Optimizing Selenium Delivery in Grapevines: Foliar vs. Rhizosphere Fertilization Effects on Photosynthetic Efficiency, Fruit Metabolites, and VOCs of ‘Muscat Hamburg’ Grape (Vitis vinifera L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Photosynthetic Pigments Content and Fluorescence Analysis
2.4. Fruit Appearance and Biochemical Properties
2.5. Volatile Organic Compounds Analyses
2.6. Statistical Analysis
3. Results
3.1. Leaf Photosynthetic Pigments
3.2. Leaf Chlorophyll Fluorescence
3.3. Fruit Appearance Traits
3.4. Fruit Flavor Qualities
3.5. Berry Antioxidant Substances
3.6. Volatile Organic Compounds
3.7. Correlation Between Fertilization Method and Grape Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| t(Fm) | Time required to reach maximum fluorescence after illumination following dark adaptation |
| Fv | Variable Fluorescence |
| Fo | Fixed Fluorescence |
| Fm | Maximum fluorescence |
| F1, F2, F3, F4, F5 | The fluorescence values at 50 μs, 100 μs, 300 μs, 2 ms, and 30 ms are represented as the O/J/I/P phases |
| Fv/Fm | Maximum efficiency of photochemistry in Photosystem II |
| Fv/Fo | Photosystem II activity |
| Vj | The relative variable fluorescence intensity at the J-phase |
| Vi | The relative variable fluorescence intensity at the I-phase |
| Sm | The normalized O-J-I-P fluorescence induction curve, the fluorescence intensity F equal to FM, and the area between the curve and the y-axis |
| Sm/(tFm) | Reduction rate of the plastoquinone pool |
| (ABS/TRo/ETo/DIo)/RC | Energy absorbed per reaction center/Energy captured by the reaction center/Energy used for electron transport/Energy dissipated as heat |
| (ABS/TRo/ETo/DIo)/CSo | Energy absorbed per unit excited state area/Energy captured by reaction centers/Energy used for electron transport/Energy dissipated as heat (at t = 0) |
| (ABS/TRo/ETo/DIo)/CSm | Energy absorbed per unit excited state area/Energy captured by reaction centers/Energy used for electron transport/Energy dissipated as heat (at t = m) |
| φPo | Maximum efficiency of photochemistry (at t = 0) |
| φDo | Quantum Ratio of Heat Dissipation (at t = 0) |
| φEo | Quantum Yield of Electron Transport (at t = 0) |
| ψO | The ratio of excitons used to drive electron transport beyond QA to other electron acceptors in the electron transport chain, to the excitons used to reduce QA, among the excitons captured by the reaction center (at t = 0). |
| PIABS | Photochemical Performance Index based on Absorption |
| PI total | Overall Photosynthetic Performance Index |
