Microbial Effects on the Production of Aquaponically Grown Lettuce
Abstract
:1. Introduction
2. Materials and Methods
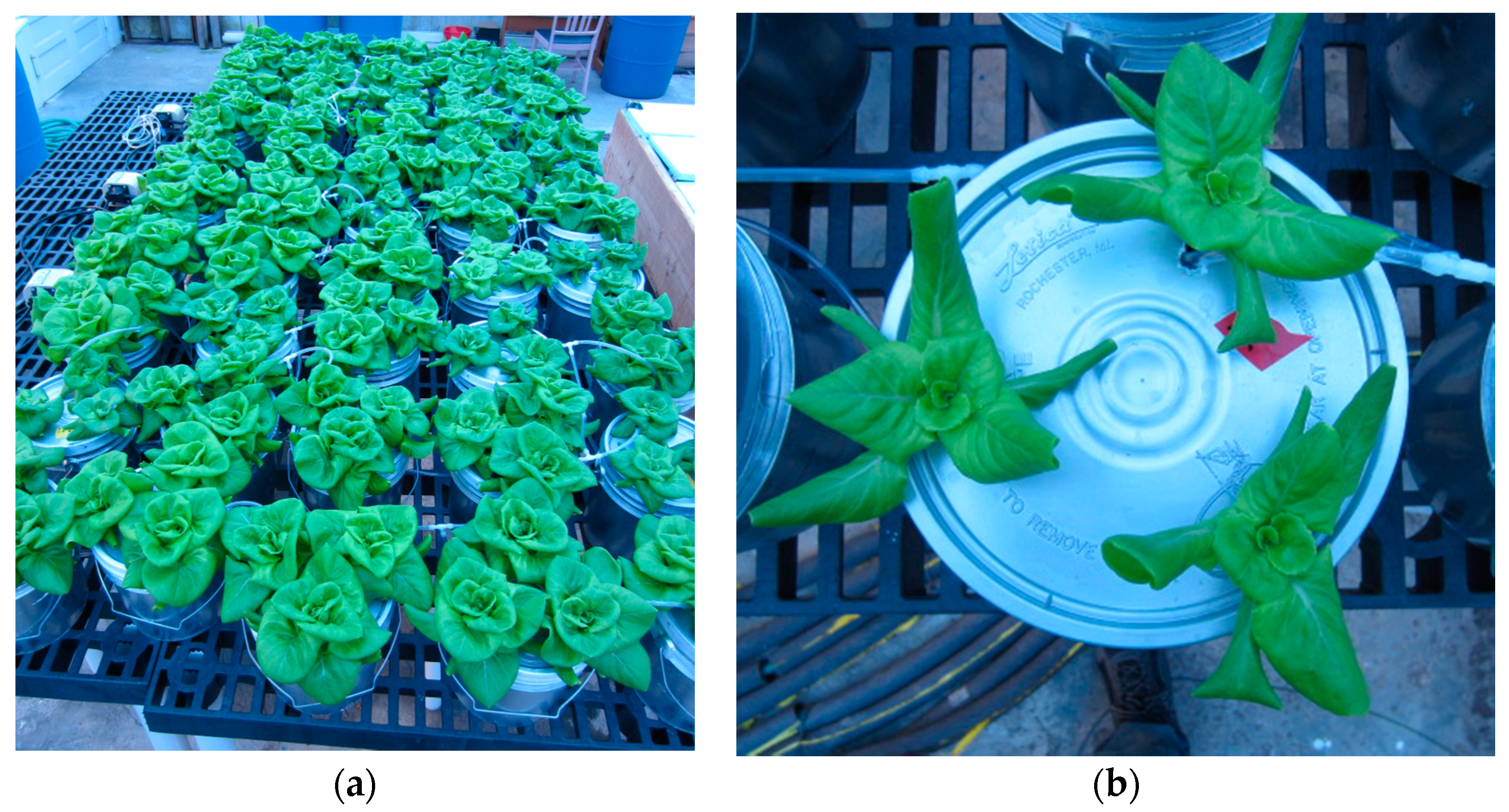
2.1. Experimental Setup
2.2. Treatments
2.3. Experimental Protocol
2.4. Measurements
2.5. Statistical Analysis
3. Results
3.1. Sterilization Verification
3.2. Lettuce Response to Treatment
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pantanella, E.; Cardarelli, M.; Colla, G.; Rea, E.; Marcucci, A. Aquaponics vs. Hydroponics: Production and Quality of Lettuce Crop. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC 2010): International Symposium on Greenhouse 2010 and Soilless Cultivation, Lisbon, Portugal, 22–27 August 2010; Castilla, N., Ed.; International Society for Horticultural Science: Leuven, Belgium, 2012; pp. 887–893. [Google Scholar]
- Timmons, M.; Ebeling, J. Recirculating Aquaculture, 3rd ed.; Ithaca Publishing Company LLC: Ithaca, NY, USA, 2013. [Google Scholar]
- Rakocy, J.E.; Shultz, R.C.; Bailey, D.S.; Thoman, E.S. Aquaponic Production of Tilapia and Basil: Comparing a Batch and Staggered Cropping System. In Proceedings of the South Pacific Soilless Culture Conference, Palmerston North, New Zealand, 10–13 February 2003; Nichols, M.A., Ed.; International Society for Horticultural Science: Leuven, Belgium, 2004; pp. 63–69. [Google Scholar]
- Goddek, S.; Schmautz, Z.; Scott, B.; Delaide, B.; Keesman, K.J.; Wuertz, S.; Junge, R. The effect of anaerobic and aerobic fish sludge supernatant on hydroponic lettuce. Agronomy 2016, 6, 37. [Google Scholar] [CrossRef]
- Rakocy, J.E. Aquaponics—Integrating Fish and Plant Culture; Oklahoma Cooperative Extension Service: Stillwater, MN, USA, 2012. [Google Scholar]
- Tokuyama, T.; Mine, A.; Kamiyama, K.; Yabe, R.; Satoh, K.; Matsumoto, H.; Takahashi, R.; Itonaga, K. Nitrosomonas communis strain YNSRA, an ammonia-oxidizing bacterium, isolated from the reed rhizoplane in an aquaponics plant. J. Biosci. Bioeng. 2004, 98, 309–312. [Google Scholar] [CrossRef]
- Anderson, T.S.; de Villiers, D.; Timmons, M.B. Growth and tissue elemental composition response of butterhead lettuce (Lactuca sativa, cv. Flandria) to Hydroponic and Aquaponic Conditions. Horticulturae 2017, 3, 43. [Google Scholar] [CrossRef]
- Sonneveld, C.; Straver, N. Nutrient solutions for vegetables and flowers grown in water of substrates. In Series: Voedingsoplossingen Glastuinbouw; Proefstation voor Tuinbouw onder Glas te Naaldwijk: Naaldwijk, The Netherlands, 1994; Volume 8, pp. 1–45. [Google Scholar]
