Abstract
The efficacy of 1-methylcyclopropene (1-MCP) in maintenance of apple fruit quality can differ depending on apple cultivar, ethylene content at harvest, 1-MCP concentration, the interval between harvest and 1-MCP application, and the number of applications. In this study, we investigated whether the 1-MCP concentration and its application frequency differently affected fruit quality attributes of the two new apple cultivars ‘Arisoo’ and ‘Picnic’. The fruits were treated with 1-MCP (as single or double treatments) at the rate of 0 μL L−1 (control), 0.5 μL L−1, 1 μL L−1, 0.5 + 0.5 μL L−1, and 1 + 1 μL L−1 for 18 h, and they were stored at 0 °C up to six months. Comparatively, the fruit qualities of 1-MCP treated fruits were higher than that of the control during cold storage, with a higher suppression of internal ethylene content and a slower reduction of titratable acidity in 1-MCP treatments than the controls throughout the cold storage in both cultivars, regardless of the concentration and number of applications. Reduction of fruit firmness and soluble solid contents were maintained by single application of 0.5 μL L−1 1-MCP in ‘Arisoo’ apple, while double application of 0.5 + 0.5 μL L−1 was needed in ‘Picnic’ cultivar. The effective concentration for weight-loss reduction was found to be the single application of 1 μL L−1 1-MCP in both ‘Arisoo’ and ‘Picnic’. Conclusively, 1-MCP as single treatment at the rate of 0.5 μL L−1 could be sufficient in inhibiting ethylene action and maintaining fruit quality losses during cold storage, particularly in ‘Arisoo’. However, double application of 1-MCP might be necessary for some quality attributes in ‘Picnic’ apple.
1. Introduction
Apple (Malus domestica Borkh.) is one of the most representative fruits in Korea, which accounted for 20.5% of Korea’s fruit production in 2019, with a cultivation area of 32,954 ha and a production capacity of 508,000 tons in 2019–2020 [1,2]. As for 2019, ‘Fuji’ is the most cultivated apple in Korea, accounting for 67.2% of total apple cultivation, more than two-thirds of other cultivars. Following the ‘Fuji’ apple, ‘Hongro’ and ‘Tsugaru’ are popular apple cultivars in Korea. However, recently, the demand for cultivation or consumption of new apple cultivars is increasing from various consumers. Comparing the status of Korean apple cultivars in 2012 and 2019, ‘Fuji’ apple’s cultivation decreased from 70.5% to 67.9%, and other cultivars increased from 6.3% to 7.3% [3]. Therefore, many studies on new breeding varieties and quality maintenance are necessary to meet the demand in the apple market.
In Korea, ‘Arisoo’ (‘Yoko’ × ‘Senshu’) and ‘Picnic’ (‘Fuji’ × ‘Sansa’) apples are new cultivars that have high-quality attributes and were bred in 2013 and 2011, respectively [4]. ‘Arisoo’ is a large apple cultivar with about 285 g fruit weight, and the harvest time is from early to mid-September. The advantage of this apple is that it is well colored, even at high temperatures, during the maturity stage. Around the commercial harvesting time, the ‘Arisoo’ apple has about 14.0% soluble solid content (SSC) and 0.33% titratable acidity (TA). ‘Picnic’ is a small-sized apple cultivar that has about 223 g fruit weight, and the estimated harvest time is from early to mid-September. SSC and TA of the ‘Picnic’ apple are 14.2% and 0.43%, respectively, at the commercial harvest time. Additionally, both apple cultivars have excellent palatability because of their SSC/TA ratio [4].
The apple is a climacteric fruit in which ethylene production rapidly increases during the ripening stage [5]. Ethylene is a major factor that regulates various physiological and biological reactions during fruit ripening, such as increased respiration, formation of volatile compounds, fruit softening, and its related cell wall enzyme activities causing rapid ripening and the reduction of postharvest qualities [6,7,8,9,10,11]. Furthermore, higher ethylene production reduces firmness, fruit weight, and organic acid content during storage or distribution, which causes a sharp drop in marketability [12]. Therefore, ethylene plays a crucial role in apple fruit shelf life, and control of ethylene production is essential for quality maintenance.
To control ethylene, 1-methylcyclopropene (1-MCP) is used as an ethylene inhibitor [13,14,15]. Previous reports indicated that 1-MCP had excellent effects not only in suppressing ethylene action during storage but also in maintaining the quality of fruits such as fruit softening, fruit firmness, and TA [13,16,17,18,19,20,21,22]. However, the apple has an extensive range of cultivars with different ripening rates, harvest standards, postharvest procedures, and storage periods [23]. Therefore, it is typical that factors, including cultivars, metabolism and cell wall degradation, maturity, storage type and temperature, the time between harvest and application of 1-MCP, and repeated 1-MCP treatment, can influence the effectiveness of 1-MCP [24,25]. It has been reported that the effect of 1-MCP may be insignificant or shorter in some cultivars compared to others [24,26].
