Altered Carbohydrate Allocation Due to Soil Water Deficit Affects Summertime Flowering in Meiwa Kumquat Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Observation of Flowering Behavior and Determination of Total Soluble Sugars and Starch
2.3. Sugar Composition of the Xylem Tissue and Bark Extracts of the Scaffold Branch
2.4. Statistical Analyses
3. Results
3.1. Changes in the Soil Water Content and Leaf Water Potential during SWD Treatment
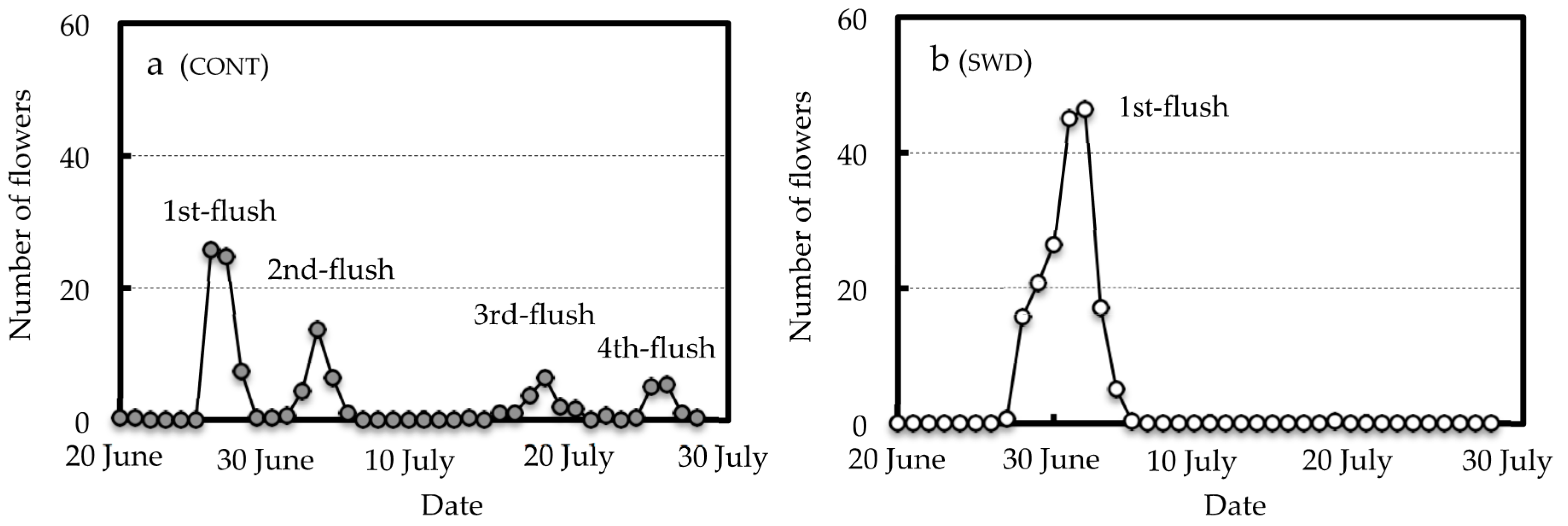
3.2. Effect of SWD Treatment on Flowering Behavior
3.3. Effects of SWD Treatment on the Soluble Sugar, Starch and Water Contents of the Plant Parts
3.4. Relationships between Sugar Content of the Current Spring Stems and the Number of First-Flush Flowers or Leaf Water Potential of the Current Spring Leaves
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hadano, Y. Saibaigijutsu no kiso. Kinkan. In Kajuenngei Dai Hyakka; Jyoryoku Tokusan Kaju, Nousangyosonbunka Kyokai: Tokyo, Japan, 2000; Volume 15, pp. 117–119. [Google Scholar]
- Abbott, C.E. Blossom-bud differentiation in citrus trees. Am. J. Bot. 1935, 22, 476–485. [Google Scholar] [CrossRef]
- Iwahori, S.; Tominaga, S. Increase in first-flush flowering of ‘Meiwa’ kumquat, Fortunella crassifolia Swingle, trees by paclo-butrazol. Sci. Hortic. 1986, 28, 347–353. [Google Scholar] [CrossRef]
- Iwasaki, N.; Hayasaki, K.; Tanaka, S. Effect of Water Stress on Flowering of Meiwa Kumquat Trees. Environ. Control Biol. 2000, 38, 105–109. [Google Scholar] [CrossRef]
- Iwasaki, N.; Yamaguchi, T. Flowering and Yield of Meiwa Kumquat Trees Are Affected by Duration of Water stress. Environ. Control Biol. 2004, 42, 241–245. [Google Scholar] [CrossRef]
- Iwasaki, N.; Hagiwara, H.; Yasuda, N.; Ono, T. Effects of deficit soil water and deficit vapor-pressure on flowering of Meiwa kumquat trees. J. SASJ 2012, 43, 8–14. [Google Scholar]
- Iwasaki, N.; Nakano, Y.; Suzuki, K.; Mochizuki, A. Relationships between the number of first-flush flowers and leaf water potential or leaf ABA content affected by varying degrees of water stress in Meiwa kumquat (Fortunella crassifolia Swingle). Environ. Control Biol. 2017, 55, 59–64. [Google Scholar] [CrossRef][Green Version]
- Iwasaki, N.; Hiratsuka, S. Effect of trunk size on the relationship between drought stress and first-flush flower number in Meiwa kumquat. Environ. Control Biol. 2019, 57, 1–7. [Google Scholar] [CrossRef][Green Version]
- Iwasaki, N.; Hori, K.; Ikuta, Y. Xylem plays an important role in regulating the leaf water potential and fruit quality of Meiwa kumquat (Fortunella crassifolia Swingle) trees under drought conditions. Agric. Water Manag. 2019, 214, 47–54. [Google Scholar] [CrossRef]
