The Aconitum carmichaelii F3′5′H Gene Overexpression Increases Flavonoid Accumulation in Transgenic Tobacco Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
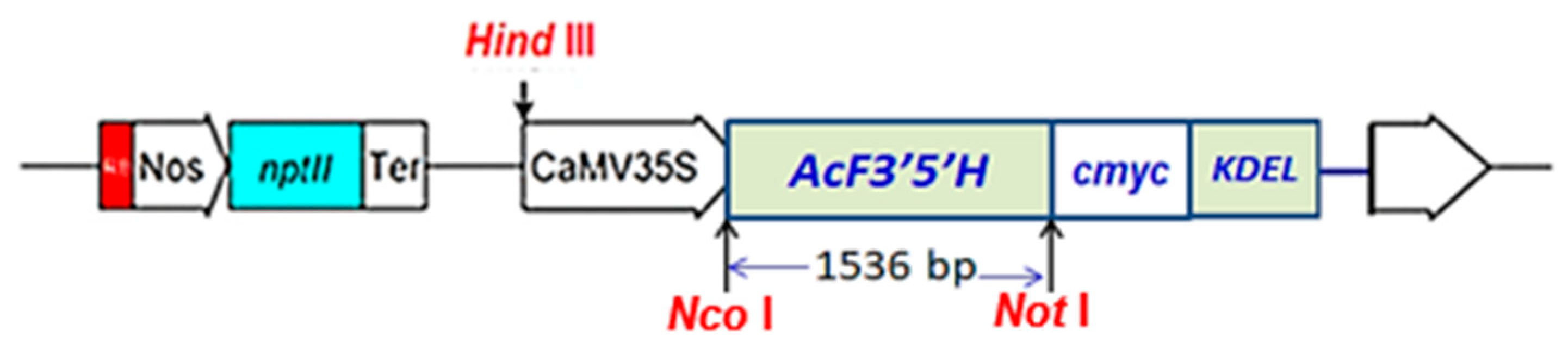
2.2. Transferring the A. Carmichaelii F3′5′H Gene into Tobacco through A. Tumefaciens
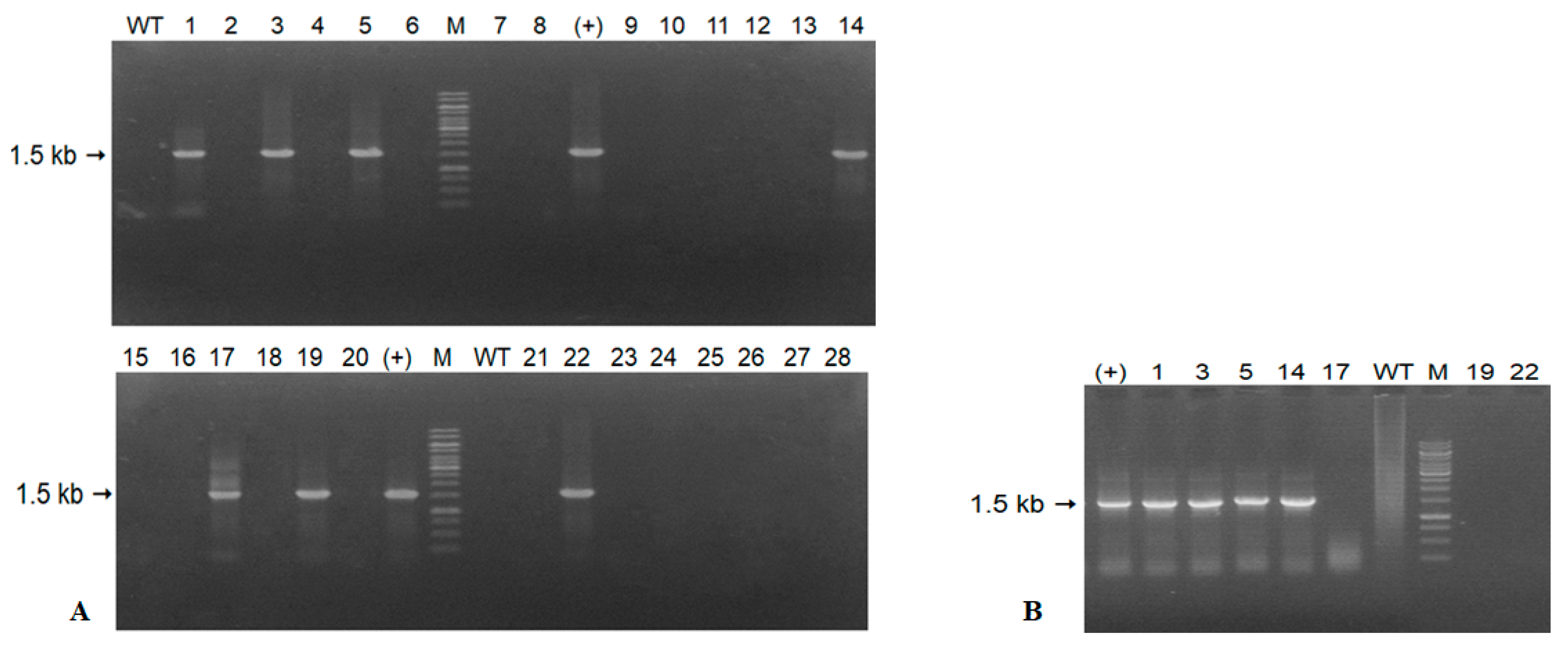
2.3. Confirmation of the Transgene in Transformed Tobacco
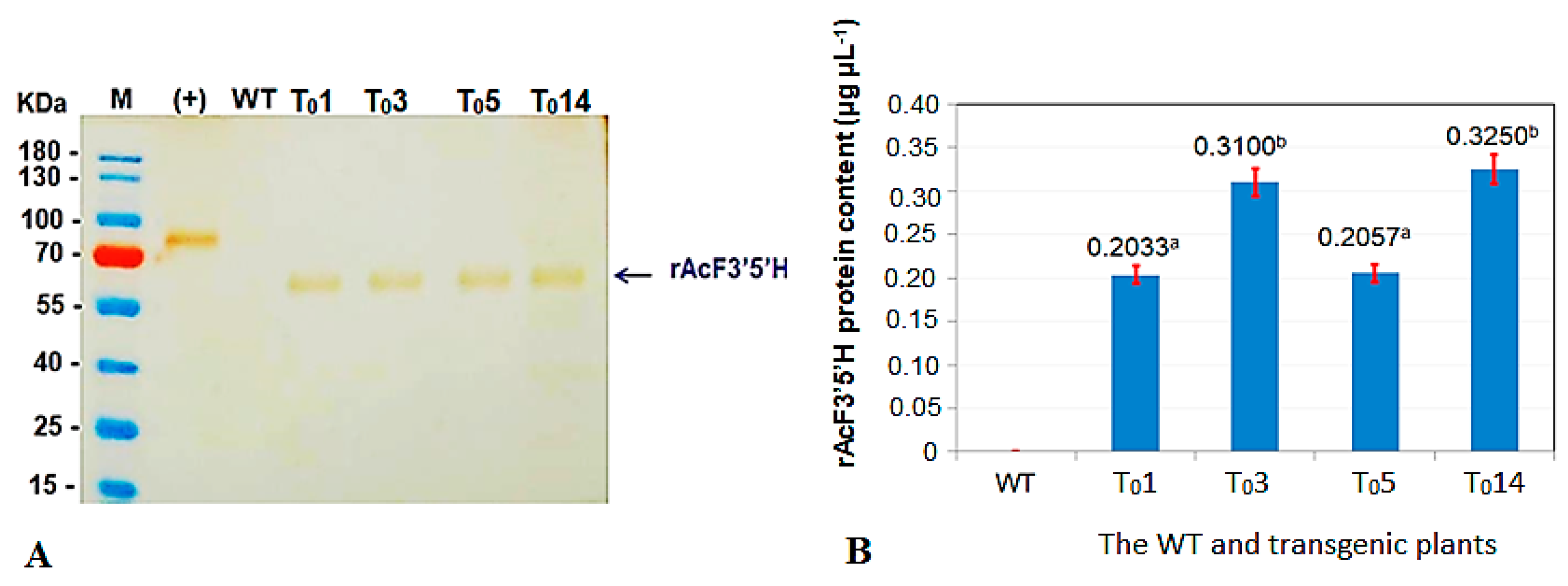
2.4. Confirmation of the Recombinant AcF3′5′H Protein Expression in Genetically Modified (GM) Tobacco Plants
2.5. Content Analysis of total Flavonoid in GM Tobacco and Non-Transformed Plants
2.6. Statistical Analysis
3. Results
3.1. Results of Expression of the A. Carmichaelii Flavonoid 3′5′-Hydroxylase Gene in GM Tobacco Plants
3.2. Content of Flavonoid in Leaves of GM Tobacco Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Do, T.L. Medicinal Plants and Medicines in Viet Nam; Medical Publishing House: Hanoi, Vietnam, 2004. [Google Scholar]
- Czemmel, S.; Stracke, R.; Weisshaar, B.; Cordon, N.; Harris, N.; Walker, A.; Robinson, S.; Bogs, J. The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berries. Plant Physiol. 2009, 151, 1513–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, Y.; Nakano-Shimada, R.; Ohbayashi, M.; Okinaka, Y.; Kiyokawa, S.; Kikuchi, Y. Expression of chimeric P450 genes encoding flavonoid-3′, 5′-hydroxylase in transgenic tobacco and petunia plants. FEBS Lett. 1999, 461, 241–245. [Google Scholar] [CrossRef] [Green Version]
