Untargeted GC-TOFMS Analysis Reveals Metabolomic Changes in Salvia miltiorrhiza Bunge Leaf and Root in Response to Long-Term Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Drought Treatment
2.2. Sample Preparation
2.3. GC-TOFMS Analysis
2.4. Data Preprocessing and Annotation
2.5. Multivariate and Univariate Statistical Analysis
3. Results
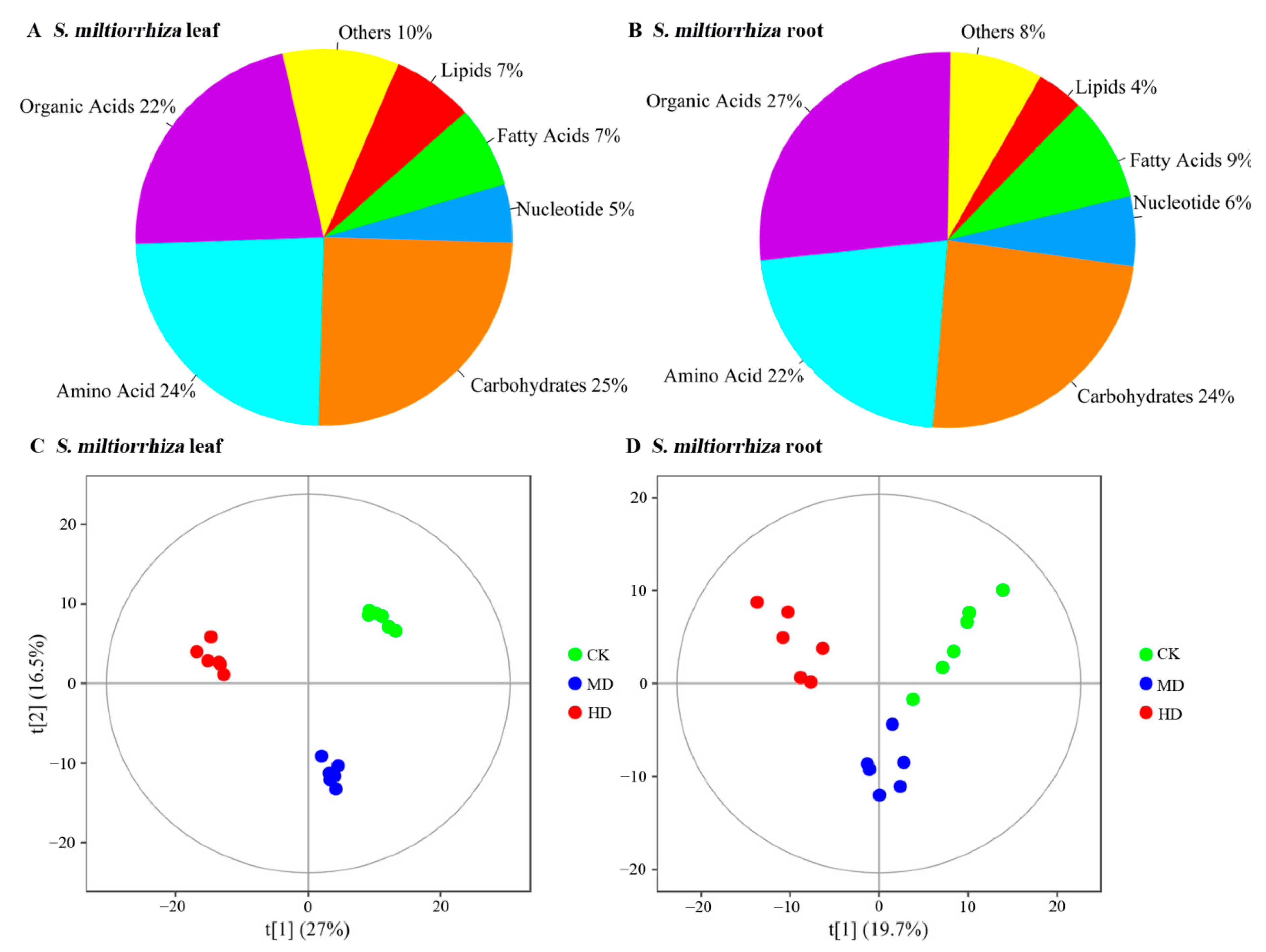
3.1. Metabolite Profiles of S. miltiorrhiza Leaf and Root Identified by GC-TOFMS Technology
3.2. Identification of Differential Metabolites in S. miltiorrhiza Leaf and Root
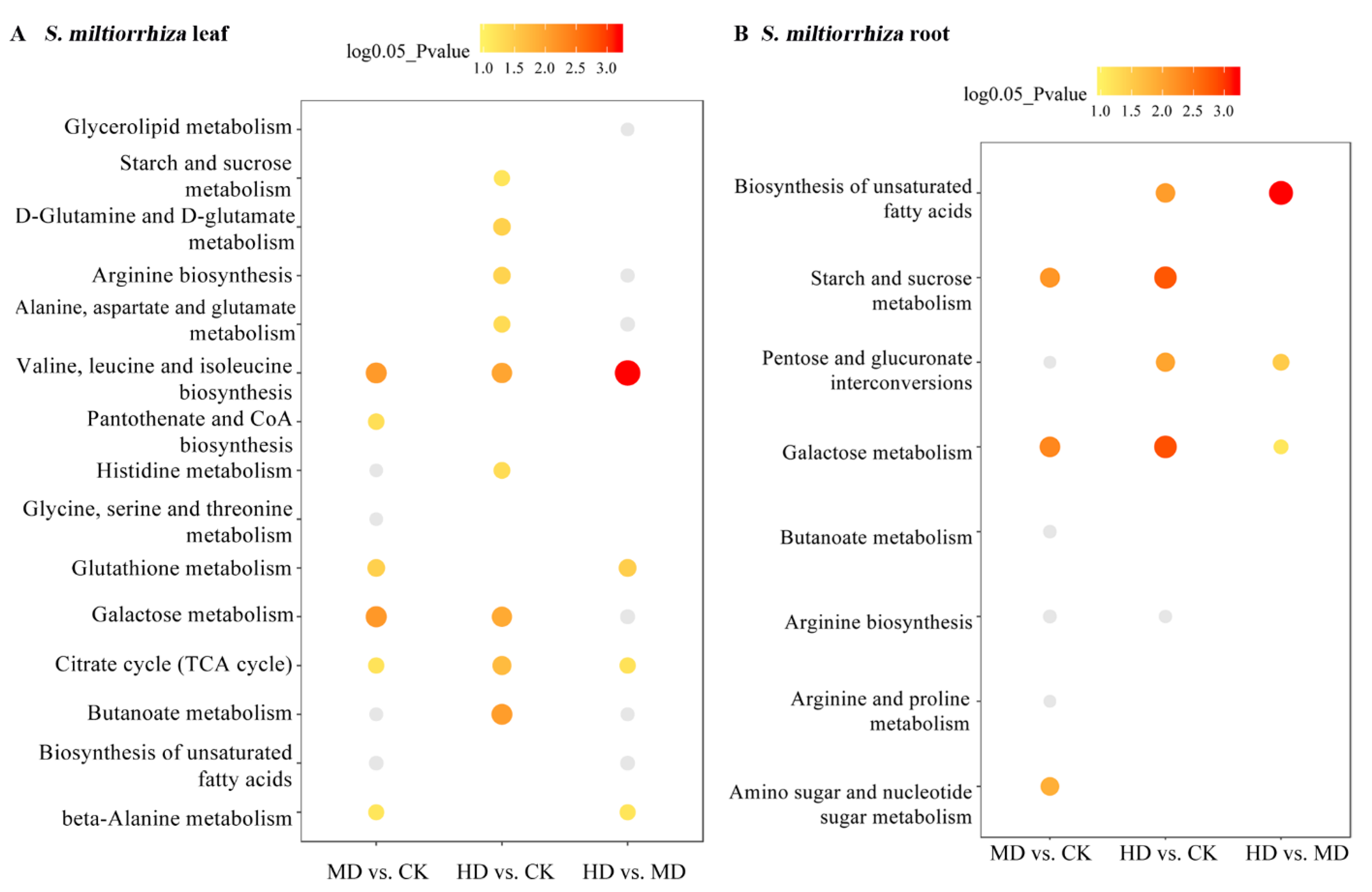
3.3. KEGG Pathway Annotation
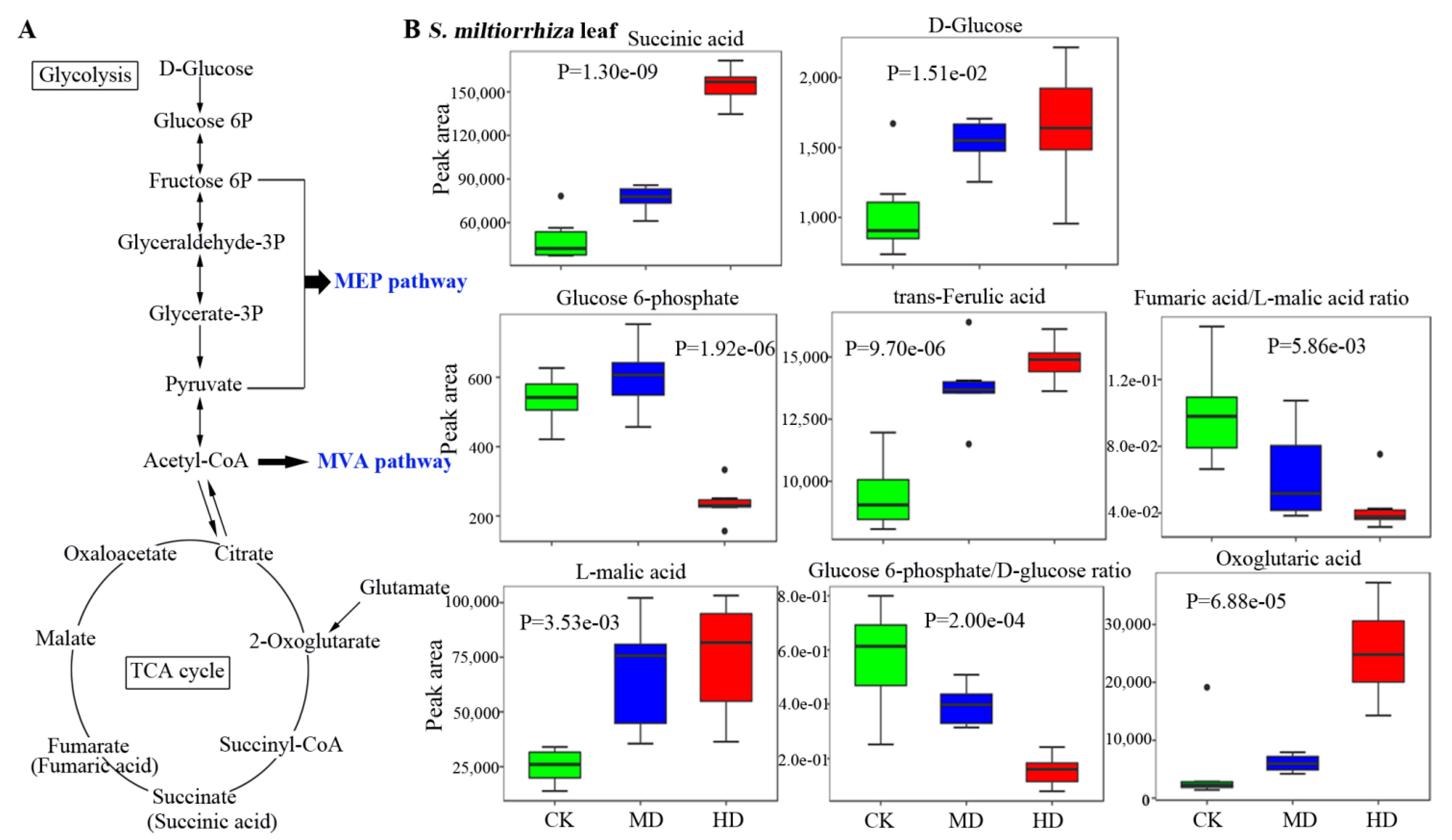
3.4. Metabolic Profiles of Metabolites Related to the Biosynthesis of Terpenoids in S. miltiorrhiza
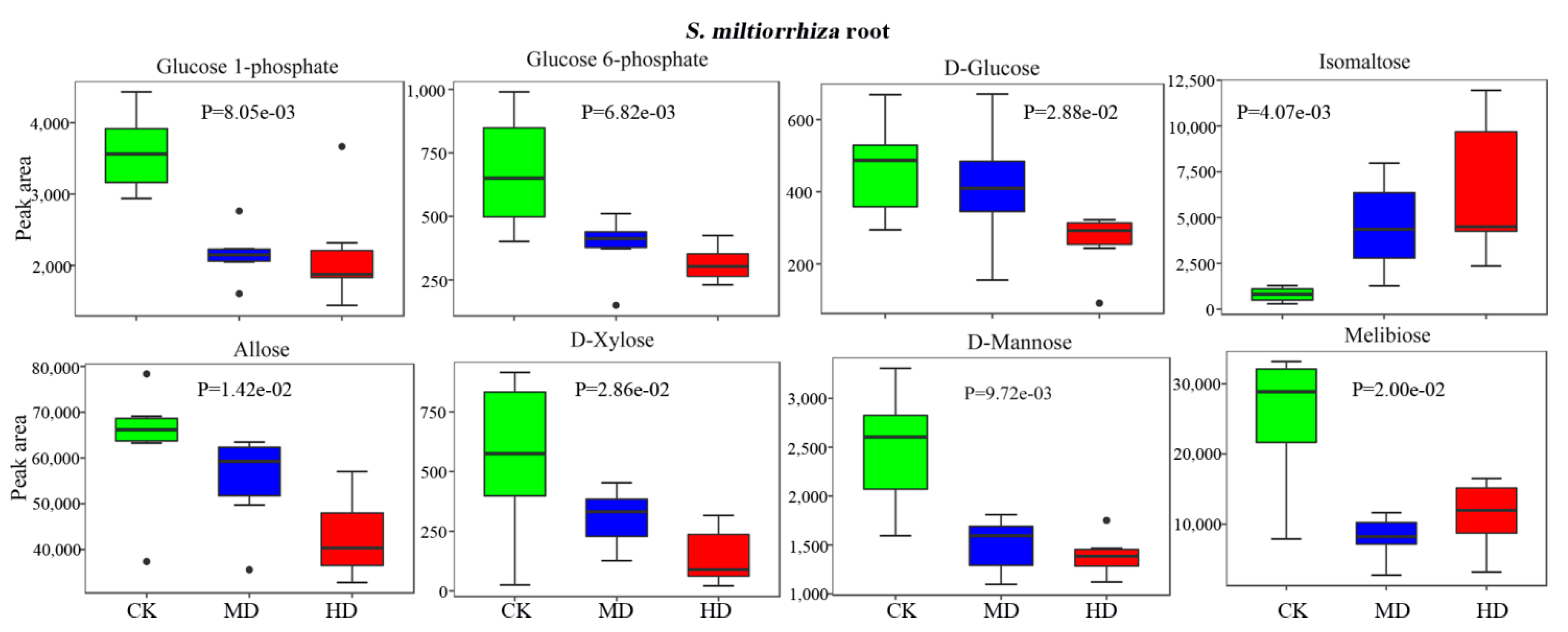
3.5. Drought Decreases the Bioactive Constituents in S. miltiorrhiza Root
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FC | fold change; |
| GC-TOFMS | gas chromatography time-of-flight mass spectrometry; |
| HD | high drought; |
| KEGG | Kyoto Encyclopedia of Genes and Genomes; |
| MD | moderate drought; |
| MEP | 2-methyl-d-erythritol 4-phosphate; |
| MVA | mevalonic acid; |
| NF-κB | nuclear factor-kappa B; |
| PCA | principal component analysis; |
| PLS-DA | partial least squares discrimination analysis; |
| TCA | citrate cycle; |
| TCM | traditional Chinese medicine; |
| VCAM-1 | vascular cell adhesion molecule-1; |
| VIP | variable importance in projection; |
References
- Lin, Y.-S.; Peng, W.-H.; Shih, M.-F.; Cherng, J.-Y. Anxiolytic effect of an extract of Salvia miltiorrhiza Bunge (Danshen) in mice. J. Ethnopharmacol. 2021, 264, 113285. [Google Scholar] [CrossRef]