A single application of 1-MCP at a rate of 1 μL L−1 has been traditionally accepted as an adequate dosage [27,28]. Dose-response studies highlighted that a concentration from 0.5 to 1 μL L−1 1-MCP is necessary for a maximal response in apples [29]. However, other studies showed that high concentrations of 1-MCP, up to 20 μL L−1, can improve its efficacy on ethylene synthesis and extend the storage period of fruits [28]. Recently, double application of 1-MCP in ‘McIntosh’, ‘Empire’, and ‘Northern Spy’ apples during controlled atmosphere storage exhibited better results than single treatment in reducing internal ethylene content and maintaining firmness and TA [25]. These suggest the way to explore new 1-MCP commercial dosage, including concentration and number of applications. Additionally, since the effects of 1-MCP are variable and often cultivar-specific, it is essential to determine the suitable concentration and number of applications according to the apple cultivar to be treated.
In Korea, recently, the cultivation of two new apple cultivars, namely ‘Arisoo’ and ‘Picnic’, has been started. There are few scientific studies regarding the application of 1-MCP on ethylene perception and other quality attributes in them. Therefore, the main objective of this study was to investigate the effects of 1-MCP treatments, at different concentrations and frequency of applications, on fruit quality attributes in midseason harvested ‘Arisoo’ and ‘Picnic’ apples during long-term cold storage.
2. Materials and Methods
2.1. Chemicals and Equipment
All the chemicals used in this experiment were analytical grades and as follows: 1-MCP powder (3.3% (w/w), SmartFreshTM, AgroFresh, Yakima, WA, USA), 0.1 N sodium hydroxide (NaOH) (Daejung Chemical Co., Shiheung, Korea), malic acid (Daejung Chemical Co., Shiheung, Korea), potassium iodide (Junsei Chemical Co., Tokyo, Japan), and iodine (Daejung Chemical Co., Shiheung, Korea). The following equipment was used: digital balance (AND Co., Daejeon, Korea), Rheometer (Compac-100II, Sun Scientific Co., Tokyo, Japan), gas chromatography (GC-2010, Shimadzu Co., Tokyo, Japan), Porapak Q column (80/100 2-m, RASTEK, Bellefonte, PA, USA), digital refractometer (PR-201, Atago Co., Tokyo, Japan), titrator (DL-15, Mettler-Toledo Ltd., Zurich, Switzerland), and chroma-meter (CR-200, Minolta Co., Tokyo, Japan).
2.2. Plant Materials and Treatments
‘Arisoo’ and ‘Picnic’ apples were harvested on 5th and 13th September 2018, respectively, from the apple orchard (36° 41′ 42.3404″ N, 128° 7′ 53.6719″ E) at the apple research institute in Mungyeong, Republic of Korea. After harvest, a total of 810 uniform-sized fruits (405 fruits per cultivar), which were free from visible infection and injuries, were carefully selected. First of all, the fruit quality attributes at harvest were measured using 15 fruits per cultivar. Then, the selected fruits of each cultivar were divided into five treatment groups. The first group (90 fruits per cultivar) was used as the untreated control. The second and third groups (90 fruits per group for each cultivar) were treated with 1-MCP once at the rate of 0.5 μL L−1 and 1 μL L−1, respectively. The fourth and fifth groups (60 fruits per group for each cultivar) were treated with 1-MCP twice at the rate of 0.5 + 0.5 μL L−1 and 1 + 1 μL L−1, respectively.
For all 1-MCP treatments, the fruits were exposed to the desired rate of 1-MCP for 18 h in an enclosed container. For single treatments, the fruits were treated immediately after harvest and then stored. For double treatments, the fruits were treated immediately after harvest and then treated again at two months after storage. All the fruits were stored under the same condition for six months at 0 °C ± 1 °C with 90–95% relative humidity (RH).
The commercial product of 1-MCP (3.3% w/w) in powder form (SmartFreshTM, AgroFresh, Yakima, WA, USA) was used for all 1-MCP treatments. 1-MCP powders (0.1242 g and 0.2484 g) were used to prepare the concentrations of 0.5 and 1 μL L−1, respectively. To generate the 1-MCP gas, distilled water (10 mL) was added to the 1-MCP powder in a beaker according to the manufacturer’s instructions. The gas released from the 1-MCP solution was considered equivalent to the desired final concentration.
2.3. Measurements of Fruit Quality Attributes
Fruit quality attributes were determined at one-month intervals after being treated with 1-MCP until the end of the storage. For each time of determination, 15 fruits per treatment per cultivar were measured for quality attributes. Before carrying out the measurements, the fruits were removed from cold storage and stabilized at room temperature for one day.
2.3.1. Fruit Weight Loss and Flesh Firmness
Percent weight loss was determined as the decrease in fresh weight before and after storage. Flesh firmness was measured using a Rheometer (Compac-100II, Sun Scientific Co., Tokyo, Japan) equipped with an 11-mm plunger. Each fruit was measured thrice in the equatorial regions. The data were averaged and expressed in newtons (N).
2.3.2. Internal Ethylene Content
For IEC, a 1 mL gas sample was withdrawn from the core cavity of each fruit using a syringe with a hypodermic 3.8 cm needle. Then, gas samples were measured using gas chromatography (GC-2010, Shimadzu Co., Tokyo, Japan), equipped with a flame ionization detector, and fitted with a Porapak Q (80/100 2-m, RASTEK, Bellefonte, PA, USA) column. For each treatment, IEC was measured in triplicate and then averaged.