- Anderson, T.S.; Martini, M.; de Villiers, D.; Timmons, M.B. Growth and tissue elemental composition response of Butterhead lettuce (Lactuca sativa, cv. Flandria) to hydroponic conditions at different pH and alkalinity. Horticulturae 2017, 3, 41. [Google Scholar] [CrossRef]
- Vandam, D.; Anderson, T.S.; de Villiers, D.; Timmons, M.B. Growth and tissue elemental composition response of spinach (Spinacia oleracea) to hydroponic and aquaponic water quality conditions. Horticulturae 2017, 3, 32. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 3. [Google Scholar] [CrossRef]
- Consolidated Sterilizer Systems. The Definitive Guide to Steam Sterilization Cycles; Consolidated Sterilizer Systems: Boston, MA, USA; p. 10. Available online: https://consteril.com/steam-sterilization-cycles-guide/ (accessed on 7 August 2017).
- Turner, D.E.; Daugherity, E.K.; Altier, C.; Maurer, K.J. Efficacy and limitations of an ATP-based monitoring system. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 190–195. [Google Scholar] [PubMed]
- Ling, J.; Chen, S. Impact of organic carbon on nitrification performance of different biofilters. Aquac. Eng. 2005, 33, 150–162. [Google Scholar] [CrossRef]
- Chen, Y.; Aviad, T. Effects of humic substances on plant growth 1. In Humic Substances in Soil and Crop Sciences; MacCarthy, P., Clapp, C.E., Malcolm, R.L., Bloom, P.R., Eds.; Selected Readings, SSSA: Madison, WI, USA, 1990; pp. 161–186. [Google Scholar]


| Treatment | Description |
|---|---|
| H5 | ½ Sonneveld nutrient solution at pH 5.8 (control treatment) |
| H7 | ½ Sonneveld nutrient solution at pH 7.0 |
| A7 | Untreated aquaponic water at pH 7.0 |
| SA7 | Sterilized aquaponic water at pH 7.0 |
| Element (mg/L) | A7 | H5 | H7 |
|---|---|---|---|
| Macronutrients | |||
| K | 214 | 216 | 219 |
| Ca | 87.6 a | 93.8 b | 94.0 b |
| Mg | 22.6 c | 12.8 a | 13.1 a |
| S | 21.0 | 19.3 | 19.6 |
| Na | 19.34 c | 4.73 a | 5.59 a |
| P | 12.4 | 30.5 | 31.1 |
| NO3-N | 135.1 | 149.7 | 154.7 |
| TAN | 1.1 | 8.9 | 9.5 |
| Micronutrients | |||
| Fe | 2.891 c | 0.868 a | 0.870 a |
| Sr | 0.552 | 0.586 | 0.590 |
| Zn | 0.517 b | 0.182 a | 0.184 a |
| Cu | 0.045 | 0.050 | 0.050 |
| Co | 0.003 b | 0.001 a | 0.001 a |
| Mn | 0.033 a | 0.162 c | 0.157 c |
| Mo | 0.002 | 0.025 | 0.026 |
| Other elements | |||
| Al | 0.100 | 0.090 | 0.090 |
