Measuring Camellia Petal Color Using a Portable Color Sensor
Abstract
1. Introduction
2. Materials and Methods
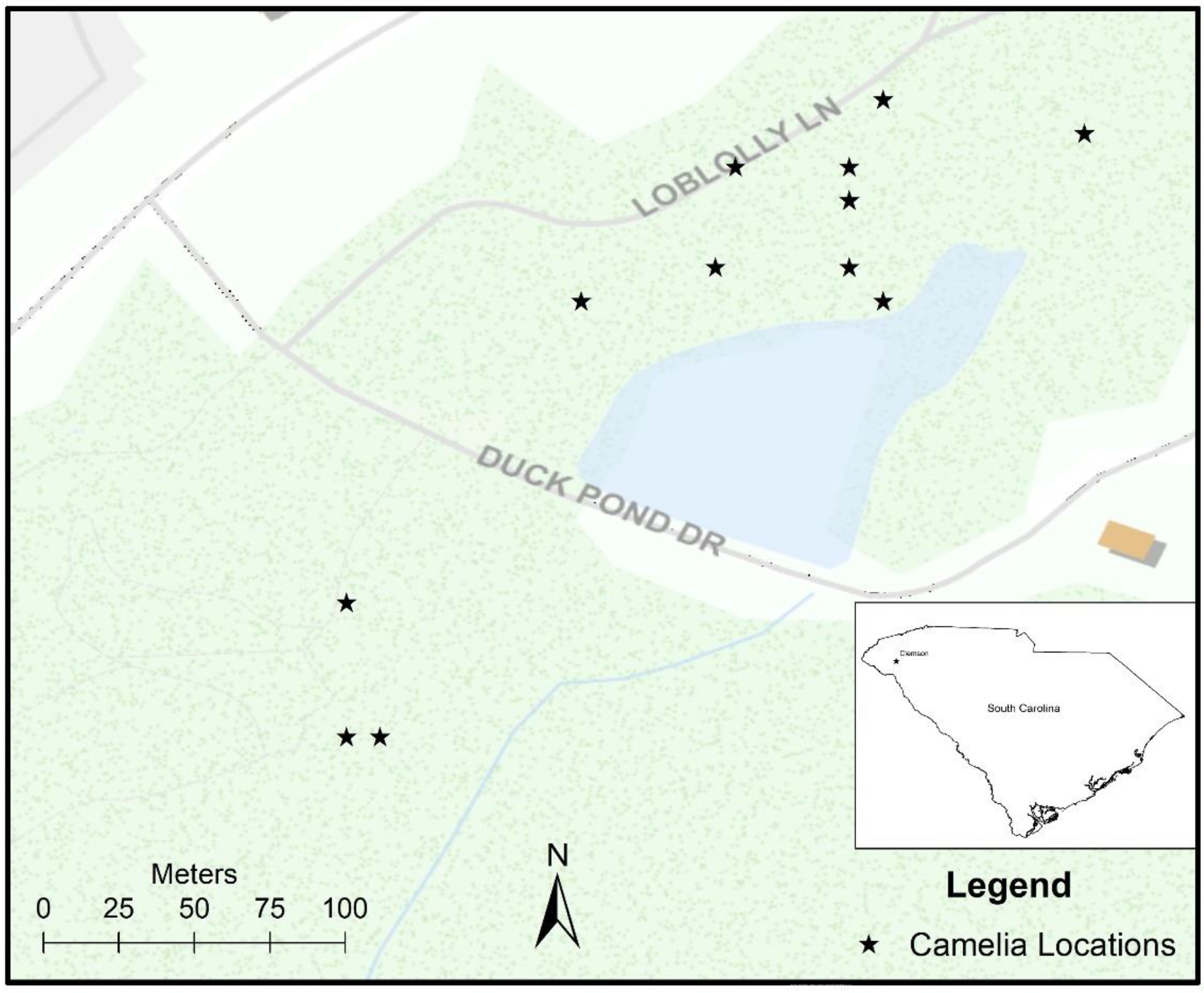
2.1. Site Description
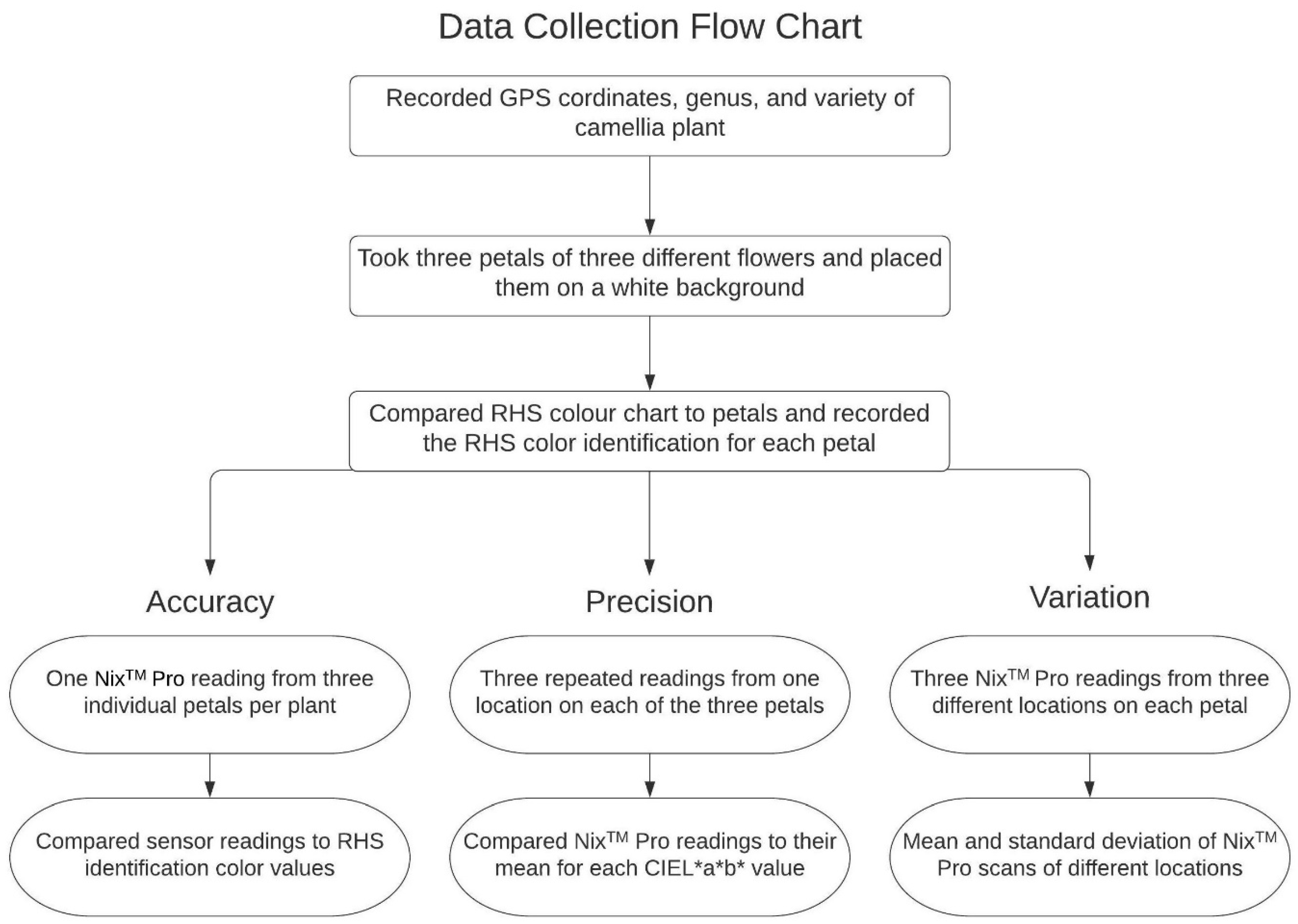
2.2. Data Collection
2.3. Statistical Analysis
3. Results and Discussion
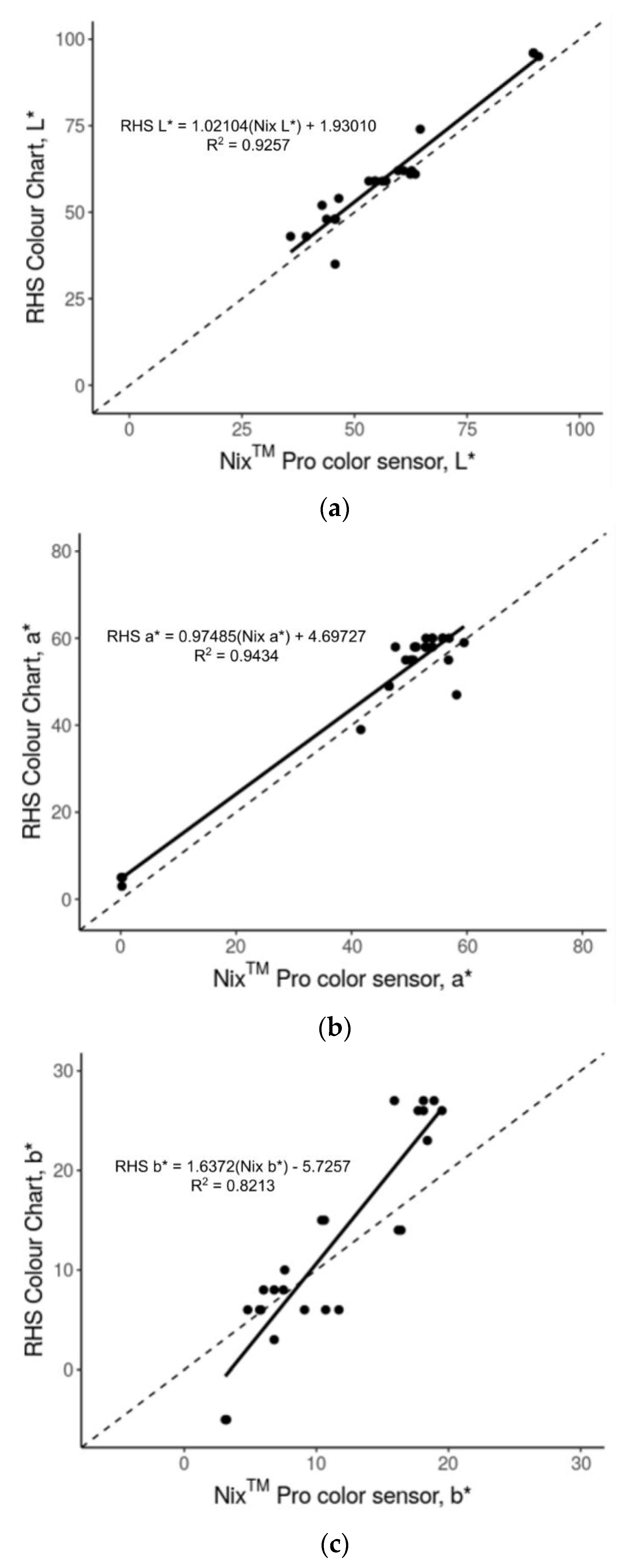
3.1. Accuracy
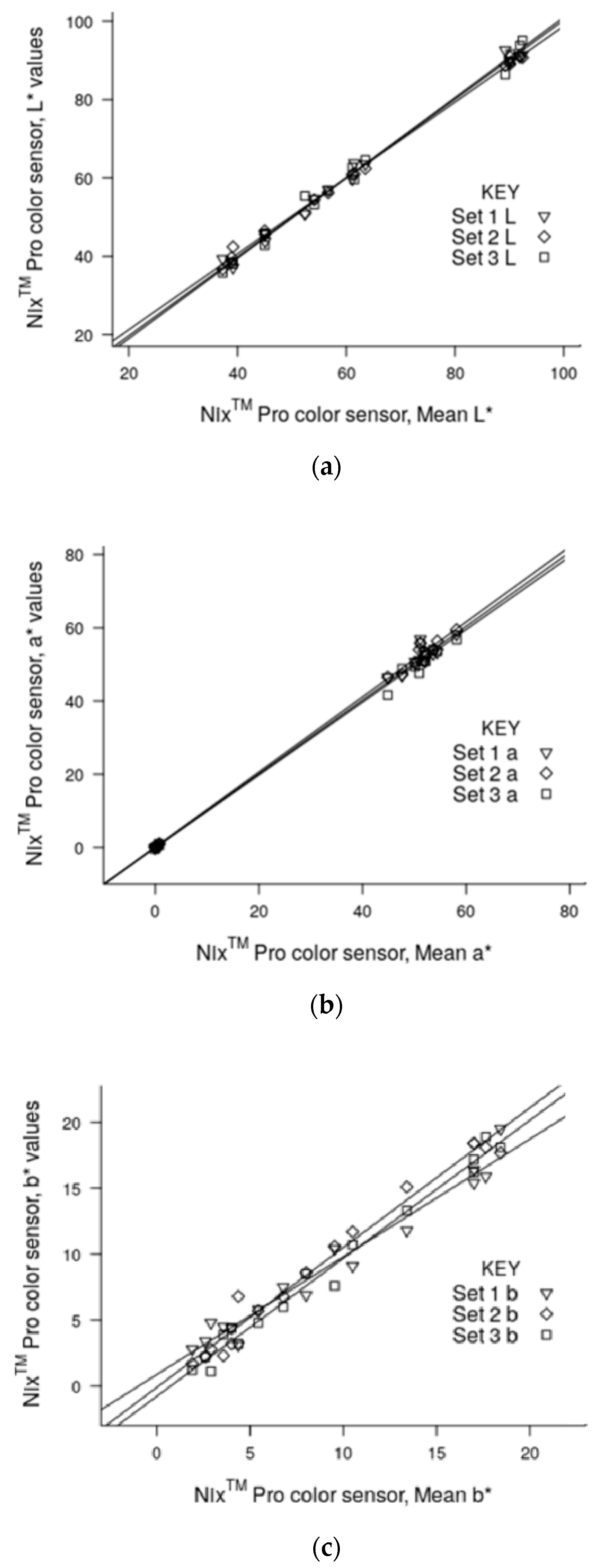
3.2. Precision
3.3. Variation
3.4. Sensor-Based Color Measurements in Horticulture
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Kooi, C.; Elzenga, J.T.M.; Staal, M.; Stavenga, D.G. How to colour a flower: On the optical principles of flower coloration. Proc. R. Soc. B 2016, 283, 20160429. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.; Roger, T.; Vorhagen, F.W. Comparative analysis of the quantization of color spaces on the basis of the CIELAB color-difference formula. ACM Trans. Graph. (TOG) 1997, 16, 109–154. [Google Scholar] [CrossRef]
- Ganesan, P.; Rajini, V.; Rajkumar, R.I. Segmentation and edge detection of color images using CIELAB color space and edge detectors. In Proceedings of the INTERACT-2010, Chennai, India, 3–5 December 2010; pp. 393–397. [Google Scholar] [CrossRef]
- Ayala-Silva, T.; Meerow, A.W. Evaluation of flower color using a colorimeter and the Royal Horticultural Society charts. Interam. Soc. Trop. Hortic. 2006, 50, 138–144. [Google Scholar]
- Zhao, D.; Tao, J. Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant Sci. 2015, 6, 261. [Google Scholar] [CrossRef] [PubMed]
- Nassau, K. The Fifteen Causes of Color: The Physics and Chemistry of Color; Wiley: Hoboken, NJ, USA, 1987. [Google Scholar] [CrossRef]
- Agostini, T.; Galmonte, A. Perceptual organization overcomes the effects of local surround in determining simultaneous lightness contrast. Psychol. Sci. 2002, 13, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Mylechreest, M. Thomas Andrew Knight and the founding of the Royal Horticultural Society. J. Gard. Hist. 1984, 12, 132–137. [Google Scholar] [CrossRef]
- A Horticultural Colour Chart. 1936. Available online: https://www.nature.com/articles/138322d0 (accessed on 15 April 2020).
- RHS Media. Royal Horticultural Society (RHS) Colour Chart, 6th ed.; Royal Horticultural Society: London, UK, 2015. [Google Scholar]
- NixTM Pro. 2020. Available online: https://www.nixsensor.com/ (accessed on 10 April 2020).