- Kang, H.S.; Chung, H.Y.; Jung, J.H.; Kang, S.S.; Choi, J.S. Antioxidant effect of Salvia miltiorrhiza. Arch. Pharmacal Res. 1997, 20, 496–500. [Google Scholar] [CrossRef]
- Wang, X.; Gao, A.; Jiao, Y.; Zhao, Y.; Yang, X. Antitumor effect and molecular mechanism of antioxidant polysaccharides from Salvia miltiorrhiza Bunge in human colorectal carcinoma LoVo cells. Int. J. Biol. Macromol. 2018, 108, 625–634. [Google Scholar] [CrossRef]
- Shi, M.; Huang, F.; Deng, C.; Wang, Y.; Kai, G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 2019, 59, 953–964. [Google Scholar] [CrossRef]
- Li, Z.-M.; Xu, S.-W.; Liu, P.-Q. Salvia miltiorrhiza Burge (Danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wang, X.; Wang, D.; Zou, Z.; Liang, Z. Effect of drought stress on growth and accumulation of active constituents in Salvia miltiorrhiza Bunge. Ind. Crop. Prod. 2011, 33, 84–88. [Google Scholar] [CrossRef]
- Lisar, S.Y.; Bakhshayeshan-Agdam, H.; Li, X.; Liu, F.; Burgess, P.; Huang, B.; Merewitz, E.; Zivcak, M.; Brestic, M.; Sytar, O.; et al. Drought Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1. [Google Scholar] [CrossRef]
- Zampieri, M.; Ceglar, A.; Dentener, F.; Toreti, A. Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales. Environ. Res. Lett. 2017, 12, 064008. [Google Scholar] [CrossRef]
- Chen, X. Effects of Drought Stress on Growth, Yield and Quality of Different Barley Genotypes. Master’s Thesis, Zhejiang University, Hangzhou, Zhejiang, 2015. [Google Scholar]
- Zhu, Z.; Liang, Z.; Han, R. Saikosaponin accumulation and antioxidative protection in drought-stressed Bupleurum chinense DC. plants. Environ. Exp. Bot. 2009, 66, 326–333. [Google Scholar] [CrossRef]