2.3.3. Soluble Solids Content and Titratable Acidity
Soluble solid content (SSC) of fresh juice samples was measured using a digital refractometer (PR-201α, Atago Co., Tokyo, Japan). To determine TA, a 5 mL fruit juice sample was mixed with 45 mL distilled water, then titrated using 0.1 N NaOH to a pH of 8.1 with a titrator (DL-15, Mettler-Toledo Ltd., Zurich, Switzerland) and expressed as % malic acid equivalents. All the measurements were replicated three times.
2.3.4. Starch Index
The starch index was determined by observing its reaction with the Cornell starch index [30] after analyzing the color of the flesh visually, compared with standards, using a 1–8 scale. Measurements were replicated for three times, and data were averaged.
2.3.5. Color Properties
Peel color variables of fruits were measured three times on the equatorial region per fruit. The lightness (L*), red/green value (a*), and blue/yellow value (b*) on the sunlit sides of fruits were determined using a chroma-meter (CR-200, Minolta Co., Tokyo, Japan).
2.4. Statistical Analysis
The statistical analyses were conducted using the SPSS program (IBM SPSS Statistics 25, SPSS Inc., New York, NY, USA). The data are expressed as mean ± standard error (SE). Where error bars were not indicated in the figures, the SE does not exceed the sample size. Mean comparisons were conducted using the least significant difference test at a 5% significance level.
3. Results
3.1. Fruit Weight Loss and Flesh Firmness
The fruit weights and flesh firmness of two apple cultivars, ‘Arisoo’ and ‘Picnic’, at harvest, are indicated in Table 1. The fruit weight of ‘Arisoo’ at harvest was higher than that of ‘Picnic’, and the opposite was observed in the flesh firmness.

Table 1.
Fruit quality attributes in ‘Arisoo’ and ‘Picnic’ at harvest.
Fruit weight loss increased with the increase in storage time in all treatments in both apple cultivars (Figure 1A,B). In ‘Arisoo’, at the end of storage, the highest weight loss was observed in the control and double treatment of 1-MCP at the rate of 1 + 1 μL L−1. The lowest weight loss was observed in a single treatment of 1-MCP at the rate of 1 μL L−1 (Figure 1A). In ‘Picnic’, at the end of storage, the lowest weight loss was observed in a single treatment at the rate of 1 μL L−1 and double treatment at the rate of 1 + 1 μL L−1 of 1-MCP (Figure 1B).
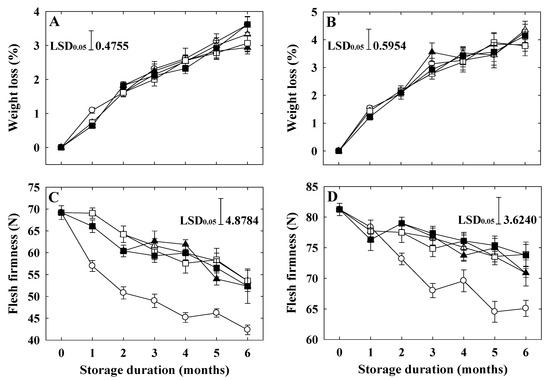
Figure 1.
Fruit weight loss and flesh firmness of ‘Arisoo’ (A,C) and ‘Picnic’ (B,D) apples during cold storage at 0 ± 1 °C with 90–95% relative humidity for up to six months. Fruits were treated with 0.5 μL L−1 1-MCP (∆) and 1 μL L−1 1-MCP (▲) at harvest, and 0.5 + 0.5 μL L−1 1-MCP (□) and 1 + 1 μL L−1 1-MCP (■) at harvest and 2 months after storage, and untreated as control (○). Data are presented as mean ± standard error (n = 15).
Flesh firmness indicated a decreasing trend from all treatments in both cultivars throughout cold storage (Figure 1C,D). However, the firmness of 1-MCP-treated fruits remained significantly higher than that of the control until the end of storage. In ‘Arisoo’ apples, fruits treated one time with 1-MCP at the rate of 0.5 μL L−1 indicated a higher flesh firmness value than other treatments after six months of storage (Figure 1C). However, in ‘Picnic’ apples, the firmness of fruits treated twice with 1-MCP at the rates of 0.5 + 0.5 and 1 + 1 μL L−1, respectively, were significantly higher than those treated with only one time 1-MCP treatment (Figure 1D).
3.2. Internal Ethylene Content
The IEC of untreated fruits in both cultivars increased rapidly during storage (Figure 2A,B). The IEC of ‘Arisoo’ was significantly higher than that of ‘Picnic’ at harvest (Table 1), throughout, and still at the end of the storage (Figure 2A,B). All 1-MCP treatments resulted in lower IEC in both cultivars, regardless of the concentration and number of applications. Treating with 1-MCP significantly limited the IEC during long-time cold storage (Figure 2A,B).