- Stiglitz, R.Y.; Mikhailova, E.A.; Sharp, J.L.; Post, C.J.; Schlautman, M.A.; Gerard, P.D.; Cope, M.P. Predicting soil organic carbon and total nitrogen at the farm scale using quantitative color sensor measurements. Agronomy 2018, 8, 212. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Hopkins, D.L. A comparison of the Nix Colour Sensor ProTM and HunterLab MiniScanTM colorimetric instruments when assessing aged beef colour stability over 72 h display. Meat Sci. 2019, 147, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, E.A.; Stiglitz, R.Y.; Post, C.J.; Schlautman, M.A.; Sharp, J.L.; Gerard, P.D. Predicting soil organic carbon and total nitrogen in the Russian Chernozem from depth and wireless color sensor measurements. Eurasian Soil Sci. 2017, 50, 1414–1419. [Google Scholar] [CrossRef]
- Stiglitz, R.; Mikhailova, E.; Post, C.; Schlautman, M.; Sharp, J.L. Evaluation of an inexpensive sensor to measure soil color. Comput. Electron. Agr. 2016, 121, 141–148. [Google Scholar] [CrossRef]
- Carter, K. Camellias. Available online: https://ucanr.edu/sites/urbanhort/files/80153.pdf (accessed on 15 April 2020).
- Robards, K.; Prenzler, P.; Ryan, D.; Zhong, H. Camellia Oil and Tea Oil. Available online: https://www.sciencedirect.com/science/article/pii/B9781893997974500176 (accessed on 15 April 2020).
- Haque, M.; South Carolina Botanical Garden. Scencyclopedia.org. Available online: http://www.scencyclopedia.org/sce/entries/south-carolina-otanical-garden/ (accessed on 10 April 2020).
- Discover South Carolina. South Carolina Botanical Garden. Available online: https://discoversouthcarolina.com/products/2703 (accessed on 10 April 2020).
- U.S. Climate Data. Weather Averages Clemson, South Carolina. Available online: https://www.usclimatedata.com/climate/clemson/south-carolina/united-states/ussc0059 (accessed on 10 April 2020).
- Royal Horticultural Society Colour Charts Edition V. Available online: http://rhscf.orgfree.com (accessed on 9 August 2020).
- International Camellia Society. 2020. The On-line Camellia Registry. Available online: https://internationalcamellia.org/international-camellia-register?camelliaId=13256&state=b (accessed on 10 April 2020).
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 10 April 2020).
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer: New York, NY, USA, 2008; ISBN 978-0-387-75968-5. Available online: http://lmdvr.r-forge.r-project.org (accessed on 10 April 2020).
- Wickham, H. Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 10 April 2020).
- Murakami, P.F. An Instructional Guide for Leaf Color Analysis Using Digital Imaging Software; U.S. Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2005; Volume 327. [Google Scholar]
- Kendal, D.; Hauser, C.E.; Garrard, G.E.; Jellinek, S.; Giljohann, K.M.; Moore, J.L. Quantifying plant colour and colour difference as perceived by humans using digital images. PLoS ONE 2013, 8, e72296. [Google Scholar] [CrossRef]