- Jia, X.; Sun, C.; Li, G.; Li, G.; Chen, G. Effects of progressive drought stress on the physiology, antioxidative enzymes and secondary metabolites of Radix Astragali. Acta Physiol. Plant 2015, 37, 1–14. [Google Scholar] [CrossRef]
- Xue, J.; Zhou, S.; Wang, W.; Huo, L.; Zhang, L.; Fang, X.; Yang, Z. Water availability effects on plant growth, seed yield, seed quality in Cassia obtusifolia L., a medicinal plant. Agric. Water Manag. 2018, 195, 104–113. [Google Scholar] [CrossRef]
- Cao, M.; Liu, Y.; Jiang, W.; Meng, X.; Zhang, W.; Chen, W.; Peng, D.; Xing, S. UPLC/MS-based untargeted metabolomics reveals the changes of metabolites profile of Salvia miltiorrhiza bunge during Sweating processing. Sci. Rep. 2020, 10, 19524. [Google Scholar] [CrossRef]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind. Crop. Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The roles of environmental factors in regulation of oxidative stress in plant. BioMed Res. Int. 2019, 2019, 9732325. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Younis, A.; Ramzan, F.; Ramzan, Y.; Zulfiqar, F.; Ahsan, M.; Lim, K.B. Molecular Markers Improve Abiotic Stress Tolerance in Crops: A Review. Plants 2020, 9, 1374. [Google Scholar] [CrossRef]
- Chai, F.; Liu, W.; Xiang, Y.; Meng, X.; Sun, X.; Cheng, C.; Liu, G.; Duan, L.; Xin, H.; Li, S. Comparative metabolic profiling of Vitis amurensis and Vitis vinifera during cold acclimation. Hortic. Res. 2019, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Villar, M.; Ayllón, N.; Alberdi, P.; Moreno, A.; Moreno, M.; Tobes, R.; Mateos-Hernández, L.; Weisheit, S.; Bell-Sakyi, L.; de la Fuente, J. Integrated metabolomics, transcriptomics and proteomics identifies metabolic pathways affected by Anaplasma phagocytophilum infection in tick cells. Mol. Cell. Proteom. 