| dVG/dto | The net closure rate of the photoreaction center at 100 μs; |
| dV/dto | The net closure rate of the photoreaction center at 300 μs |
| N | Time-dependent turnover number of QA |
| RC | Active reaction center |
References
- Feng, R.W.; Wei, C.Y.; Tu, S.X. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Fontanella, M.C.; D’Amato, R.; Regni, L.; Proietti, P.; Beone, G.M.; Businelli, D. Selenium speciation profiles in biofortified sangiovese wine. J. Trace Elem. Med. Biol. 2017, 43, 87–92. [Google Scholar] [CrossRef]
- Tedeschini, E.; Proietti, P.; Timorato, V.; D’Amato, R.; Nasini, L.; Buono, D.D.; Businelli, D.; Frenguelli, G. Selenium as stressor and antioxidant affects pollen performance in Olea europaea. Flora 2015, 215, 16–22. [Google Scholar] [CrossRef]
- Zhu, S.M.; Liang, Y.L.; Gao, D.K.; An, X.J.; Kong, F.C. Spraying foliar selenium fertilizer on quality of table grape (Vitis vinifera L.) from different source varieties. Sci. Hortic. 2017, 218, 87–94. [Google Scholar] [CrossRef]
- Cartes, P.; Jara, A.A.; Pinilla, L.; Rosas, A.; Mora, M.L. Selenium improves the antioxidant ability against aluminium-induced oxidative stress in ryegrass roots. Ann. Appl. Biol. 2010, 156, 297–307. [Google Scholar] [CrossRef]
- Regni, L.; Micheli, M.; Del Pino, A.M.; Palmerini, C.A.; D’Amato, R.; Facchin, S.L.; Famiani, F.; Peruzzi, A.; Mairech, H.; Proietti, P. The first evidence of the beneficial effects of Se-supplementation on in vitro cultivated olive tree explants. Plants 2021, 10, 1630. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, R.; De Feudis, M.; Hasuoka, P.E.; Regni, L.; Pacheco, P.H.; Onofri, A.; Businelli, D.; Proietti, P. The selenium supplementation influences olive tree production and oil stability against oxidation and can alleviate the water deficiency effects. Front. Plant Sci. 2018, 9, 1191. [Google Scholar] [CrossRef] [PubMed]
- Regni, L.; Palmerini, C.A.; Del Pino, A.M.; Businelli, D.; D’Amato, R.; Mairech, H.; Marmottini, F.; Micheli, M.; Pacheco, P.H.; Proietti, P. Effects of selenium supplementation on olive under salt stress conditions. Sci. Hortic. 2021, 278, 109866. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Biochem. 2019, 137, 144–153. [Google Scholar] [CrossRef]