| Ba | 0.018 | 0.016 | 0.011 |
| As | 0.010 | 0.006 | 0.006 |
| Pb | 0.010 c | 0.000 a | 0.000 a |
| Cd | 0.004 | 0.003 | 0.003 |
| V | 0.0029 | 0.0025 | 0.0025 |
| Treatment | Relative Light Units |
|---|---|
| H5, H7 | 47,093 |
| A7 | 2,537,609 |
| SA7 | 8484 |
| Trial | Treatment | n | Fresh Weight (g) | Dry Weight (g) | DW/FW |
|---|---|---|---|---|---|
| 1 | H5 | 30 | 24.9 (3.71) | 1.61 (0.192) | 0.065 (0.004) |
| 1 | H7 | 30 | 13.6 (2.43) | 1.06 (0.140) | 0.079 (0.009) |
| 1 | A7 | 30 | 14.1 (4.52) | 1.04 (0.260) | 0.077 (0.013) |
| 1 | SA7 | 30 | 22.5 (2.99) | 1.40 (0.151) | 0.063 (0.003) |
| 2 | H5 | 30 | 34.6 (6.53) | 1.74 (0.310) | 0.051 (0.002) |
| 2 | H7 | 30 | 15.2 (5.03) | 1.00 (0.254) | 0.068 (0.008) |
| 2 | A7 | 30 | 33.5 (6.41) | 1.67 (0.273) | 0.050 (0.003) |
| 2 | SA7 | 30 | 31.8 (9.11) | 1.62 (0.426) | 0.052 (0.004) |
| Combined | H5 | 60 | 29.8 (7.19) | 1.68 (0.264) | 0.058 (0.008) |
| Combined | H7 | 60 | 14.4 (4.01) | 1.03 (0.206) | 0.073 (0.010) |
| Combined | A7 | 60 | 23.8 (11.2) | 1.35 (0.414) | 0.064 (0.017) |
| Combined | SA7 | 60 | 27.2 (8.18) | 1.51 (0.336) | 0.057 (0.007) |
| Treatment | Fresh Weight (g) | Dry Weight (g) | Fraction Dry |
|---|---|---|---|
| H5 | 29.76 a | 1.676 a | 0.0577 c |
| H7 | 14.38 c | 1.031 c | 0.0734 a |
| A7 | 23.77 b | 1.354 b | 0.0637 b |
| SA7 | 27.16 a,b | 1.514 a,b | 0.0571 c |
| Treatment | Fresh Weight Ratio | Dry Weight Ratio | Dry to Fresh Ratio |
|---|---|---|---|
| H5 | 1.39 | 1.08 | 0.78 |
| H7 | 1.12 | 0.94 | 0.85 |
| A7 | 2.38 | 1.61 | 0.65 |
| SA7 | 1.41 | 1.16 | 0.83 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wielgosz, Z.J.; Anderson, T.S.; Timmons, M.B. Microbial Effects on the Production of Aquaponically Grown Lettuce. Horticulturae 2017, 3, 46. https://doi.org/10.3390/horticulturae3030046
Wielgosz ZJ, Anderson TS, Timmons MB. Microbial Effects on the Production of Aquaponically Grown Lettuce. Horticulturae. 2017; 3(3):46. https://doi.org/10.3390/horticulturae3030046
Chicago/Turabian StyleWielgosz, Zachary J., Tyler S. Anderson, and Michael B. Timmons. 2017. "Microbial Effects on the Production of Aquaponically Grown Lettuce" Horticulturae 3, no. 3: 46. https://doi.org/10.3390/horticulturae3030046
APA StyleWielgosz, Z. J., Anderson, T. S., & Timmons, M. B. (2017). Microbial Effects on the Production of Aquaponically Grown Lettuce. Horticulturae, 3(3), 46. https://doi.org/10.3390/horticulturae3030046