- Zabala, G.; Zou, J.; Tuteja, J.; Gonzalez, D.; Clough, S.J.; Vodkin, L. Transcriptome changes in the phenylpropanoid pathway of Glycine max in response to Pseudomonas syringae infection. BMC Plant Biol. 2006, 6. [Google Scholar] [CrossRef] [Green Version]
- Heller, W.; Forkmann, G. Biosynthesis of flavonoids. In The Flavonoids: Advances in Research Since 1986; Harborne, J.B., Ed.; Chapman & Hall: London, UK, 1993; pp. 499–536. [Google Scholar]
- Menting, J.G.T.; Scopes, R.K.; Stevenson, T.W. Characterization of flavonoid 3′,5′-hydroxylase in microsomal membrane fraction of Petunia hybrida flowers. Plant Physiol. 1994, 106, 633–642. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Huang, H.; Meng, L.; Hu, K.; Dai, S.L. Isolation and functional analysis of a homolog of flavonoid 3′5′-hydroxylase gene from Pericallis × hybrida. Physiol. Plant 2013, 149, 151–159. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Gao, L.; Yu, O.; Wang, X.; He, X.; Jiang, X.; Liu, Y.; Xia, T. Functional analysis of Flavonoid 3′5′-hydroxylase from Tea plant (Camellia sinensis): Critical role in the accumulation of catechims. BMC Plant Biol. 2014, 374, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.; Wang, L.; Zhang, C.; Wu, L.; Li, H.; Zhang, F.; Cheng, H. Transcriptome Analysis Reveals Key Flavonoid 