- Wang, Y.N.; Yi, C.J.; Wang, Y.X.; Wang, X. Effects of selenium fertilizer on fruit quality and plant resistance of blueberry. In Proceedings of the 1st International Conference on Environment Prevention and Pollution Control Technology (EPPCT), Tokyo, Japan, 9–11 November 2018; IOP Publishing Ltd.: Bristol, UK, 2018. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium enrichment enhances the quality and shelf life of Basil leaves. Plants-Basel 2020, 9, 801. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.; Leduc, D.L. Phytoremediation of selenium using transgenic plants. Curr. Opin. Biotechnol. 2009, 20, 207–212. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; pp. 123–150. [Google Scholar]
- Nadeem, S.; Muhammad, A.; Asif Kamran, M.; Muhammad, I.; Atif Riaz, M.; Kashif, K.; Saddam, H. Selenium biofortification in food crops: Key mechanisms and future perspectives. J. Food Compos. Anal. 2020, 93, 103615. [Google Scholar] [CrossRef]
- Zhu, S.M.; Liang, Y.L.; Mu, L.; An, X.J.; Yin, H.F. 1-Methylcyclopropene on fruit quality of Se-enriched grape (Vitis vinifera L.) during shelf life period. Agronomy 2020, 10, 1411. [Google Scholar] [CrossRef]
- Lin, L.J.; Li, Z.Y.; Wang, J.; Liang, D.; Xia, H.; Lv, X.L.; Tang, Y.; Wang, X.; Deng, Q.X.; Liao, M.A. 24-epibrassinolide promotes selenium uptake in grapevine under selenium stress. Sci. Hortic. 2023, 308, 111564. [Google Scholar] [CrossRef]
- Liu, L.; Han, J.X.; Deng, L.L.; Zhou, H.X.; Bie, Y.H.; Jing, Q.H.; Lin, L.J.; Wang, J.; Liao, M.A. Effects of diethyl aminoethyl hexanoate on the physiology and selenium absorption of grape seedlings. Acta Physiol. Plant. 2021, 43, 115. [Google Scholar] [CrossRef]
- Feng, T.; Chen, S.S.; Gao, D.Q.; Liu, G.Q.; Bai, H.X.; Li, A.; Peng, L.X.; Ren, Z.Y. Selenium improves photosynthesis and protects photosystem II in pear (Pyrus bretschneideri), grape (Vitis vinifera), and peach (Prunus persica). Photosynthetica 2015, 53, 609–612. [Google Scholar] [CrossRef]
- Liu, Y.P.; Ren, G.; Deng, B.; Di, J.B.; Wang, Y. Unveiling the mechanisms of aroma metabolism in selenium-treated broccoli through transcriptome sequencing analyses. Sci. Hortic. 2023, 314, 111930. [Google Scholar] [CrossRef]
- Golubkina, N.; Logvinenko, L.; Konovalov, D.; Garsiya, E.; Fedotov, M.; Alpatov, A.; Shevchuk, O.; Skrypnik, L.; Sekara, A.; Caruso, G. Foliar application of selenium under nano silicon on Artemisia annua: Effects on yield, antioxidant status, essential oil, artemisinin content and mineral composition. Horticulturae 2022, 8, 597. [Google Scholar] [CrossRef]
