169, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.J.; Booth, S.L.; van den Heuvel, E.; Stoecklin, E.; Baka, A.; Vermeer, C. The role of menaquinones (vitamin K2) in human health. Br. J. Nutr. 2013, 110, 1357–1368. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, M.S.; Na, W.R.; Sohn, C.M. Estimation of vitamin K intake in Koreans and determination of the primary vitamin K-containing food sources based on the fifth Korean National Health and Nutrition Examination Survey (2010–2011). Nutr. Res. Pract. 2013, 7, 503–509. [Google Scholar] [CrossRef][Green Version]
- Juanola-Falgarona, M.; Salas-Salvadó, J.; Martínez-González, M.Á.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary Intake of Vitamin K Is Inversely Associated with Mortality Risk. J. Nutr. 2014, 144, 743–750. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; Institute of Medicine: Washington, DC, USA, 2001. [Google Scholar]
- Ku, K.M.; Choi, J.H.; Kushad, M.M.; Jeffery, E.H.; Juvik, J.A. Pre-harvest methyl jasmonate treatment enhances cauliflower chemoprotective attributes without a loss in postharvest quality. Plant Foods Hum. Nutr. 2013, 68, 113–117. [Google Scholar] [CrossRef]
- Ku, K.M.; Choi, J.H.; Kim, H.S.; Kushad, M.M.; Jeffery, E.H.; Juvik, J.A. Methyl jasmonate and 1-methylcyclopropene treatment effects on quinone reductase inducing activity and post-harvest quality of broccoli. PLoS ONE 2013, 8, e77127. [Google Scholar] [CrossRef]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A. Influence of seasonal variation and methyl jasmonate mediated induction of glucosinolate biosynthesis on quinone reductase activity in broccoli florets. J. Agric. Food Chem. 2013, 61, 9623–9631. [Google Scholar] [CrossRef]
- Ku, K.M.; Juvik, J.A. Environmental stress and methyl jasmonate-mediated changes in flavonoid concentrations and antioxidant activity in broccoli florets and kale leaf tissues. Hortscience 2013, 48, 996–1002. [Google Scholar] [CrossRef]
- Barickman, T.C.; Ku, K.-M.; Sams, C.E. Differing precision irrigation thresholds for kale (Brassica oleracea L. var. acephala) induces changes in physiological performance, metabolites, and yield. Environ. Exp. Bot. 2020, 180, 104253. [Google Scholar] [CrossRef]
- Park, M.H.; Chang, E.H.; Yang, H.J.; Lee, J.S.; Do, G.R.; Song, H.J.; Chang, M.S.; Ku, K.M. Modified Atmosphere and Humidity Film Reduces Browning Susceptibility of Oriental Melon Suture Tissue during Cold Storage. Foods 2020, 9, 1329. [Google Scholar] [CrossRef]
- Simpson, T.; Ku, K.M. Metabolomics and Physiological Approach to Understand Allelopathic Effect of Horseradish Extract on Onion Root and Lettuce Seed as Model Organism. Plants 2021, 10, 1992. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, M.Y.; Shon, J.C.; Kwon, Y.S.; Liu, K.H.; Lee, C.H.; Ku, K.M. Untargeted and targeted metabolomics analyses of blackberries–Understanding postharvest red drupelet disorder. Food Chem. 2019, 300, 125169. [Google Scholar] [CrossRef]
- Kim, M.J.; Chiu, Y.C.; Kim, N.K.; Park, H.M.; Lee, C.H.; Juvik, J.A.; Ku, K.M. Cultivar-Specific Changes in Primary and Secondary Metabolites in Pak Choi (Brassica Rapa, Chinensis Group) by Methyl Jasmonate. Int. J. Mol. Sci. 2017, 18, 1004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ku, K.M. Biomarkers-based classification between green teas and decaffeinated green teas using gas chromatography mass spectrometer coupled with in-tube extraction (ITEX). Food Chem. 2019, 271, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.C.; Matak, K.; Ku, K.M. Methyl jasmonate treated broccoli: Impact on the production of glucosinolates and consumer preferences. Food Chem. 2019, 299, 125099. [Google Scholar] [CrossRef] [PubMed]
- Balcke, G.U.; Handrick, V.; Bergau, N.; Fichtner, M.; Henning, A.; Stellmach, H.; Tissier, A.; Hause, B.; Frolov, A. An UPLC-MS/MS method for highly sensitive high-throughput analysis of phytohormones in plant tissues. Plant Methods 2012, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Choi, J.N.; Kim, J.; Kim, J.K.; Yoo, L.G.; Lee, S.J.; Hong, Y.S.; Lee, C.H. Metabolomics analysis reveals the compositional differences of shade grown tea (Camellia sinensis L.). J. Agric. Food Chem. 2010, 58, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Kim, J.; Park, H.J.; Liu, K.H.; Lee, C.H. Application of metabolomics in the analysis of manufacturing type of pu-erh tea and composition changes with different postfermentation year. J. Agric. Food Chem. 2010, 58, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Guidlines for the Use of Chlorine Bleach as a Sanitizer in Food Processing Operations. Available online: https://extension.okstate.edu/fact-sheets/guidelines-for-the-use-of-chlorine-bleach-as-a-sanitizer-in-food-processing-operations.html (accessed on 9 December 2021).