3′-Hydroxylase and Flavonoid 3′,5′-Hydroxylase Genes in Affecting the Ratio of Dihydroxylated to Trihydroxylated Catechins in Camellia sinensis. PLoS ONE 2015, 10, e0137925. [Google Scholar] [CrossRef] [Green Version]
- Okinaka, Y.; Shimada, Y.; Nakano-Shimada, R.; Ohbayashi, M.; Kiyokawa, S.; Kikuchi, Y. Selective Accumulation of Delphinidin Derivatives in Tobacco Using a Putative Flavonoid 3′,5′-Hydroxylase cDNA from Campanula medium. Biosci. Biotechnol. Biochem. 2003, 67, 161–165. [Google Scholar] [CrossRef]
- Hu, B.; Yao, H.; Peng, X.; Wang, R.; Li, F.; Wang, Z.; Zhao, M.; Jin, L. Overexpression of Chalcone Synthase Improves Flavonoid Accumulation and Drought Tolerance in Tobacco. Preprints 2019, 2019060103. [Google Scholar] [CrossRef] [Green Version]
- Vu, T.N.T.; Le, T.H.T.; Hoang, P.H.; Sy, D.T.; Vu, T.T.T.; Chu, H.M. Overexpression of the Glycine max chalcone isomerase (GmCHI) gene in transgenic Talinum paniculatum plants. Turk. J. Bot. 2018, 42, 551–558. [Google Scholar] [CrossRef] [Green Version]
- Vu, D.L.; Bui, T.T.; Nguyen, T.H.; Nguyen, T.V. Flavonoids and other compound isolated from leaves of Aconitum carmichaeli Debx. growing in Viet Nam. J. Chem. Pharm. Res. 2015, 7, 228–234. Available online: https://www.jocpr.com/abstract/flavonoids-and-other-compound-isolated-from-leaves-of-aconitum-carmichaelidebx-growing-in-viet-nam-4395.html (accessed on 20 May 2021).