- Ma, Y.; Yin, J.J.; Wang, J.Y.; Liu, X.; He, J.R.; Zhang, R.; Rao, S.; Cong, X.; Xiong, Y.; Wu, M.C. Selenium speciation and volatile flavor compound profiles in the edible flowers, stems, and leaves of selenium-hyperaccumulating vegetable Cardamine violifolia. Food Chem. 2023, 427, 136710. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, G.; Zhao, X.; Wang, W.; Di, J.; Wang, Y.; Lin, W. Selenium-chitosan treatment affects amino acid content and volatile components of red globe grape during storage. J. Food Process. Preserv. 2023, 2023, 3848092. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Advances in Photosynthesis and Respiration. Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Souza, A.F.C.; Martins, J.P.R.; Gontijo, A.; Falqueto, A.R. Selenium improves the transport dynamics and energy conservation of the photosynthetic apparatus of in vitro grown Billbergia zebrina (Bromeliaceae). Photosynthetica 2019, 57, 931–941. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.H.; Tian, S.F.; Wang, R.; Wang, C.X. Impact of the plant growth-promoting rhizobacterium Streptomyces saraceticus strain 31 on berry quality of ‘Benifuji’ Grape: Improvements through the reconfiguration of fine root morphology and vessel anatomy. HortScience 2024, 59, 1077–1087. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Kiani, R.; Arzani, A.; Maibody, S. Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Front. Plant Sci. 2021, 12, 646221. [Google Scholar] [CrossRef]
- Hunter, J.J.; Volschenk, C.G.; Mania, E.; Castro, A.V.; Booyse, M.; Guidoni, S.; Pisciotta, A.; Di Lorenzo, R.; Novello, V.; Zorer, R. Grapevine row orientation mediated temporal and cumulative microclimatic effects on grape berry temperature and composition. Agric. For. Meteorol. 2021, 310, 108660. [Google Scholar] [CrossRef]
- Chen, T.C.; Xu, T.; Shen, L.Y.; Zhang, T.Y.; Wang, L.R.; Chen, Z.H.; Wu, Y.Y.; Yang, J. Effects of girdling and foliar fertilization with K on physicochemical parameters, phenolic and volatile composition in ‘Hanxiangmi’ table grape. Horticulturae 2022, 8, 388. [Google Scholar] [CrossRef]
- Ji, X.H.; Wang, B.L.; Wang, X.D.; Wang, X.L.; Liu, F.Z.; Wang, H.B. Differences of aroma development and metabolic pathway gene expression between Kyoho and 87-1 grapes. J. Integr. Agric. 2021, 20, 1525–1539. [Google Scholar] [CrossRef]
- Pezzarossa, B.; Remorini, D.; Gentile, M.L.; Massai, R. Effects of foliar and fruit addition of sodium selenate on selenium accumulation and fruit quality. J. Sci. Food Agric. 2012, 92, 781–786. [Google Scholar] [CrossRef]
- Zhong, Y.; Cheng, J.J. Effects of selenite on unicellular green microalga Chlorella pyrenoidosa: Bioaccumulation of selenium, enhancement of photosynthetic pigments, and amino acid production. J. Agric. Food Chem. 2017, 65, 10875–10883. [Google Scholar] [CrossRef]