- Ono, T.; Hagiwara, H.; Iwasaki, N. Effects of water stress on leaf water potential, flowering, carbohydrate content and ABA content in Meiwa kumquat trees. Hort. Res. 2010, 9, 209–213. [Google Scholar] [CrossRef][Green Version]
- Ono, T.; Hagiwara, H.; Yasuda, N.; Takekawa, H.; Iwasaki, N. Relationship between Atmospheric Relative Humidity and Effectiveness of Water Stress on Flowering in Meiwa Kumquat Trees. Hort. Res. 2012, 11, 81–85. [Google Scholar] [CrossRef][Green Version]
- Chapotin, S.M.; Razanameharizaka, J.H.; Holbrook, N.M. Baobab trees (Adansonia) in Madagascar use stored water to flush new leaves but not to support stomatal opening before the rainy season. New Phytol. 2006, 169, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Van den Bilcke, N.; Simbo, D.J.; Samson, R. Water relations and drought tolerance of young African tamarind (Tamarindus indica L.) trees. S. Afr. J. Bot. 2013, 88, 352–360. [Google Scholar] [CrossRef]
- Van den Bilcke, N.; Smedt, S.D.; Simbo, D.J.; Samson, R. Sap flow and water use in African baobab (Adansonia digitata L.) seedling in response to drought stress. S. Afr. J. Bot. 2013, 88, 438–446. [Google Scholar] [CrossRef]
- Sade, N.; Gebremedhin, A.; Monshelion, M. Risk-taking plants Anisohydric behavior as a stress-resistance trait. Plant Signal. Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.F.; Boyer, J.S. Osmoregulation, solute distribution, and growth in soybean seedlings having low water potentials. Planta 1981, 151, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Ooshiro, A. A simple and rapid analysis of starch content in root and shoot of Satsuma mandarin by using colorimetric determination. Jpn. J. Soil Sci. Plant Nutr. 2001, 72, 81–84. [Google Scholar]
- García-Tejero, I.; Jiménez-Bocanegra, J.A.; Martínez, G.; Romero, R.; Durán-Zuazo, V.H.; Muriel-Fernández, J.L. Positive impact of regulated deficit irrigationon yield and fruit quality in a commercial citrus orchard Citrus sinensis (L.) Osbeck cv. Salustiano. Agric. Water Manag. 2010, 97, 614–622. [Google Scholar] [CrossRef]
- González-Altozano, P.; Castel, J.R. Effects of regulated deficit irrigation on “Clementina de Nules” citrus trees. I. Yield and fruit quality effect. J. Hortic. Sci. Biotech. 1999, 74, 706–713. [Google Scholar] [CrossRef]
- Panigrahi, P.; Srivastava, A.K. Effective management of irrigation water in citrus orchards under a water scarce hot sub-humid region. Sci. Hortic. 2016, 210, 6–13. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.; Romero, P.; Navarro, J.; Botía, P. Response of sweet orange cv. ‘lane late’ to deficit irrigation strategy in two rootstocks. II: Flowering, fruit growth, yield and fruit quality. Irrig. Sci. 2008, 26, 519–529. [Google Scholar] [CrossRef]