- Garcia, J.E.; Greentree, A.D.; Shrestha, M.; Dorin, A.; Dyer, A.G. Flower colours through the lens: Quantitative measurement with visible and ultraviolet digital photography. PLoS ONE 2014, 9, e96646. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Chakraborty, S. Use of diffuse reflectance spectroscopy and Nix pro color sensor in combination for rapid prediction of soil organic carbon. Comput. Electron. Agric. 2020, 176, 105630. [Google Scholar] [CrossRef]
- Griesbach, R.J.; Austin, S. Comparison of the Munsell and Royal Horticultural Society’s color charts in describing flower color. Taxon 2005, 54, 771–773. [Google Scholar] [CrossRef]
- Kasajima, I. Measuring plant colors. Plant Biotechnol. 2019, 36, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Voss, D.H. Relating colorimeter measurement of plant color to the Royal Horticultural Society Colour Chart. HortScience 1992, 27, 1256–1260. [Google Scholar] [CrossRef]

| Variety | Information |
|---|---|
| Color: White | |
| Snow on the Mountain | Yashiro, Kôken, 1841, Kokon Yôrankô, vol. 310. Originated in Japan. |
| Chastity | Woodroof 1947, SCCS., Bulletin, vol. 8, No. 6, p. 4; Valley Garden Supply Catalogue,1946–1947. |
| Masterpiece | Yokoyama & Kirino, 1989, Nihon no Chinka, p. 26, colour photo and description. |
| Ivory Tower | SCCS., 1968, Camellia Nomenclature, p. 71. |
| Nuccio’s Pearl | Nuccio’s Nurseries Catalogue, 1978, p. 12: (N #730l). |
| Color: Pink | |
| Marie Bracey | American Camellia Yearbook, 1957, p. 301, Reg. No. 292. |
| In the Pink | Kramer Nursery Catalogue, 1971: Rose-pink formal double. American Camellia Yearbook, 1979, p. 107, Reg. No. 1541. |
| Ruth Lennon | American Camellia Yearbook, 1991, p. 80, Reg. No. 2214, colour photo between pages 80–81. |
| Celestina | Anonymous, Mar. 1832, Revue Horticole, p. 203–204: Double. Light rose, fine form. Originated by Cunningham, England. |
| Show Time | Nuccio’s Nurseries Catalogue, 1978: Light pink semi-double. American Camellia Yearbook, 1979, p. 111, Reg. No. 1528. |
| Tiffany | Womak, 1962–1963, American Camellia Yearbook, p. 1. |
| Color: Red | |
| Scarlet Glory | American Camellia Yearbook, 1984, p. 181, Reg. No. 1953. |
| Barbara Morgan | Fruitland Nursery Catalogue, 1946–1947, p. 29; Fendig, 1952, American Camellia Catalogue. |
| Mathotiana | Anonymous, 1847, Gardeners’ Chronicle, (27):434. Spae, 1847, Société Royale d’Agriculture et de Botanique de Gand, Annales (1847), 3:459–460, pl. 170. |
| Dr. Clifford Parks | American Camellia Yearbook, 1972, p. 128, Reg. No. 1210. |
| Dr. W. G. Lee | Gerbing Azalea Nursery Catalogue Supplement, 1943–1944 as ‘Dr Lee’s No. 43’. |
| Joe Holland | Magnolia Gardens and Nursery Catalogue, 1942–1943. |
| Variety | Petal 1 | Petal 2 | Petal 3 |
|---|---|---|---|
| Color: White | |||