2015, 14, 3154–3172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Chen, Y.; Guo, Q.; Zhu, G.; Wang, C.; Liu, Z. Effects of drought stress on the growth, physiology and secondary metabolite production in pinellia ternata thunb. Pak. J. Bot 2021, 53, 833–840. [Google Scholar] [CrossRef]
- Jang, S.-A.; Park, D.W.; Kwon, J.E.; Song, H.S.; Park, B.; Jeon, H.; Sohn, E.-H.; Koo, H.J.; Kang, S.C. Quinic acid inhibits vascular inflammation in TNF-α-stimulated vascular smooth muscle cells. Biomed. Pharmacother. 2017, 96, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Muthamil, S.; Balasubramaniam, B.; Balamurugan, K.; Pandian, S.K. Synergistic effect of quinic acid derived from Syzygium cumini and undecanoic acid against Candida spp. biofilm and virulence. Front. Microbiol. 2018, 9, 2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heikkilä, E.; Hermant, A.; Thevenet, J.; Bermont, F.; Kulkarni, S.S.; Ratajczak, J.; Santo-Domingo, J.; Dioum, E.H.; Canto, C.; Barron, D. The plant product quinic acid activates Ca2+-dependent mitochondrial function and promotes insulin secretion from pancreatic beta cells. Br. J. Pharmacol. 2019, 176, 3250–3263. [Google Scholar]
- Chu, S.; Liu, W.; Lu, Y.; Yan, M.; Guo, Y.; Chang, N.; Jiang, M.; Bai, G. Sinigrin Enhanced Antiasthmatic Effects of Beta Adrenergic Receptors Agonists by Regulating cAMP-Mediated Pathways. Front. Pharmacol. 2020, 11, 723. [Google Scholar] [CrossRef]