- Filek, M.; Gzyl-Malcher, B.; Zembala, M.; Bednarska, E.; Laggner, P.; Kriechbaum, M. Effect of selenium on characteristics of rape chloroplasts modified by cadmium. J. Plant Physiol. 2010, 167, 28–33. [Google Scholar] [CrossRef]
- Malik, J.A.; Kumar, S.; Thakur, P.; Sharma, S.; Kaur, N.; Kaur, R.; Pathania, D.; Bhandhari, K.; Kaushal, N.; Singh, K.; et al. Promotion of growth in Mungbean (Phaseolus aureus Roxb.) by selenium is associated with stimulation of carbohydrate metabolism. Biol. Trace Elem. Res. 2011, 143, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bansal, A.; Dhillon, S.K.; Dhillon, K.S. Comparative effects of selenate and selenite on growth and biochemical composition of rapeseed (Brassica napus L.). Plant Soil 2010, 329, 339–348. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E.; Kalemba, E. The protective role of selenium in recalcitrant Acer saccharium L. seeds subjected to desiccation. J. Plant Physiol. 2011, 168, 220–225. [Google Scholar] [CrossRef]
- Malik, J.A.; Goel, S.; Kaur, N.; Sharma, S.; Singh, I.; Nayyar, H. Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ. Exp. Bot. 2012, 77, 242–248. [Google Scholar] [CrossRef]
- Habibi, G. Selenium ameliorates salinity stress in Petroselinum crispum by modulation of photosynthesis and by reducing shoot Na accumulation. Russ. J. Plant Physiol. 2017, 64, 368–374. [Google Scholar] [CrossRef]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1428–1438. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Lukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Chen, Q.W.; Guan, T.S.; Yun, L.; Li, R.N.; Recknagel, F. Online forecasting chlorophyll a concentrations by an auto-regressive integrated moving average model: Feasibilities and potentials. Harmful Algae 2015, 43, 58–65. [Google Scholar] [CrossRef]
- Sucu, S.; Yagci, A. Effects of selenium treatments on physical and chemical traits of some grape cultivars. Erwerbs-Obstbau 2023, 65, 2055–2062. [Google Scholar] [CrossRef]
- Zhu, S.M.; Liang, Y.L.; An, X.J.; Kong, F.C.; Yin, H.F. Response of fruit quality of table grape (Vitis vinifera L.) to foliar selenium fertilizer under different cultivation microclimates. Eur. J. Hortic. Sci. 2019, 84, 332–342. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, S.H.; Huang, X.; Zhang, F.B.; Pang, Y.W.; Huang, Q.Y.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Proietti, P.; Nasini, L.; Del Buono, D.; D’Amato, R.; Tedeschini, E.; Businelli, D. Selenium protects olive (Olea europaea L.) from drought stress. Sci. Hortic. 2013, 164, 165–171. [Google Scholar] [CrossRef]