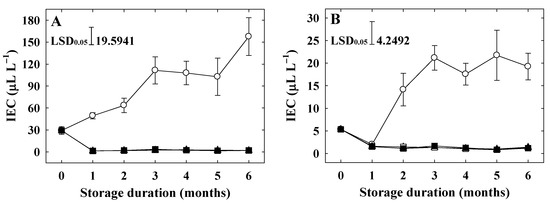
Figure 2.
Internal ethylene content (IEC) of ‘Arisoo’ (A) and ‘Picnic’ (B) apples during cold storage at 0 ± 1 °C with 90–95% relative humidity for up to six months. Fruits were treated with 0.5 μL L−1 1-MCP (∆) and 1 μL L−1 1-MCP (▲) at harvest, and 0.5 + 0.5 μL L−1 1-MCP (□) and 1 + 1 μL L−1 1-MCP (■) at harvest and 2 months after storage, and untreated as control (○). Data are presented as mean ± standard error (n = 15).
3.3. Soluble Solids Content and Titratable Acidity
The SSC of ‘Arisoo’ was lower than that of ‘Picnic’ at harvest (Table 1). The SSC of both cultivars changed with the storage duration. In ‘Arisoo’, the application of 1-MCP at a rate of 0.5 μL L−1 for both single and double applications consistently maintained the high level of SSC throughout the whole storage period. At the end of storage, a single application of 1-MCP at a rate of 0.5 μL L−1 showed the highest SSC compared with control and other treatments (Figure 3A). However, in ‘Picnic’, the highest SSC at six months after cold storage was observed in fruits applied twice with 1-MCP at the rates of 0.5 + 0.5 μL L−1 and 1 + 1 μL L−1, respectively (Figure 3B).
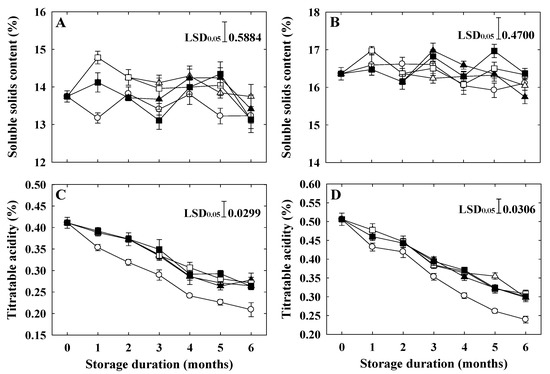
Figure 3.
Soluble solids content and titratable acidity of ‘Arisoo’ (A,C) and ‘Picnic’ (B,D) apples during cold storage at 0 ± 1 °C with 90–95% relative humidity for up to six months. Fruits were treated with 0.5 μL L−1 1-MCP (∆) and 1 μL L−1 1-MCP (▲) at harvest, and 0.5 + 0.5 μL L−1 1-MCP (□) and 1 + 1 μL L−1 1-MCP (■) at harvest and 2 months after storage, and untreated as control (○). Data are presented as mean ± standard error (n = 15).
The TA of ‘Picnic’ was higher than that of ‘Arisoo’ at harvest (Table 1). The TA of both cultivars showed decreasing trends during storage. However, compared to untreated (control) ones, fruits applied with 1-MCP maintained significantly higher TA, regardless of the concentration and number of applications (Figure 3C,D).
3.4. Starch Index
The starch index of ‘Arisoo’ was higher than that of ‘Picnic’ at harvest (Table 1). Compared to the control, the application of 1-MCP had an insignificant effect on the starch index of both varieties, regardless of the concentration and number of applications (Figure 4A,B). In ‘Arisoo’, the starch index increased slightly for four months and then became stable after that in all the treatments, including control (Figure 4A). However, in ‘Picnic’, the significant and rapid increase in the starch index was observed in all treated and untreated fruits (Figure 4B).
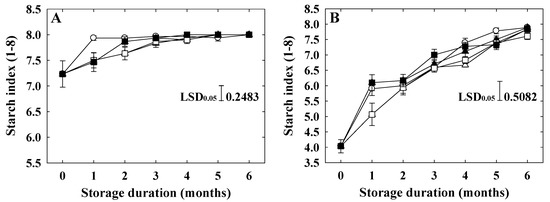
Figure 4.
Starch index of ‘Arisoo’ (A) and ‘Picnic’ (B) apples during cold storage at 0 ± 1 °C with 90–95% relative humidity for up to six months. Fruits were treated with 0.5 μL L−1 1-MCP (∆) and 1 μL L−1 1-MCP (▲) at harvest, and 0.5 + 0.5 μL L−1 1-MCP (□) and 1 + 1 μL L−1 1-MCP (■) at harvest and 2 months after storage, and untreated as control (○). Data are presented as mean ± standard error (n = 15).
3.5. Color Properties
In the ‘Arisoo’ apple, all the peel color variables, including lightness (L*), redness (a*), and yellowness (b*), slightly increased at the end of the storage period (Figure 5A,C,E). However, in ‘Picnic’, all the color properties decreased at the end (Figure 5B,D,F). Although the storage period affected the color properties, no significant difference was observed among the treatments in both varieties until the end of the storage.