- Lee, H.-W.; Lee, C.G.; Rhee, D.-K.; Um, S.H.; Pyo, S. Sinigrin inhibits production of inflammatory mediators by suppressing NF-κB/MAPK pathways or NLRP3 inflammasome activation in macrophages. Int. Immunopharmacol. 2017, 45, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Park, B.; Lee, H.-W.; Park, H.J.; Koo, H.J.; Kim, B.O.; Sohn, E.-H.; Um, S.H.; Pyo, S. Sinigrin attenuates the progression of atherosclerosis in ApoE−/− mice fed a high-cholesterol diet potentially by inhibiting VCAM-1 expression. Chem. Biol. Interact. 2017, 272, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Ommati, M.M.; Heidari, R.; Ghanbarinejad, V.; Abdoli, N.; Niknahad, H. Taurine treatment provides neuroprotection in a mouse model of manganism. Biol. Trace Elem. Res. 2019, 190, 384–395. [Google Scholar] [CrossRef]
- Guo, J.; Ma, Y. Biosynthetic Pathway of Tanshinones in Salvia miltiorrhiza. In The Salvia miltiorrhiza Genome; Springer: Berlin/Heidelberg, Germany, 2019; pp. 129–139. [Google Scholar]
- Fu, L.; Han, B.; Zhou, Y.; Ren, J.; Cao, W.; Patel, G.; Kai, G.; Zhang, J. The anticancer properties of tanshinones and the pharmacological effects of their active ingredients. Front. Pharmacol. 2020, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhu, W.; Kong, X.; Chen, X.; Sun, X.; Zhang, W.; Zhang, R. Tanshinone IIA inhibits glucose metabolism leading to apoptosis in cervical cancer. Oncol. Rep. 2019, 42, 1893–1903. [Google Scholar] [CrossRef]
- Qin, T.; Rasul, A.; Sarfraz, A.; Sarfraz, I.; Hussain, G.; Anwar, H.; Riaz, A.; Liu, S.; Wei, W.; Li, J. Salvianolic acid A & B: Potential cytotoxic polyphenols in battle against cancer via targeting multiple signaling pathways. Int. J. Biol. Sci. 2019, 15, 2256. [Google Scholar]