- Owusu-Sekyere, A.; Kontturi, J.; Hajiboland, R.; Rahmat, S.; Aliasgharzad, N.; Hartikainen, H.; Seppänen, M. Influence of selenium (Se) on carbohydrate metabolism, nodulation and growth in alfalfa (Medicago sativa L.). Plant Soil 2013, 373, 541–552. [Google Scholar] [CrossRef]
- Luo, X.Y.; Wu, B.; Peng, P.; Ren, L.L.; Xia, M.L.; Chen, J.H. Determining and analyzing content of invert sugar and reducing sugar and total sugar and vitamin C to ganzhou selenium-rich navel oranges. China Food Addit. 2011, 4, 203–208. (In Chinese) [Google Scholar]
- Haghighi, M.; Ramezani, M.R.; Rajaii, N. Improving oxidative damage, photosynthesis traits, growth and flower dropping of pepper under high temperature stress by selenium. Mol. Biol. Rep. 2019, 46, 497–503. [Google Scholar] [CrossRef]
- Del Pino, A.M.; Regni, L.; Reale, L.; Micheli, M.; Datti, A.; Proietti, P.; Palmerini, C.A. Selenium preserves cytosolic-Ca2+ homeostasis in olive callus cells during oxidative stress. Plant Cell, Tissue Organ Cult. 2023, 154, 519–525. [Google Scholar] [CrossRef]
- Karimi, R.; Ghabooli, M.; Rahimi, J.; Amerian, M. Effects of foliar selenium application on some physiological and phytochemical parameters of Vitis vinifera L. cv. Sultana under salt stress. J. Plant Nutr. 2020, 43, 2226–2242. [Google Scholar] [CrossRef]
- Gul, H.; Kinza, S.; Shinwari, Z.K.; Hamayun, M. Effect of selenium on the biochemistry of Zea mays under salt stress. Pak. J. Bot. 2017, 49, 25–32. [Google Scholar]
- Hawrylak-Nowak, B.; Dresler, S.; Rubinowska, K.; Matraszek-Gawron, R.; Woch, W.; Hasanuzzaman, M. Selenium biofortification enhances the growth and alters the physiological response of lamb’s lettuce grown under high temperature stress. Plant Physiol. Biochem. 2018, 127, 446–456. [Google Scholar] [CrossRef]
- Yue, X.; Ju, Y.; Zhang, H.; Wang, Z.; Xu, H.; Zhang, Z. Integrated transcriptomic and metabolomic analysis reveals the changes in monoterpene compounds during the development of Muscat Hamburg (Vitis vinifera L.) grape berries. Food Res. Int. 2022, 162, 112065. [Google Scholar] [CrossRef] [PubMed]
- Javadi, F.; Kalatejari, S.; Diyanat, M. Effect of foliar or soil application of selenium on some morphological and physiological traits of garden pansy (Viola xwittrockiana Gams) grown under salinity stress. Acta Agric. Slov. 2020, 115, 357–368. [Google Scholar] [CrossRef]
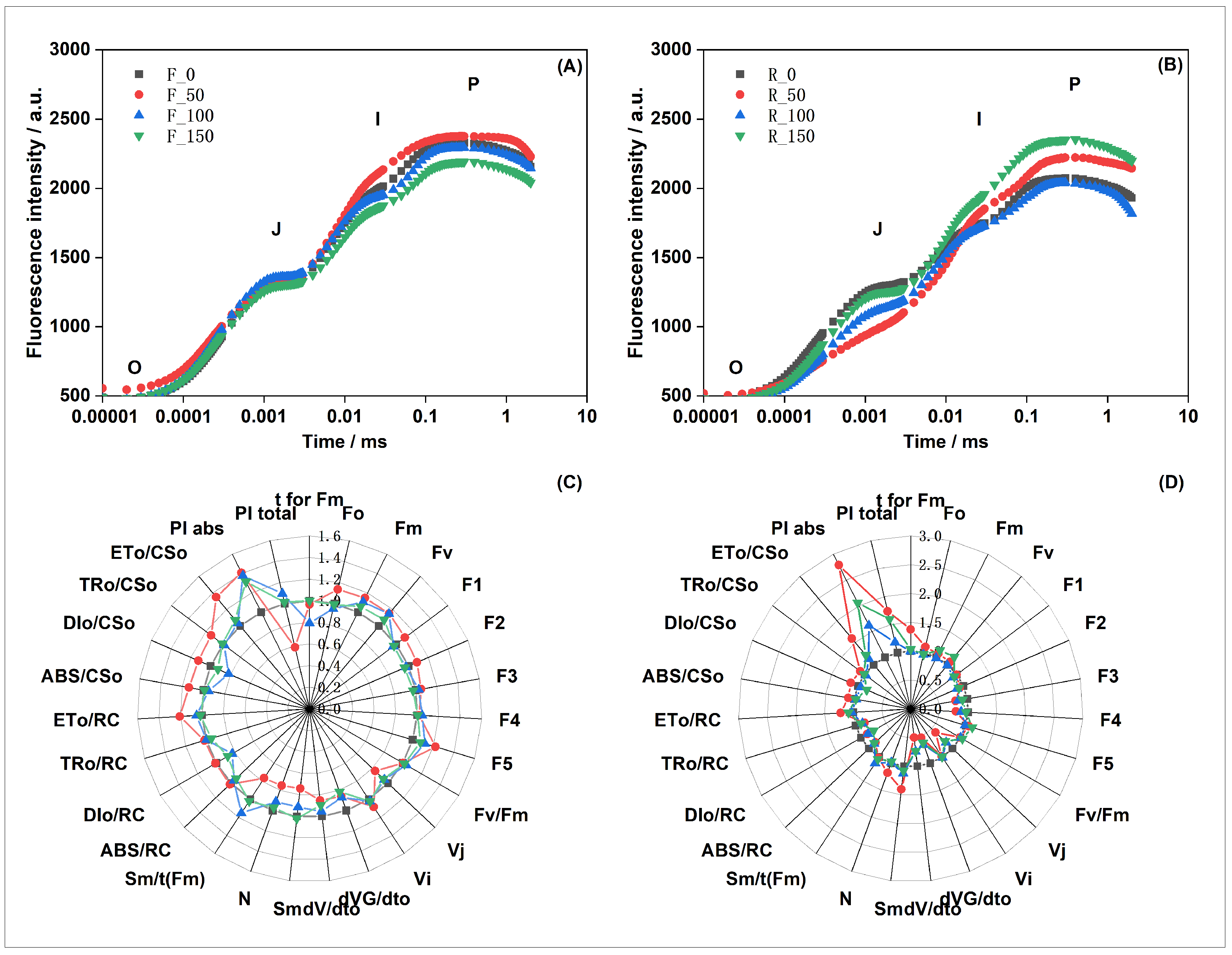

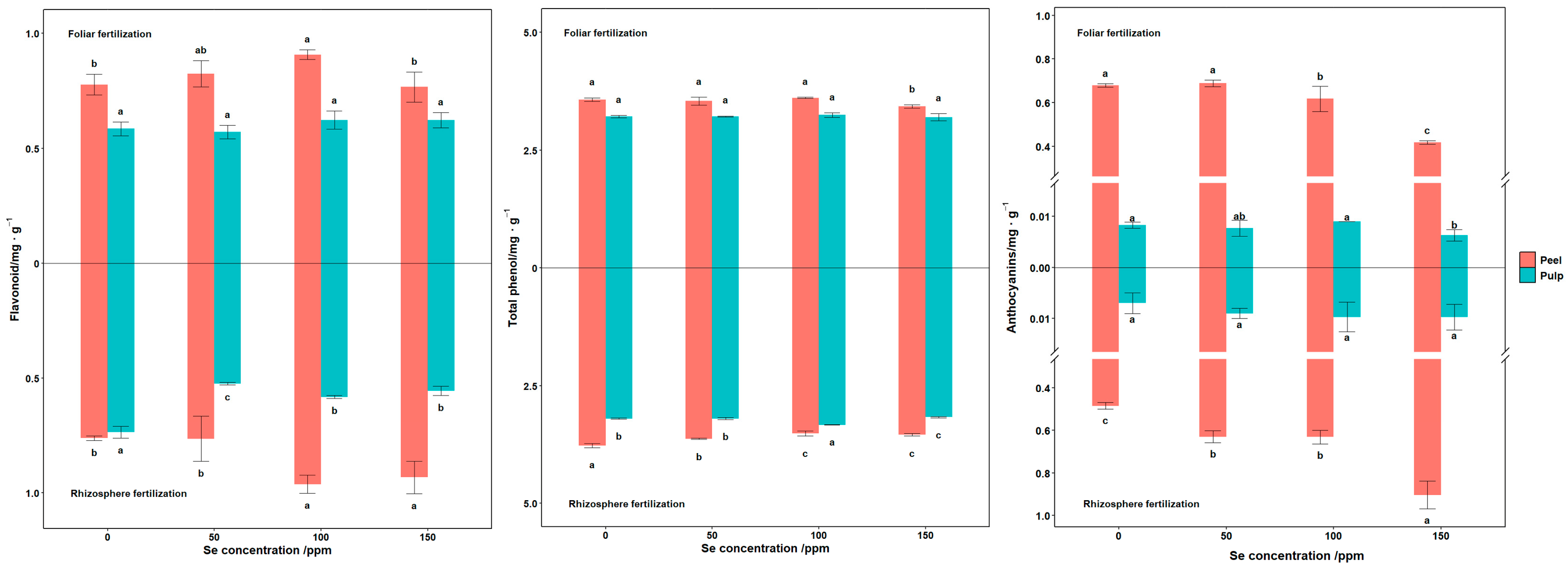

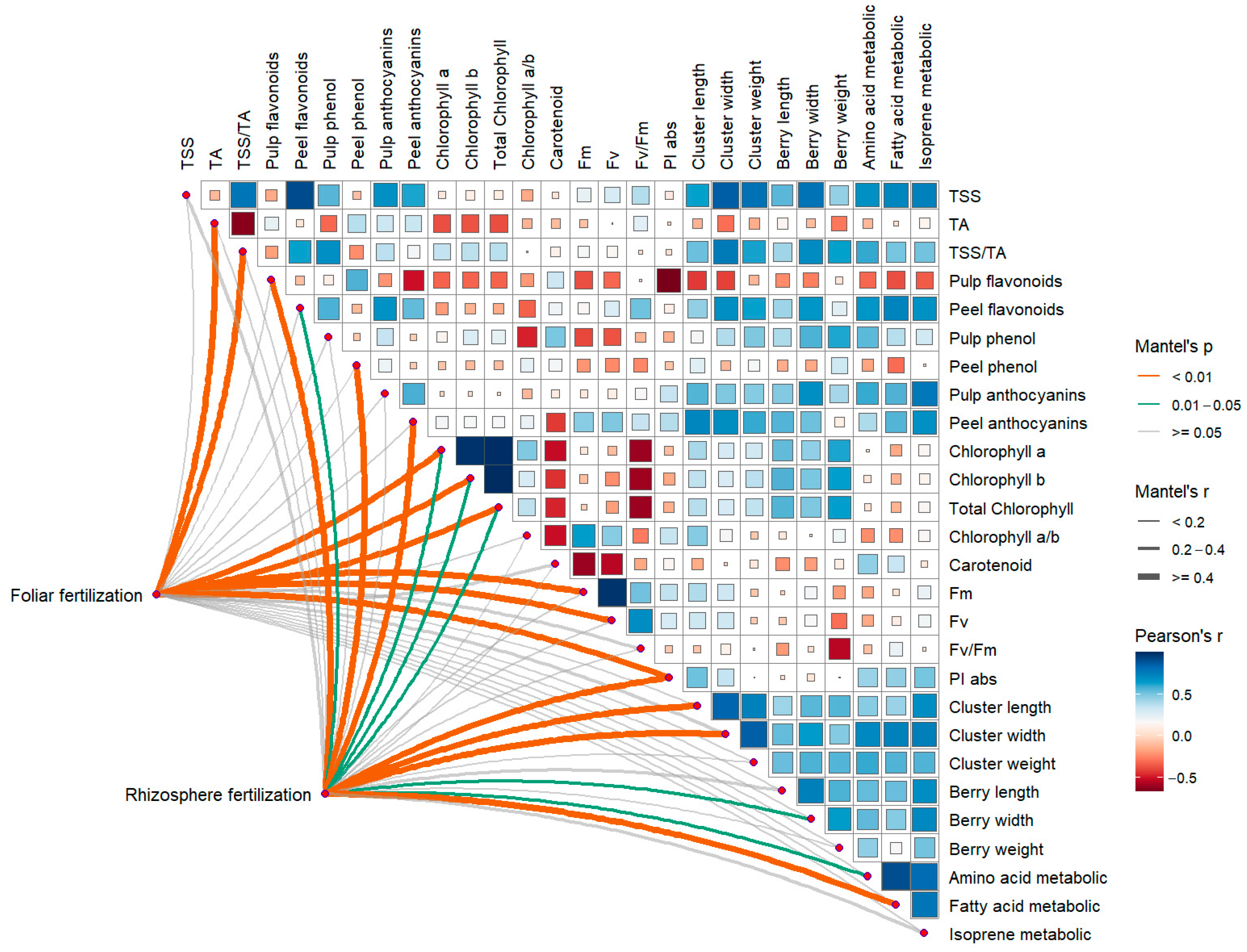
| (A) | ||||||
| Period | Se Concentration /ppm | Chlorophyll a /mg·g−1FW | Chlorophyll b /mg·g−1FW | Total Chlorophyll /mg·g−1FW | Chlorophyll a/b | Carotenoid /mg·g−1FW |