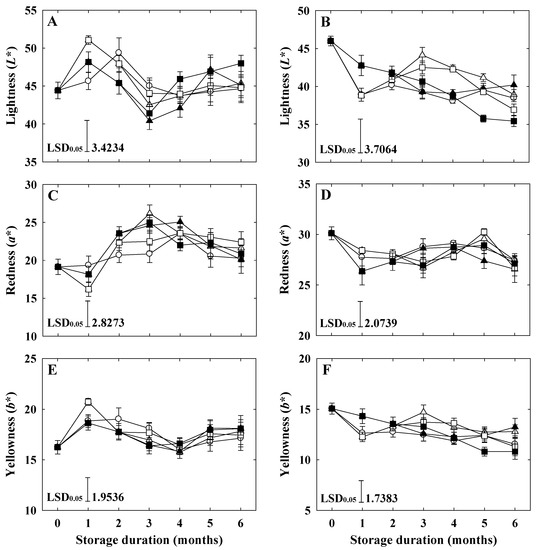
Figure 5.
Fruit peel color properties (L*, a*, and b*) of ‘Arisoo’ (A,C,E) and ‘Picnic’ (B,D,F) apples during cold storage at 0 ± 1 °C with 90–95% relative humidity for up to six months. Fruits were treated with 0.5 μL L−1 1-MCP (∆) and 1 μL L−1 1-MCP (▲) at harvest, and 0.5 + 0.5 μL L−1 1-MCP (□) and 1 + 1 μL L−1 1-MCP (■) at harvest and 2 months after storage, and untreated as control (○). Data are presented as mean ± standard error (n = 15). L*: lightness; a*: red/green value; b*: blue/yellow value.
3.6. Pearson’s Correlation Coefficient Analysis
Pearson’s correlation coefficient analysis was conducted to clarify the relationship between the quality attributes of each 1-MCP treatment for two apple cultivars at the end of the cold storage (Figure 6 and Figure 7). The fruit quality attributes were found to be correlated with each other. In ‘Arisoo’ apples applied with single treatments of 0.5 µL L−1 and 1 µL L−1 1-MCP (Figure 6), the positive correlations between IEC and weight loss were highly significant (p < 0.01 and p < 0.001, respectively). IEC was negatively correlated with TA, but not significant. In both treatments, fruit firmness and SSC were positively and significantly correlated (p < 0.05) (Figure 6). The positive correlation between TA and SSC was significant (p < 0.05) in double treatment of 1+1 µL L−1 1-MCP (Figure 6). In ‘Picnic’ apples treated with single applications of 0.5 µL L−1 1-MCP, fruit firmness was positively correlated with SSC (p < 0.01) (Figure 7). When treated with single application of 1 µL L−1 1-MCP, a negative correlation (p < 0.05) between weight loss and TA was observed (Figure 7). The IEC was found to be negatively correlated (p < 0.05) with TA, which was in turn positively correlated (p < 0.05) with fruit firmness (Figure 7).
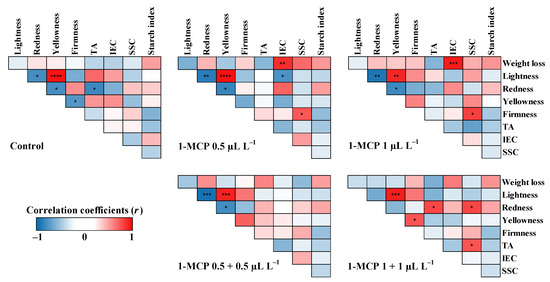
Figure 6.
Pearson’s correlation coefficient (r) for fruit quality attributes in ‘Arisoo’ apples treated with 1-MCP at different concentrations and number of applications during cold storage at 0 ± 1 °C with 90–95% relative humidity for up to six months. Red and blue colors represent positive and negative correlation between variables, respectively. Asterisks (*, **, ***, or ****) represent statistically significant correlation at p < 0.05, 0.01, 0.001, or 0.0001, respectively. TA: titratable acidity; IEC: internal ethylene content; SSC: soluble solids content; L*: lightness; a*: red/green value; b*: blue/yellow value.
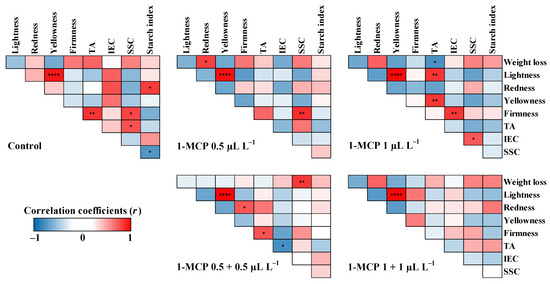
Figure 7.
Pearson’s correlation coefficient (r) for fruit quality attributes in ‘Picnic’ apples treated with 1-MCP at different concentrations and number of applications during cold storage at 0 ± 1 °C with 90–95% relative humidity for up to six months. Red and blue colors represent positive and negative correlation between variables, respectively. Asterisks (*, **, ***, or ****) represent statistically significant correlation at p < 0.05, 0.01, 0.001, or 0.0001, respectively. TA: titratable acidity; IEC: internal ethylene content; SSC: soluble solids content; L*: lightness; a*: red/green value; b*: blue/yellow value.