| Snow on the Mountain | NN155A | NN155D | NN155D |
| Chastity | NN155A | NN155D | NN155D |
| Masterpiece | NN155D | NN155D | NN155D |
| Ivory Tower | NN155D | NN155A | NN155D |
| Ivory Tower | NN155D | NN155D | NN155D |
| Nuccio’s Pearl | NN155D | N155D | N155D |
| Color: Pink | |||
| Marie Bracey | 58C | 58C | 58C |
| Marie Bracey | 55A | 55A | 55B |
| In the Pink | 47D | 47D | 52D |
| Ruth Lennon | 55A | 55A | 55A |
| Ruth Lennon | 55A | 55A | 55A |
| Celestina | 58C | 58C | 58C |
| Show Time | 62C | 62D | 62C |
| Tiffany | 54C | 54C | 54D |
| Color: Red | |||
| Scarlet Glory | 45B | 45B | 45B |
| Scarlet Glory | 53C | 53B | 53B |
| Barbara Morgan | 53C | 53C | 53C |
| Mathotiana | 53C | 52A | 53C |
| Dr. Clifford Parks | 45B | 45B | 45B |
| Dr. W. G. Lee | 46C | 46C | 46C |
| Joe Holland | 50A | 50B | 50A |
| RHS Colour | L* | a* | b* |
|---|---|---|---|
| NN155A Yellowish White | 95 | 3 | 3 |
| N155D White | 92 | 6 | −1 |
| NN155D White | 96 | 5 | −5 |
| NN155B White | 95 | 4 | −1 |
| N45B Moderate Red | 35.3 | 56.6 | 18.1 |
| N45C Moderate Red | 39.5 | 54.6 | 17.5 |
| 53C Strong Red | 46 | 56 | 14 |
| 58C Strong Purplish Red | 59 | 58 | 6 |
| 62C Light Purplish Pink | 80 | 29 | −5 |
| 62D Pale Purplish Pink | 86 | 17 | −3 |
| 54C Strong Pink | 68 | 37 | 3 |
| 54D Moderate Purplish Pink | 78 | 28 | 3 |
| 55A Deep Purplish Pink | 62 | 55 | 8 |
| 55B Strong Purplish Pink | 69 | 43 | −1 |
| 45B Vivid Red | 43 | 60 | 27 |
| 46C Vivid Red | 48 | 60 | 26 |
| 50A Strong Red | 50 | 59 | 24 |
| 50B Deep Pink | 57 | 53 | 18 |
| 51A Strong Red | 52 | 55 | 14 |
| 52A Vivid Red | 54 | 59 | 23 |
| 53C Strong Red | 46 | 56 | 14 |
| 52D Strong Pink | 74 | 39 | 10 |
| 47D Deep Pink | 61 | 49 | 15 |
| 46D Deep Yellowish Pink | 52 | 56 | 21 |
| Variety | L* | a* | b* |
|---|---|---|---|
| Color: White | |||
| Nuccio’s Pearl | |||
| - Petal 1 | 88.3 (0.1) | 1.9 (0.0) | 3.9 (0.0) |
| - Petal 2 | 87.3 (0.1) | 1.0 (0.0) | 9.0 (0.0) |
| - Petal 3 | 87.5 (0.0) | 1.6 (0.0) | 5.7 (0.0) |
| Color: Pink | |||
| Show Time | |||
| - Petal 1 | 73.2 (0.1) | 34.8 (0.1) | 5.1 (0.0) |
| - Petal 2 | 79.0 (0.0) | 23.8 (0.0) | 1.9 (0.0) |
| - Petal 3 | 66.3 (0.1) | 33.9 (0.1) | 3.3 (0.0) |
| Tiffany | |||
| - Petal 1 | 69.1 (0.1) | 38.9 (0.1) | 5.3 (0.0) |
| - Petal 2 | 69.6 (0.1) | 40.8 (0.0) | 6.0 (0.0) |
| - Petal 3 | 65.7 (0.6) | 41.9 (0.2) | 7.8 (0.1) |
| Color: Red | |||
| Scarlet Glory | |||
| - Petal 1 | 38.0 (0.0) | 46.1 (0.1) | 12.7 (0.0) |
| - Petal 2 | 38.4 (0.0) | 47.8 (0.0) | 11.8 (0.0) |
| - Petal 3 | 44.1 (0.1) | 55.0 (0.0) | 17.8 (0.0) |
| Joe Holland | |||
| - Petal 1 | 46.8 (0.2) | 51.2 (0.1) | 12.2 (0.1) |
| - Petal 2 | 50.1 (0.1) | 49.4 (0.1) | 10.6 (0.0) |
| - Petal 3 | 44.1 (0.6) | 50.0 (0.0) | 7.9 (0.0) |
| Variety | L* | a* | b* |
|---|---|---|---|
| Color: White | |||
| Snow on the Mountain | 92.4 (2.4) | −0.1 (0.3) | 4.0 (0.7) |