| BBCH73 | 0 | 3.15 ± 0.04 b | 7.70 ± 0.24 a | 10.85 ± 0.27 a | 0.41 ± 0.01 b | 2.36 ± 0.21 ab |
| 50 | 3.49 ± 0.14 a | 7.91 ± 0.07 a | 11.40 ± 0.21 a | 0.44 ± 0.01 a | 2.15 ± 0.09 b | |
| 100 | 2.36 ± 0.03 c | 5.83 ± 0.20 b | 8.18 ± 0.23 b | 0.41 ± 0.01 b | 2.88 ± 0.64 a | |
| 150 | 2.36 ± 0.13 c | 5.71 ± 0.30 b | 8.07 ± 0.42 b | 0.41 ± 0.01 b | 2.77 ± 0.18 ab | |
| BBCH81 | 0 | 3.65 ± 0.06 b | 8.76 ± 0.15 b | 12.41 ± 0.19 b | 0.42 ± 0.02 a | 3.31 ± 0.24 a |
| 50 | 3.92 ± 0.09 a | 9.25 ± 0.14 a | 13.17 ± 0.23 a | 0.42 ± 0.01 a | 2.97 ± 0.26 b | |
| 100 | 3.75 ± 0.05 ab | 8.86 ± 0.12 b | 12.61 ± 0.17 b | 0.42 ± 0.01 a | 3.87 ± 0.12 a | |
| 150 | 3.22 ± 0.04 b | 7.71 ± 0.08 b | 10.93 ± 0.12 b | 0.42 ± 0.02 a | 3.50 ± 0.08 a | |
| BBCH89 | 0 | 2.34 ± 0.15 a | 5.56 ± 0.26 a | 7.90 ± 0.40 a | 0.42 ± 0.02 a | 2.70 ± 0.50 a |
| 50 | 2.39 ± 0.13 a | 5.89 ± 0.25 a | 8.28 ± 0.38 a | 0.41 ± 0.01 a | 3.20 ± 0.30 a | |
| 100 | 1.86 ± 0.11 b | 4.50 ± 0.11 b | 6.36 ± 0.22 b | 0.41 ± 0.01 a | 2.98 ± 0.51 a | |
| 150 | 1.72 ± 0.09 b | 4.10 ± 0.30 b | 5.82 ± 0.39 b | 0.42 ± 0.02 a | 2.88 ± 0.21 a | |
| (B) | ||||||
| Period | Se Concentration /ppm | Chlorophyll a /mg·g−1FW | Chlorophyll b /mg·g−1FW | Total Chlorophyll /mg·g−1FW | Chlorophyll a/b | Carotenoid /mg·g−1FW |
| BBCH73 | 0(CK) | 2.46 ± 0.08 b | 5.86 ± 0.16 b | 8.31 ± 0.24 b | 0.42 ±0.01 ab | 3.40 ± 0.13 b |
| 50 | 2.78 ± 0.15 a | 6.52 ± 0.06 a | 9.29 ± 0.17 a | 0.43 ± 0.02 a | 3.15 ± 0.18 b | |
| 100 | 2.74 ± 0.01 a | 6.86 ± 0.09 a | 9.60 ± 0.10 a | 0.40 ± 0.01 b | 3.77 ± 0.13 a | |
| 150 | 2.34 ± 0.11 b | 5.58 ± 0.31 b | 7.92 ± 0.42 b | 0.42 ± 0.01 ab | 2.64 ± 0.27 c | |
| BBCH81 | 0(CK) | 3.60 ± 0.13 ab | 8.60 ± 0.19 b | 12.19 ± 0.32 b | 0.42 ± 0.01 a | 4.08 ± 0.32 a |
| 50 | 3.86 ± 0.07 a | 9.20 ± 0.18 a | 13.06 ± 0.14 a | 0.42 ± 0.01 a | 4.19 ± 0.24 a | |
| 100 | 3.57 ± 0.19 b | 8.60 ± 0.32 b | 12.16 ± 0.51 b | 0.41 ± 0.02 a | 3.27 ± 0.28 b | |
| 150 | 3.45 ± 0.16 b | 8.34 ± 0.37 b | 11.79 ± 0.53 b | 0.41 ± 0.02 a | 4.34 ± 0.13 a | |
| BBCH89 | 0(CK) | 2.38 ± 0.05 a | 5.76 ± 0.19 a | 8.14 ± 0.23 a | 0.41 ± 0.01 a | 8.31 ± 0.27 ab |
| 50 | 2.53 ± 0.05 a | 6.00 ± 0.49 a | 8.53 ± 0.48 a | 0.42 ± 0.04 a | 8.53 ± 0.48 a | |
| 100 | 2.11 ± 0.16 b | 5.07 ± 0.23 b | 7.18 ± 0.15 b | 0.42 ± 0.05 a | 7.18 ± 0.11 b | |
| 150 | 1.94 ± 0.13 b | 4.54 ± 0.37 b | 6.48 ± 0.46 c | 0.43 ± 0.03 a | 6.48 ± 0.46 b | |
| (A) | ||||||
| Se Concentration /ppm | Berry Length /mm | Berry Width /mm | Berry Weight /g | Cluster Length /cm | Cluster Width /cm | Cluster Weight /g |