4. Discussion
1-Methylcyclopropene (1-MCP) is an ethylene inhibitor widely used to maintain fruit quality during storage of climacteric fruits [28]. 1-MCP mainly inhibits the ethylene sensitivity by binding to ethylene receptors, forming an ethylene-receptor complex, and delays the fruit ripening [13,31,32]. In this study, the effects of concentration and number of applications of 1-MCP on major quality features of two newly bred apple cultivars, namely ‘Arisoo’ and ‘Picnic’, during six-month cold storage, were studied. The relationship of 1-MCP concentration, number of applications, and apple cultivar were visible mainly in fruit firmness, weight loss, and SSC. However, for IEC and TA, the positive effects of 1-MCP were significant in both cultivars but uninfluenced by the concentration and number of applications. For starch index and color properties, the differences were insignificant and negligible in both cultivars.
In the ‘Arisoo’ apple, a single application of 1-MCP at the rate of 0.5 μL L−1 was sufficient, while double application at the rate of 0.5 + 0.5 μL L−1 was needed in the ‘Picnic’ apple for maintaining firmness and SSC after six months in cold storage. The decrease in firmness and SSC at the end of the storage showed a positive correlation between these quality attributes, which was supported by correlation results. The effectiveness of 1-MCP on fruit firmness and SSC was found to be shorter in ‘Picnic’ than in ‘Arisoo’. 1-MCP as an ethylene action inhibitor can directly influence the ethylene production, enzyme activities involved in ethylene biosynthesis, formation of volatile compounds, and fruit-softening process dependent on ethylene [7,11]. The effects of 1-MCP can vary depending on apple cultivars, showing shorter effects in some cultivars than others. This may be due to differences in metabolic rates, genetics, IEC at harvest, and their abilities to regenerate new ethylene receptors [24,26]. The high ethylene concentration at harvest can reduce the effectiveness of 1-MCP [26]. However, this could not be the main reason for the shorter effect of 1-MCP in ‘Picnic’ apples than ‘Arisoo’ in this study, since it has a lower IEC than that of ‘Arisoo’ at harvest.
1-MCP can also inhibit the activities of cell wall degrading enzymes, namely polygalacturonase, pectin methylesterase, cellulase, β-galactosidase, and α-L-arabinofuranosidase, leading to the maintenance of cell wall and fruit firmness [21,22,28]. The shorter activity of 1-MCP on fruit firmness in ‘Picnic’ than in ‘Arisoo’ might be related to the differences in cell wall components and activities of pectin-degrading enzymes between the two cultivars, which are to be further investigated. SSC is positively associated with weight loss, which increases with the decrease in the transpiration rate related to blockage of receptors by 1-MCP [21,33,34]. The shorter effect of 1-MCP on SSC in ‘Picnic’ than in ‘Arisoo’ might be indirectly due to less blockage of ethylene receptors by 1-MCP in that cultivar compared with ‘Arisoo’.
Single application of 1-MCP at the rate of 1 μL L−1 reduced fruit weight loss in both cultivars, showing that the dosage lower than that should not be used to maintain fruit weight during cold storage. Yoo et al. [35] also reported similar results that weight loss was more influenced by 1 μL L−1 than 0.5 μL L−1 1-MCP in ‘Honggeum’ apples. Weight loss is related to transpiration rate, which in turn is affected by the extent of ethylene-receptor blockage by 1-MCP [21,33,34]. Therefore, to reduce weight loss, the dosage of 1-MCP applied should be sufficient for complete blockage of receptors.
Interestingly, all 1-MCP treatments in this study could suppress IEC and maintain TA in both varieties compared with untreated controls. Even the lowest concentration at a minimum number of applications, i.e., single application of 0.5 μL L−1 1-MCP, exhibited a significant effect on IEC and TA. These findings were supported by previous studies [20,34] that even a small amount of 1-MCP can efficiently control the IEC and maintain TA during storage. For most climacteric fruits, the minimum effective concentration of 1-MCP was known to be 1 μL L−1 [28]. Additionally, multiple or repeated applications of 1-MCP have been suggested to be used for quality maintenance during long-term cold storage [25]. Alternatively, some studies showed that 1-MCP at the rate of 1 μL L−1 or multiple applications could be associated with certain storage disorders while keeping apples for a long time [25,27].
There was no significant effect of 1-MCP, regardless of concentration and number of applications, on starch index and color properties on the sunlit sides of both cultivars. Similar to this study, the effect of 1-MCP was inconclusive on color properties and starch index in the ‘Szampion’ apple [36], on color properties in the ‘Honggeum’ apple [35], and the ‘Gamhong’ and ‘Golden Delicious’ apple [34,37]. Although ethylene mainly influences the ripening process and postharvest quality during storage, other growth regulators and their interactions with ethylene should also be considered [11]. Additionally, cultivars, species, and maturity of fruit may also manipulate certain effects of 1-MCP application [38,39]. These might be the reason for not observing the effect of 1-MCP, an ethylene inhibitor, on starch index and fruit peel color variables of the cultivars in this study.