- Ku, K.-M.; Kim, M.J.; Jeffery, E.H.; Kang, Y.-H.; Juvik, J.A. Profiles of glucosinolates, their hydrolysis products, and quinone reductase inducing activity from 39 arugula (Eruca Sativa Mill.) accessions. J. Agric. Food Chem. 2016, 64, 6524–6532. [Google Scholar] [CrossRef]
- Frazie, M.D.; Kim, M.J.; Ku, K.M. Health-Promoting Phytochemicals from 11 Mustard Cultivars at Baby Leaf and Mature Stages. Molecules 2017, 22, 1749. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Ku, K.M.; Kim, J. Postharvest variation of major glucosinolate and their hydrolytic products in Brassicoraphanus ‘BB1’. Postharvest Biol. Technol. 2019, 154, 70–78. [Google Scholar] [CrossRef]
- Kjaer, A.; Ohashi, M.; Wilson, J.; Djerassi, C. Mass spectra of isothiocyanates. Acta Chem. Scand. 1963, 17, 2143–2154. [Google Scholar] [CrossRef]
- Spencer, G.F.; Daxenbichler, M.E. Gas chromatography-mass spectrometry of nitriles, isothiocyanates and oxazolidinethiones derived from cruciferous glucosinolates. J. Sci. Food Agric. 1980, 31, 359–367. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Chae, S.H.; Lee, Y.S.; Kim, J.H.; Han, T.H.; Ku, K.M. Metabolite and Elastase Activity Changes in Beach Rose (Rosa rugosa) Fruit and Seeds at Various Stages of Ripeness. Plants 2021, 10, 1283. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Han, N.; Su’udi, M.; Kim, J. The major aliphatic glucosinolate content in Korean radish during vegetative and reproductive growth. Hortic. Environ. Biotechnol. 2015, 56, 152–158. [Google Scholar] [CrossRef]
- Hanlon, P.R.; Barnes, D.M. Phytochemical composition and biological activity of 8 varieties of radish (Raphanus sativus L.) sprouts and mature taproots. J. Food Sci. 2011, 76, C185–C192. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Kim, M.-B.; Lim, S. Formation and Stabilization of Raphasatin and Sulforaphene from Radish Roots by Endogenous Enzymolysis. Prev. Nutr. Food Sci. 2015, 20, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Lim, S.; Kim, J.; Lee, E.J. The mechanism of deterioration of the glucosinolate-myrosynase system in radish roots during cold storage after harvest. Food Chem. 2017, 233, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Ahn, J.-C.; Lee, E.J.; Kim, J. Antiproliferation effect of sulforaphene isolated from radish (Raphanus sativus L.) seeds on A549 cells. Appl. Biol. Chem. 2020, 63, 75. [Google Scholar] [CrossRef]
- Hanlon, P.R.; Webber, D.M.; Barnes, D.M. Aqueous extract from Spanish black radish (Raphanus sativus L. Var. niger) induces detoxification enzymes in the HepG2 human hepatoma cell line. J. Agric. Food. Chem. 2007, 55, 6439–6446. [Google Scholar] [CrossRef]
- Saldeen, K.; Saldeen, T. Importance of tocopherols beyond alpha-tocopherol: Evidence from animal and human studies. Nutr. Res. 2005, 25, 877–889. [Google Scholar] [CrossRef]
- Shahidi, F.; de Camargo, A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef]
- Okano, T.; Shimomura, Y.; Yamane, M.; Suhara, Y.; Kamao, M.; Sugiura, M.; Nakagawa, K. Conversion of phylloquinone (vitamin K-1) into menaquinone-4 (vitamin K-2) in mice. J. Biol. Chem. 2008, 283, 11270–11279. [Google Scholar] [CrossRef]