- Haberstroh, S.; Kreuzwieser, J.; Lobo-do-Vale, R.; Caldeira, M.C.; Dubbert, M.; Werner, C. Terpenoid emissions of two Mediterranean woody species in response to drought stress. Front. Plant Sci. 2018, 9, 1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiber, A.; Duan, Q.; Jansen, K.; Verena Junker, L.; Kammerer, B.; Rennenberg, H.; Ensminger, I.; Gessler, A.; Kreuzwieser, J. Drought effects on root and needle terpenoid content of a coastal and an interior Douglas fir provenance. Tree Physiol. 2017, 37, 1648–1658. [Google Scholar] [CrossRef]


| Class | Name | HMDBID | KeggID | HD versus CK | MD versus CK | ||
|---|---|---|---|---|---|---|---|
| p Value | FC | p Value | FC | ||||
| S. miltiorrhiza leaf | |||||||
| Amino acids | Ratio of l-Glutamic acid to Oxoglutaric acid | HMDB00148 HMDB00208 | C00025 C00026 | 5.50e-03 | 0.06 | 2.40e-02 | 0.35 |
| l-Lysine | HMDB00182 | C00047 | 3.50e-03 | 2.58 | 4.50e-04 | 1.85 | |
| l-Leucine | HMDB00687 | C00123 | 5.00e-06 | 0.11 | 2.00e-04 | 0.40 | |
| l-Histidine | HMDB00177 | C00135 | 6.10e-03 | 4.47 | 6.50e-06 | 2.98 | |
| l-Alloisoleucine | HMDB00557 | NA | 1.60e-02 | 21.98 | 2.50e-05 | 41.37 | |
| Carbohydrates | Sucrose | HMDB00258 | C00089 | 2.70e-02 | 2.48 | 1.20e-04 | 2.46 |
| Sorbitol | HMDB00247 | C00794 | 4.90e-02 | 0.11 | 4.20e-02 | 0.07 | |
| Mannitol | HMDB00765 | C00392 | 3.30e-02 | 1.94 | 1.60e-02 | 1.53 | |
| Lactulose | HMDB00740 | C07064 | 2.10e-02 | 3.90 | 2.30e-05 | 3.05 | |
| d-Xylose | HMDB00098 | C00181 | 5.80e-03 | 1.95 | 8.00e-03 | 1.78 | |
| d-Glucose | HMDB00122 | C00031 | 2.40e-02 | 1.81 | 1.40e-02 | 1.71 | |
| beta-Lactose | HMDB41627 | C01970 | 5.30e-03 | 2.13 | 1.30e-04 | 6.38 | |
| Fatty acids | Docosahexaenoic acid | HMDB02183 | C06429 | 2.70e-03 | 0.16 | 2.80e-02 | 0.49 |