5. Conclusions
It can be concluded that single application of 0.5 μL L−1 1-MCP effectively reduces IEC and maintains fruit qualities during six-month cold storage of two newly released cultivars, particular in ‘Arisoo’. Double application of 1-MCP at 0.5 + 0.5 μL L−1 or single application at 1 μL L−1 might be needed for some quality features, namely, firmness, weight loss, and SSC in the case of the ‘Picnic’ apple. This clearly suggests that it is essential to determine the appropriate concentration and number of applications (single or multiple) of 1-MCP before applying to any new variety in such a way that desirable benefits of 1-MCP could be achieved up to its fullest capacity.
Author Contributions
Conceptualization, J.-G.K., J.Y. and I.-K.K.; methodology, J.-G.K. and J.Y.; software, J.-G.K.; validation, J.-G.K., J.Y. and N.M.W.; formal analysis, J.-G.K. and N.M.W.; investigation, J.-G.K., J.Y. and N.M.W.; resources, J.-G.K., J.Y. and I.-K.K.; data curation, J.-G.K.; writing—original draft preparation, J.-G.K. and J.Y.; writing—review and editing, J.-G.K., T.-T.M., A.H.N. and I.-K.K.; visualization, T.-T.M., A.H.N. and I.-K.K.; supervision, I.-K.K.; project administration, I.-K.K.; funding acquisition, I.-K.K. T.-T.M. and A.H.N. did major editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the 2021 research fund of Rural Development Administration, Republic of Korea (PJ01586702).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data used in this study are included in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 2019 Apple Update: Republic of Korea. Foreign Agriculture Service, United States Department of Agriculture (USDA). Available online: https://kr.usembassy.gov/wp-content/uploads/sites/75/2019-Apple-Update_Seoul_Korea-Republic-of_12-03-2019.pdf (accessed on 8 December 2019).
- KOSIS. Statistics Korea Database (2020) Fruit Production in Korea. Available online: https://kosis.kr/eng/ (accessed on 2 September 2021).
- KREI. Korea Rural Economic Institute. 2019. Available online: https://www.krei.re.kr/eng/index.do (accessed on 2 September 2021).
- Ban, S.H.; Yun, W.H.; Kim, G.H.; Kwon, S.I.; Choi, C. Genetic identification of apple cultivars bred in Korea using simple sequence repeat markers. Hortic. Environ. Biotechnol. 2014, 55, 531–539. [Google Scholar] [CrossRef]
- Lohani, S.; Trivedi, P.K.; Nath, P. Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: Effect of 1-MCP, ABA and IAA. Postharvest Biol. Technol. 2004, 31, 119–126. [Google Scholar] [CrossRef]
- Bardy, C.J. Fruit ripening. Annu. Rev. Plant Physiol. 1987, 38, 155–178. [Google Scholar] [CrossRef]
- Song, J.; Bangerth, F. The effect of harvest date on aroma compound production from ‘Golden Delicious’ apple fruit and relationship to respiration and ethylene production. Postharvest Biol. Technol. 1996, 8, 259–269. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–340. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Bouzayen, M.; Latche, A.; Nath, P.; Pech, J.C. Mechanism of fruit ripening. In Plant Developmental Biology; Pua, E.A., Davey, H.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 319–339. [Google Scholar] [CrossRef]
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2014, 65, 4561–4575. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Song, J.; Campbell-Palmer, L.; Fillmore, S.; Zhang, Z. Effect of ethylene and 1-MCP on expression of genes involved in ethylene biosynthesis and perception during ripening of apple fruit. Postharvest Biol. Technol. 2013, 78, 55–66. [Google Scholar] [CrossRef]
- Sisler, E.C.; Serek, M. Inhibitors of ethylene responses in plants at the receptor level: Recent developments. Physiol. Plant 1997, 100, 577–582. [Google Scholar] [CrossRef]
- Asif, M.H.; Trivedi, P.K.; Solomos, T.; Tucker, M. Isolation of high-quality RNA from apple (Malus domestica) fruit. J. Agric. Food Chem. 2006, 54, 5227–5229. [Google Scholar] [CrossRef]
- Lee, J.; Mattheis, J.P.; Rudell, D.R. Storage temperature and 1-methylcyclopropene treatment affect storage disorders and physiological attributes of ‘Royal Gala’ apples. HortScience 2016, 51, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Sisler, E.C.; Dupille, E.; Serek, M. Effect of 1-methylcyclopropene and methylenecyclopropane on ethylene binding and ethylene action on cut carnations. Plant Growth Regul. 1996, 18, 79–86. [Google Scholar] [CrossRef]
- Fan, X.; Blankenship, S.M.; Mattheis, J.P. 1-Methylcyclopropene inhibits apple ripening. J. Am. Soc. Hortic. Sci. 1999, 124, 690–695. [Google Scholar] [CrossRef] [Green Version]