- Déjadin, A.; Laurans, F.; Arnaud, D.; Breton, C.; Pilate, G.; Leple, J.C. Wood formation in Angiosperms. C. R. Biol. 2010, 333, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood formation in trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Yakushiji, H.; Nonami, H.; Fukuyama, T.; Ono, S.; Takagi, N.; Hashimoto, Y. Sugar accumulation enhanced by osmoregulation in Satsuma mandarin fruit. J. Am. Soc. Hortic. Sci. 1996, 121, 466–472. [Google Scholar] [CrossRef]
- Kasuga, J.; Fujiwara, S.; Arakawa, K. Seasonal changes in accumulation of soluble sugars in birch xylem tissues. Cryobiol. Cryotechnol. 2003, 49, 185–189. [Google Scholar]
- Santarius, K.A. The protective effect of sugars on chloroplast membranes during temperature and water stress and its relationship to frost, desiccation and heat resistance. Planta 1973, 113, 105–114. [Google Scholar] [CrossRef]
- Garcia-Luis, A.; Fornes, F.; Guardiola, J.L. Leaf carbohydrates and flower formation in Citrus. J. Am. Soc. Hortic. Sci. 1995, 120, 222–227. [Google Scholar] [CrossRef]
- Yahata, D.; Oba, Y.; Kuwahara, M. Changes in carbohydrate level, α-amylase activity, indoleacetic acid and gibberellin-like substances in the summer shoots of Wase Satsuma mandarin trees grown indoors during flower-bud differentiation. J. Jpn. Soc. Hortic. Sci. 1995, 64, 527–533. [Google Scholar] [CrossRef][Green Version]
- Nii, N.; Okamoto, S. Effects of leaf age and defoliation on tree growth, flowering and fruit set of Satsuma mandarin. J. Jpn. Soc. Hortic. Sci. 1973, 42, 7–12. [Google Scholar] [CrossRef][Green Version]
- Marquat, C.; Vandamme, M.; Gendraud, M.; Petel, G. Dormancy in vegetative buds of peach: Relation between carbohydrate absorption potentials and carbohydrate concentration in the bud during dormancy and its release. Sci. Hortic. 1999, 79, 151–162. [Google Scholar] [CrossRef]
- Nakajima, Y.; Susanto, S.; Hasegawa, K. Influence of water stress in autumn on flower induction and fruiting in young pomelo trees (Citrus grandis (L.) Osbeck). J. Jpn. Soc. Hortic. Sci. 1993, 62, 15–20. [Google Scholar] [CrossRef]
- Astegiano, E.D.; Maestri, M.; Estevao, M.D.M. Water stress and dormancy release in flower buds of Coffea arabica L.: Water movement into the buds. J. Hortic. Sci. 1988, 63, 529–533. [Google Scholar] [CrossRef]
- Barbier, F.F.; Lunn, J.E.; Beveridge, C.A. Ready, steady, go! A sugar hit starts the race to shoot branching. Curr. Opin. Plant Biol. 2015, 25, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.F.; Dun, E.A.; Kerr, S.C.; Chabikwa, T.G.; Beveridge, C.A. An update on the signals controlling shoot branching. Trends Plant Sci. 2019, 24, 220–236. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, G.; Andrade, J.L.; Meinzer, F.C.; Holbrook, N.M.; Cavelier, J.; Jackson, P.; Celis, A. Stem water storage and diurnal patterns of water use in tropical forest canopy trees. Plant Cell Environ. 1998, 21, 397–406. [Google Scholar] [CrossRef]