- Furt, F.; van Oostende, C.; Widhalm, J.R.; Dale, M.A.; Wertz, J.; Basset, G.J.C. A bimodular oxidoreductase mediates the specific reduction of phylloquinone (vitamin K1) in chloroplasts. Plant J. 2010, 64, 38–46. [Google Scholar] [CrossRef]
- Bednarek, P.; Piślewska-Bednarek, M.; Svatoš, A.; Schneider, B.; Doubský, J.; Mansurova, M.; Humphry, M.; Consonni, C.; Panstruga, R.; Sanchez-Vallet, A.; et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 2009, 323, 101–106. [Google Scholar] [CrossRef]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 2009, 323, 95–101. [Google Scholar] [CrossRef]
- Mikkelsen, M.D.; Petersen, B.L.; Glawischnig, E.; Jensen, A.B.; Andreasson, E.; Halkier, B.A. Modulation of CYP79 genes and glucosinolate profiles in Arabidopsis by defense signaling pathways. Plant Physiol. 2003, 131, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Garcia, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. Plants as Biofactories: Postharvest Stress-Induced Accumulation of Phenolic Compounds and Glucosinolates in Broccoli Subjected to Wounding Stress and Exogenous Phytohormones. Front. Plant Sci. 2016, 7, 45. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, J.; Li, J.; Sun, P.; Zhang, Y.; Xin, X.; Lu, M.; Hu, J. Genome-wide transcriptomic analysis of a desert willow, Salix psammophila, reveals the function of hub genes SpMDP1 and SpWRKY33 in drought tolerance. BMC Plant Biol. 2019, 19, 356. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, M.K.; Sengar, R.S. Osmolytes: Proline metabolism in plants as sensors of abiotic stress. J. Appl. Nat. Sci. 2017, 9, 2079–2092. [Google Scholar] [CrossRef]
- Tsouvaltzis, P.; Brecht, J.K. Changes in Quality and Antioxidant Enzyme Activities of Bunched and Topped Radish (Raphanus sativus L.) Plants during Storage at 5 or 10C. J. Food Qual. 2014, 37, 157–167. [Google Scholar] [CrossRef]
- Robinson, J.; Browne, K.; Burton, W. Storage characteristics of some vegetables and soft fruits. Ann. Appl. Biol. 1975, 81, 399–408. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Seo, H.-Y.; Min, S.; Ku, K.-M. Phytonutrients and Metabolism Changes in Topped Radish Root and Its Detached Leaves during 1 °C Cold Postharvest Storage. Horticulturae 2022, 8, 42. https://doi.org/10.3390/horticulturae8010042
Liu M, Seo H-Y, Min S, Ku K-M. Phytonutrients and Metabolism Changes in Topped Radish Root and Its Detached Leaves during 1 °C Cold Postharvest Storage. Horticulturae. 2022; 8(1):42. https://doi.org/10.3390/horticulturae8010042
Chicago/Turabian StyleLiu, Mengpei, Hye-Young Seo, Sunggi Min, and Kang-Mo Ku. 2022. "Phytonutrients and Metabolism Changes in Topped Radish Root and Its Detached Leaves during 1 °C Cold Postharvest Storage" Horticulturae 8, no. 1: 42. https://doi.org/10.3390/horticulturae8010042
APA StyleLiu, M., Seo, H.-Y., Min, S., & Ku, K.-M. (2022). Phytonutrients and Metabolism Changes in Topped Radish Root and Its Detached Leaves during 1 °C Cold Postharvest Storage. Horticulturae, 8(1), 42. https://doi.org/10.3390/horticulturae8010042