| Chastity | 89.2 (3.1) | 0.7 (0.4) | 2.9 (1.9) |
| Masterpiece | 91.9 (1.6) | 0.8 (0.2) | 1.9 (0.8) |
| Ivory Tower | 90.1 (0.7) | 0.2 (0.1) | 4.4 (2.1) |
| Ivory Tower | 90.1 (1.3) | 0.03 (0.1) | 3.6 (1.1) |
| Nuccio’s Pearl | 87.6 (0.8) | 1.8 (1.0) | 6.5 (3.5) |
| Color: Pink | |||
| Marie Bracey | 54.1 (0.8) | 52.2 (1.1) | 5.4 (0.6) |
| Marie Bracey | 52.4 (2.6) | 47.6 (1.0) | 2.6 (0.7) |
| In the Pink | 63.5 (1.1) | 44.9 (2.8) | 9.5 (1.7) |
| Ruth Lennon | 61.1 (1.5) | 50.1 (0.7) | 6.8 (0.8) |
| Ruth Lennon | 61.5 (2.1) | 52.0 (1.2) | 8.0 (0.1) |
| Celestina | 56.7 (0.4) | 50.9 (3.2) | 10.5 (1.3) |
| Show Time | 72.0 (7.1) | 31.8 (6.9) | 3.5 (1.7) |
| Tiffany | 57.2 (6.1) | 40.7 (2.4) | 22.9 (13.2) |
| Color: Red | |||
| Scarlet Glory | 39.2 (2.8) | 54.4 (1.7) | 17.0 (1.5) |
| Scarlet Glory | 39.9 (3.7) | 48.3 (5.9) | 13.2 (4.4) |
| Barbara Morgan | 38.9 (0.7) | 51.8 (1.6) | 13.4 (1.7) |
| Mathotiana | 45.0 (1.9) | 58.2 (1.4) | 17.0 (1.2) |
| Dr. Clifford Parks | 37.3 (1.8) | 53.6 (0.6) | 17.6 (1.6) |
| Dr. W. G. Lee | 45.1 (1.1) | 56.2 (0.6) | 18.4 (0.9) |
| Joe Holland | 52.9 (6.1) | 50.3 (1.1) | 10.1 (1.8) |
| Variety | L* | a* | b* |
|---|---|---|---|
| Color: White | |||
| Nuccio’s Pearl | |||
| - Petal 1 | 88.0 (3.2) | 1.1 (1.1) | 5.8 (2.8) |
| - Petal 2 | 86.8 (1.7) | 1.3 (0.6) | 7.4 (3.8) |
| - Petal 3 | 86.7 (1.2) | 1.5 (1.5) | 5.6 (1.3) |
| Color: Pink | |||
| Show Time | |||
| - Petal 1 | 74.8 (3.6) | 30.0 (8.3) | 4.7 (4.1) |
| - Petal 2 | 79.8 (2.7) | 17.3 (2.6) | 4.0 (2.2) |
| - Petal 3 | 77.0 (6.1) | 23.8 (7.9) | 3.7 (1.7) |
| Tiffany | |||
| - Petal 1 | 69.7 (3.1) | 36.1 (4.7) | 7.0 (1.2) |
| - Petal 2 | 69.8 (4.5) | 36.9 (3.0) | 9.4 (1.3) |
| - Petal 3 | 71.7 (2.8) | 31.9 (2.6) | 7.1 (2.8) |
| Color: Red | |||
| Scarlet Glory | |||
| - Petal 1 | 36.8 (1.4) | 46.7 (3.7) | 12.3 (2.3) |
| - Petal 2 | 38.0 (1.0) | 47.2 (1.9) | 12.0 (0.7) |
| - Petal 3 | 44.8 (0.6) | 55.3 (0.8) | 17.1 (0.5) |
| Joe Holland | |||
| - Petal 1 | 52.9 (2.2) | 48.5 (2.2) | 9.4 (3.3) |
| - Petal 2 | 53.4 (0.9) | 48.4 (2.0) | 8.2 (1.5) |
| - Petal 3 | 46.4 (2.2) | 48.1 (1.9) | 7.2 (0.8) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Post, P.C.; Schlautman, M.A. Measuring Camellia Petal Color Using a Portable Color Sensor. Horticulturae 2020, 6, 53. https://doi.org/10.3390/horticulturae6030053
Post PC, Schlautman MA. Measuring Camellia Petal Color Using a Portable Color Sensor. Horticulturae. 2020; 6(3):53. https://doi.org/10.3390/horticulturae6030053
Chicago/Turabian StylePost, Phillip C., and Mark A. Schlautman. 2020. "Measuring Camellia Petal Color Using a Portable Color Sensor" Horticulturae 6, no. 3: 53. https://doi.org/10.3390/horticulturae6030053
APA StylePost, P. C., & Schlautman, M. A. (2020). Measuring Camellia Petal Color Using a Portable Color Sensor. Horticulturae, 6(3), 53. https://doi.org/10.3390/horticulturae6030053