- Hoang, T.T.H.; Nguyen, T.N.L.; Chu, H.M. Molecular cloning and designing transgenic construct carying Flavonoi 3′5′ hdroxylase gene isolated from Aconitum carmichaelii Debx. Plants. TNU J. Sci. Technol. 2020, 225, 43–49. [Google Scholar]
- Topping, J.F. Tobacco Transformation. In Plant Virology Protocols. Methods in Molecular Biology™; Foster, G.D., Taylor, S.C., Eds.; Humana Press: Totowa, NJ, USA, 1998; Volume 81. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Sun, H.J.; Cui, M.L.; Ma, B.; Ezura, H. Functional expression of the tastemodifying protein, miraculin, in transgenic lettuce. FEBS Lett. 2006, 580, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Kalita, P.; Barman, T.K.; Pal, T.K.; Kalita, R. Estimation of total flavonoid content (tfc) and anti oxidant activities of methanolic whole plant extract of biophytum sensitivum linn. J. Drug Deliv. Ther. 2013, 3, 33–37. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.R.; Schiffthaler, B.; Thompson, S.L.; Street, N.R.; Wang, X.R. Interspecific Plastome Recombination Reflects Ancient Reticulate Evolution in Picea (Pinaceae). Mol. Biol. Evol. 2017, 34, 1689–1701. [Google Scholar] [CrossRef]
- Ferrer, J.L.; Austin, M.B.; Stewart, C.J.; Noel, J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008, 46, 356–370. [Google Scholar] [CrossRef] [Green Version]
- He, Y.N.; Ou, S.P.; Xiong, X.; Pan, Y.; Pei, J.; Xu, R.C.; Geng, F.N.; Han, L.; Zhang, D.K.; Yang, M. Stems and leaves of Aconitum carmichaelii Debx. as potential herbal resources for treating rheumatoid arthritis: Chemical analysis, toxicity and activity evaluation. Chin. J. Nat. Med. 2018, 16, 644–652. [Google Scholar] [CrossRef]
- Kim, S.Y.; Cheon, K.S.; Im, S.M.; Kwon, O.H.; Yoo, B.S.; Kim, M.S.; Le, S.Y. Isolation and Expression Pattern of Flavonoid 3′,5′-hydroxylase Gene in Clematis patens. Flower Res. J. 2016, 24, 205–211. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, K.S.; Kim, Y.J.; Pham, A.T.; Kim, H.H.; Cho, J.W.; Park, S.U. Cloning and characterization of a flavonol synthase gene from Scutellaria baicalensis. Sci. World J. 2014, 980740. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, T.; Xin, Y.; Wang, G.; Xu, L. Overexpression of GbF3′5′H1 Provides a Potential to Improve the Content of Epicatechin and Gallocatechin. Molecules 2020, 25, 4836. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.L.; Hoang, T.T.H.; Nguyen, H.Q.; Tu, Q.T.; Tran, T.H.; Lo, T.M.T.; Vu, T.T.T.; Chu, H.M. Agrobacterium tumefaciens-mediated genetic transformation and overexpression of the flavonoid 3′5′-hydroxylase gene increases the flavonoid content of the transgenic Aconitum carmichaelii Debx. Plant. Vitro Cell. Dev. Biol. Plant 2021, 107. [Google Scholar] [CrossRef]


| Samples | Content of Flavonoids * (µg g−1) | The Increase Compared to WT (%) |
|---|---|---|
| WT | 408.43 a ± 5.11 | 0 |
| T01 | 691.20 b ± 2.02 | 69.23 |
| T03 | 907.83 c ± 5.14 | 122.23 |
| T05 | 713.60 b ± 4.21 | 74.72 |
| T014 | 900.37 c ± 0.81 | 120.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, Y.T.H.; Hoang, H.T.T.; Mai, A.T.H.; Nguyen, L.T.N.; Nguyen, Q.H.; Pham, N.T.T.; Sy, T.D.; Chu, M.H. The Aconitum carmichaelii F3′5′H Gene Overexpression Increases Flavonoid Accumulation in Transgenic Tobacco Plants. Horticulturae 2021, 7, 384. https://doi.org/10.3390/horticulturae7100384
Nguyen YTH, Hoang HTT, Mai ATH, Nguyen LTN, Nguyen QH, Pham NTT, Sy TD, Chu MH. The Aconitum carmichaelii F3′5′H Gene Overexpression Increases Flavonoid Accumulation in Transgenic Tobacco Plants. Horticulturae. 2021; 7(10):384. https://doi.org/10.3390/horticulturae7100384
Chicago/Turabian StyleNguyen, Yen Thi Hai, Hoan Thi Thu Hoang, Anh Thi Hoang Mai, Lan Thi Ngoc Nguyen, Quan Huu Nguyen, Nhan Thi Thanh Pham, Thuong Danh Sy, and Mau Hoang Chu. 2021. "The Aconitum carmichaelii F3′5′H Gene Overexpression Increases Flavonoid Accumulation in Transgenic Tobacco Plants" Horticulturae 7, no. 10: 384. https://doi.org/10.3390/horticulturae7100384
APA StyleNguyen, Y. T. H., Hoang, H. T. T., Mai, A. T. H., Nguyen, L. T. N., Nguyen, Q. H., Pham, N. T. T., Sy, T. D., & Chu, M. H. (2021). The Aconitum carmichaelii F3′5′H Gene Overexpression Increases Flavonoid Accumulation in Transgenic Tobacco Plants. Horticulturae, 7(10), 384. https://doi.org/10.3390/horticulturae7100384