| Indoles | Indoleacetic acid | HMDB00197 | C00954 | 4.90e-02 | 0.39 | 3.60e-02 | 2.48 |
| Lipids | MG182 | HMDB11568 | NA | 1.70e-02 | 3.01 | 3.20e-03 | 1.94 |
| Nucleotide | Ratio of Uridine to Cytidine | HMDB00296 HMDB00089 | C00299 C00475 | 1.30e-05 | 0.50 | 3.00e-06 | 0.24 |
| Ratio of Uracil to Uridine | HMDB00300 HMDB00296 | C00106 C00299 | 2.70e-02 | 1.76 | 1.20e-02 | 2.52 | |
| Organic acids | trans-Ferulic acid | HMDB00954 | C01494 | 5.50e-05 | 1.65 | 5.80e-04 | 1.51 |
| Succinic acid | HMDB00254 | C00042 | 3.20e-07 | 3.71 | 6.60e-03 | 1.84 | |
| Maleic acid | HMDB00176 | C01384 | 5.00e-02 | 3.82 | 6.80e-05 | 1.78 | |
| l-Malic acid | HMDB00156 | C00149 | 5.60e-03 | 3.14 | 1.00e-02 | 2.90 | |
| Fumaric acid | HMDB00134 | C00122 | 3.70e-02 | 1.37 | 2.30e-05 | 1.59 | |
| 4-Hydroxycinnamic acid | HMDB02035 | C00811 | 2.90e-02 | 2.02 | 4.50e-06 | 1.69 | |
| 3-Aminosalicylic acid | HMDB01972 | NA | 3.50e-04 | 0.08 | 2.80e-04 | 0.04 | |
| Vitamin | Pantothenic acid | HMDB00210 | C00864 | 3.10e-02 | 2.46 | 3.90e-04 | 1.77 |
| S. miltiorrhiza root | |||||||
| Amino acid | Urea | HMDB00294 | C00086 | 1.50e-04 | 0.16 | 1.60e-04 | 0.20 |
| l-Alloisoleucine | HMDB00557 | NA | 1.00e-03 | 20.07 | 1.60e-03 | 59.69 | |
| Carbohydrates | Sorbitol | HMDB00247 | C00794 | 1.30e-03 | 0.23 | 2.90e-02 | 0.50 |
| Ribonolactone | HMDB01900 | C02674 | 6.00e-04 | 0.59 | 1.60e-04 | 0.65 | |
| Ratio of l-Arabinose to l-Arabitol | HMDB00646 HMDB01851 | C00259 C00532 | 1.70e-02 | 0.11 | 4.70e-02 | 0.30 | |
| Melibiose | HMDB00048 | C05402 | 1.60e-02 | 0.42 | 6.50e-03 | 0.29 | |
| Isomaltose | HMDB02923 | C00252 | 1.90e-02 | 5.43 | 1.50e-02 | 5.26 | |
| Glucose 6-phosphate | HMDB01401 | C00092 | 1.10e-02 | 0.47 | 2.80e-02 | 0.63 | |