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Tomala, K.; Malachowska, M.; Guzek, D.; Glabska, D.; Gutkowska, K. The effects of 1-methylcyclopropene treatment on the fruit quality of ‘Idared’ apples during storage and transportation. Agriculture 2020, 10, 490. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Kwon, S.I.; Watkins, C.B.; Kang, I.K. Characterization of fruit quality attributes and cell wall metabolism in 1-methylcyclopropene (1-MCP)-treated ‘Summer King’ and ‘Green Ball’ apples during cold storage. Front. Plant Sci. 2019, 10, 1513. [Google Scholar] [CrossRef] [Green Version]
- Win, N.M.; Yoo, J.; Naing, A.H.; Kwon, J.G.; Kang, I.K. 1-Methylcyclopropene (1-MCP) treatment delays modification of cell wall pectin and fruit softening in ‘Hwangok’ and ‘Picnic’ apples during cold storage. Postharvest Biol. Technol. 2021, 180, 111599. [Google Scholar] [CrossRef]
- Watkins, C.B. Principles and practices of postharvest handling and stress. In Apples: Crop Physiology, Production and Uses; Feree, D., Warrington, I.J., Eds.; CAP Publishing: Wallingford, UK, 2003; pp. 585–615. [Google Scholar] [CrossRef]
- Watkins, C.B. Overview of 1-methylcyclopropene trials and uses for edible horticultural crops. HortScience 2008, 43, 86–94. [Google Scholar] [CrossRef]
- DeEll, J.R.; Lum, G.B.; Ehsani-Moghaddam, B. Effects of multiple 1-methylcyclopropene treatments on apple fruit quality and disorders in controlled atmosphere storage. Postharvest Biol. Technol. 2016, 111, 93–98. [Google Scholar] [CrossRef]
- Watkins, C.B.; Nock, J.F.; Lu, X. Repeated treatments of apple fruit with SmartFreshTM. N. Y. Fruit Q. 2013, 21, 11–16. [Google Scholar]
- DeEll, J.; Eshani-Moghaddam, B. Effects of rapid consecutive postharvest 1-methylcyclopropene treatments on fruit quality and storage disorders in apples. HortScience 2013, 48, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ma, Y.; Dong, C.; Terry, L.A.; Watkins, C.B.; Yu, Z.; Cheng, Z.M. Meta-analysis of the effects of 1-methylcyclopropene (1-MCP) treatment on climacteric fruit ripening. Hortic. Res. 2020, 7, 208. [Google Scholar] [CrossRef]
- Jayanty, S.S.; Canoles, M.; Beaudry, R.M. Concentration dependence of ‘Redchief Delicious’ apple fruit softening and chlorophyll fluorescence to repeated doses of 1-methylcyclopropene. J. Am. Soc. Hortic. Sci. 2004, 129, 760–765. [Google Scholar] [CrossRef] [Green Version]
- Blanpied, G.D.; Silsby, K.J. Predicting harvest date windows for apples. Cornell Coop. Ext. 1992, 221, 12. Available online: https://ecommons.cornell.edu/handle/1813/3299 (accessed on 10 September 2021).
- Sisler, E.C.; Blankenship, S.M. Method of Counteracting an Ethylene Response in Plants. U.S. Patent 5,518,988, 21 May 1996. Available online: http://europepmc.org/article/PAT/WO9533377 (accessed on 10 September 2021).
- Sisler, E.C.; Serek, M. Compounds interacting with the ethylene receptor in plants. Plant Biol. 2003, 5, 473–480. [Google Scholar] [CrossRef]
- Mahajan, B.V.C.; Sing, K.; Dhillon, W.S. Effect of 1-methylcyclopropene (1-MCP) on storage life and quality of pear fruits. J. Food Sci. Technol. 2010, 47, 351–354. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.O.; Yoo, J.; Lee, J.; Win, N.M.; Ryu, S.; Han, J.S.; Jung, H.Y.; Choung, M.G.; Kwon, Y.D.; Lee, D.H.; et al. Effects of 1-methylcyclopropene (1-MCP) and polyethylene (PE) film liner treatments on the fruit quality of cold-stored ‘Gamhong’ apples. Hortic. Environ. Biotechnol. 2018, 59, 51–57. [Google Scholar] [CrossRef]
- Yoo, J.; Jung, H.; Win, N.M.; Kwon, J.G.; Cho, Y.J.; Jung, H.Y.; Lee, D.H.; Kang, I.K. Changes in fruit quality attributes, cell wall materials, and related hydrolases activities in 1-methylcyclopropene (1-MCP)-treated ‘Honggeum’ apples during cold storage. Hortic. Sci. Technol. 2020, 38, 870–879. [Google Scholar] [CrossRef]
- Tomala, K.; Grzeda, M.; Guzek, D.; Glabska, D.; Gutkowska. The effects of preharvest 1-methylcyclopropene (1-MCP) treatment on the fruit quality parameters of cold-stored ‘Szampion’ cultivar apples. Agriculture 2020, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Saftner, R.A.; Abbott, J.A.; Conway, W.S.; Barden, C.L. Effects of 1-methylcyclopropene and heat treatments on ripening and postharvest decay in Golden Delicious apples. J. Am. Soc. Hortic. Sci. 2003, 128, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Borenstein, M.; Hedges, L.V.; Higgins, J.; Rothstein, H.R. Introduction to Meta-Analysis, 1st ed.; John Wiley & Sons, Ltd.: Oxford, UK, 2009; 421p. [Google Scholar] [CrossRef]
- Watkins, C.P. Advances in the use of 1-MCP. In Advances in Postharvest Fruit and Vegetable Technology, 1st ed.; Wills, R.B.H., Golding, J., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 117–146. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).