- Stratton, L.; Goldstein, G.; Meinzer, F.C. Stem water storage capacity and efficiency of water transport: Their functional significance in a Hawaiian dry forest. Plant Cell Environ. 2000, 23, 99–106. [Google Scholar] [CrossRef]


| 8 Jun | 11 Jun | 14 Jun | |
|---|---|---|---|
| Soil water content (%) | |||
| CONT | 41.6 z | 39.2 | 45.1 |
| SWD | 32.7 | 9.3 | 12.1 |
| Significance | ns y | * | * |
| Leaf water potential (Mpa) | |||
| CONT | −1.75 | −1.61 | −1.62 |
| SWD | −1.92 | −2.38 | −2.73 |
| Significance | ns | * | * |
| 1st-Flush Flowers z | Total Flowers | |
|---|---|---|
| CONT | 58.0 y | 113.7 |
| SWD | 177.0 | 177.3 |
| Significance | * x | ns |
| Sucrose | Glucose | Fructose | Total | |
|---|---|---|---|---|
| Xylem tissue | ||||
| CONT | 3.4 z | 1.4 | 1.7 | 6.5 |
| SWD | 13.5 (4.0) y | 9.6 (6.6) | 9.8 (5.7) | 32.8 (5.0) |
| significance | * x | ** | ** | ** |
| Bark | ||||
| CONT | 21.0 | 0.5 | 1.7 | 20.4 |
| SWD | 25.1 (1.2) | 1.1 (2.1) | 2.0 (1.2) | 28.3 (1.4) |
| significance | ns | ns | ns | ns |
| Number of 1st-Flush Flowers | Current Leaf Water Potential | |
|---|---|---|
| Scaffold branch | ||
| Xylem | 0.8385 * z | −0.8727 * z |
| Bark | 0.8395 * | −0.9011 * |
| Stems | ||
| Previous year stems | 0.8662 * | −0.9565 ** |
| Current-year spring stems | 0.9584 ** | −0.9567 ** |
| Leaves | ||
| Previous year leaves | 0.7015 | −0.9400 ** |
| Current-year spring leaves | 0.7055 | −0.7649 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, N.; Tamura, A.; Hori, K. Altered Carbohydrate Allocation Due to Soil Water Deficit Affects Summertime Flowering in Meiwa Kumquat Trees. Horticulturae 2020, 6, 49. https://doi.org/10.3390/horticulturae6030049
Iwasaki N, Tamura A, Hori K. Altered Carbohydrate Allocation Due to Soil Water Deficit Affects Summertime Flowering in Meiwa Kumquat Trees. Horticulturae. 2020; 6(3):49. https://doi.org/10.3390/horticulturae6030049
Chicago/Turabian StyleIwasaki, Naoto, Asaki Tamura, and Kyoka Hori. 2020. "Altered Carbohydrate Allocation Due to Soil Water Deficit Affects Summertime Flowering in Meiwa Kumquat Trees" Horticulturae 6, no. 3: 49. https://doi.org/10.3390/horticulturae6030049
APA StyleIwasaki, N., Tamura, A., & Hori, K. (2020). Altered Carbohydrate Allocation Due to Soil Water Deficit Affects Summertime Flowering in Meiwa Kumquat Trees. Horticulturae, 6(3), 49. https://doi.org/10.3390/horticulturae6030049