| Glucose 1-phosphate | HMDB01586 | C00103 | 5.70e-03 | 0.53 | 7.20e-04 | 0.60 | |
| d-Mannose | HMDB00169 | C00159 | 6.70e-03 | 0.53 | 1.00e-02 | 0.61 | |
| Fatty acids | Myristoleic acid | HMDB02000 | C08322 | 4.10e-02 | 0.56 | 2.30e-02 | 0.54 |
| Lipids | MG182 | HMDB11568 | NA | 5.80e-03 | 0.55 | 1.00e-03 | 0.30 |
| Nucleotide | Uridine | HMDB00296 | C00299 | 2.80e-02 | 0.52 | 4.50e-02 | 0.55 |
| Organic acids | Taurine | HMDB00251 | C00245 | 2.60e-02 | 0.42 | 4.40e-03 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Su, Y.; Li, J.; Ren, Z.; Tian, G.; Wang, J. Untargeted GC-TOFMS Analysis Reveals Metabolomic Changes in Salvia miltiorrhiza Bunge Leaf and Root in Response to Long-Term Drought Stress. Horticulturae 2021, 7, 175. https://doi.org/10.3390/horticulturae7070175
Zhang J, Su Y, Li J, Ren Z, Tian G, Wang J. Untargeted GC-TOFMS Analysis Reveals Metabolomic Changes in Salvia miltiorrhiza Bunge Leaf and Root in Response to Long-Term Drought Stress. Horticulturae. 2021; 7(7):175. https://doi.org/10.3390/horticulturae7070175
Chicago/Turabian StyleZhang, Jin, Yuekai Su, Jingyu Li, Zhenli Ren, Guoqing Tian, and Jianhua Wang. 2021. "Untargeted GC-TOFMS Analysis Reveals Metabolomic Changes in Salvia miltiorrhiza Bunge Leaf and Root in Response to Long-Term Drought Stress" Horticulturae 7, no. 7: 175. https://doi.org/10.3390/horticulturae7070175
APA StyleZhang, J., Su, Y., Li, J., Ren, Z., Tian, G., & Wang, J. (2021). Untargeted GC-TOFMS Analysis Reveals Metabolomic Changes in Salvia miltiorrhiza Bunge Leaf and Root in Response to Long-Term Drought Stress. Horticulturae, 7(7), 175. https://doi.org/10.3390/horticulturae7070175