| 0 (CK) | 19.95 ± 0.77 a | 18.77 ± 0.55 ab | 4.93 ± 0.53 ab | 22.35 ± 0.58 c | 14.80 ± 0.32 c | 634.32 ± 15.03 b |
| 50 | 19.96 ± 0.20 a | 19.31 ± 0.75 a | 5.49 ± 0.34 a | 24.93 ± 0.37 a | 19.27 ± 0.63 a | 721.09 ± 24.80 a |
| 100 | 18.33 ± 0.73 b | 18.70 ± 0.58 ab | 4.70 ± 0.64 bc | 23.33 ± 0.52 b | 16.33 ± 0.52 b | 626.08 ± 26.95 b |
| 150 | 18.76 ± 0.58 b | 18.04 ± 0.50 b | 4.24 ± 0.28 c | 19.63 ± 0.36 d | 13.17 ± 0.67 d | 617.62 ± 19.70 b |
| (B) | ||||||
| Se Concentration /ppm | Berry Length /mm | Berry Width /mm | Berry Weight /g | Cluster Length /cm | Cluster Width /cm | Cluster Weight /g |
| 0 (CK) | 18.64 ± 0.50 b | 17.67 ± 0.85 c | 4.94 ± 0.42 a | 22.35 ± 0.58 b | 14.83 ± 0.26 d | 529.88 ± 8.11 d |
| 50 | 18.97 ± 0.53 b | 18.34 ± 0.59 bc | 5.05 ± 0.43 a | 24.50 ± 0.63 a | 16.33 ± 0.55 c | 628.98 ± 21.95 c |
| 100 | 19.95 ± 0.77 a | 19.31 ± 0.75 a | 5.49 ± 0.34 a | 24.01 ± 0.89 a | 20.00 ± 0.14 a | 876.47 ± 29.32 a |
| 150 | 19.72 ± 0.43 a | 18.67 ± 0.76 ab | 4.30 ± 0.70 b | 24.33 ± 0.68 a | 19.13 ± 0.58 b | 727.24 ± 30.24 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Zhang, Y.; Yao, X.; Tian, S.; Wang, R.; Wang, C.; Jiang, J. Optimizing Selenium Delivery in Grapevines: Foliar vs. Rhizosphere Fertilization Effects on Photosynthetic Efficiency, Fruit Metabolites, and VOCs of ‘Muscat Hamburg’ Grape (Vitis vinifera L.). Horticulturae 2025, 11, 297. https://doi.org/10.3390/horticulturae11030297
Ma C, Zhang Y, Yao X, Tian S, Wang R, Wang C, Jiang J. Optimizing Selenium Delivery in Grapevines: Foliar vs. Rhizosphere Fertilization Effects on Photosynthetic Efficiency, Fruit Metabolites, and VOCs of ‘Muscat Hamburg’ Grape (Vitis vinifera L.). Horticulturae. 2025; 11(3):297. https://doi.org/10.3390/horticulturae11030297
Chicago/Turabian StyleMa, Chuang, Yuechong Zhang, Xinyu Yao, Shufen Tian, Rong Wang, Chaoxia Wang, and Jianfu Jiang. 2025. "Optimizing Selenium Delivery in Grapevines: Foliar vs. Rhizosphere Fertilization Effects on Photosynthetic Efficiency, Fruit Metabolites, and VOCs of ‘Muscat Hamburg’ Grape (Vitis vinifera L.)" Horticulturae 11, no. 3: 297. https://doi.org/10.3390/horticulturae11030297
APA StyleMa, C., Zhang, Y., Yao, X., Tian, S., Wang, R., Wang, C., & Jiang, J. (2025). Optimizing Selenium Delivery in Grapevines: Foliar vs. Rhizosphere Fertilization Effects on Photosynthetic Efficiency, Fruit Metabolites, and VOCs of ‘Muscat Hamburg’ Grape (Vitis vinifera L.). Horticulturae, 11(3), 297. https://doi.org/10.3390/horticulturae11030297





