Abstract
Some olive cultivars grown in southeastern Italy are characterized by the production of olives with a reduced level of bitterness. They are known as sweet olive cultivars and fruits are usually consumed directly or cooked without any debittering process, offering either health benefits to consumers, thanks to the high content of antioxidants, or an economic benefit to farmers for their higher price with respect to both table and oil olives. This study evaluates and compares the organoleptic, pomological, chemical, and physical parameters of seven sweet olive cultivars at different ripening degrees in the Puglia region over 8 weeks of maturity stage for two consecutive seasons (2022 and 2023). The organoleptic evaluation was performed by a restricted panel of usual consumers/experts of sweet olives. The results showed a higher preference for the olive cultivars locally named Triggiano Dolce, Cerasella, and Mele. Significant differences in weight, length, and width of the fruits were observed based on both cultivar and year. The phenolic composition of olive cultivars was significantly affected by both cultivar and harvest year, with Cazzinicchio and Cellina di Nardò having the highest total polyphenols. The analysis of water fraction extracted from olive samples by liquid chromatography coupled with mass spectrometry led to the identification of eleven compounds belonging to the secoiroids, phenylpropanoids, phenylethanolids, and flavonoids classes. The comparison of these compounds among the studied cultivars highlighted significant differences.
Keywords:
table olive; Olea europaea L.; marginal areas; Puglia; organoleptic; pomological; chemical 1. Introduction
The olive (Olea europaea L.) is one of the oldest tree species cultivated in the world, and its products are table olives and virgin olive oil, which have historically represented a foodstuff of high nutritional value for many populations and countries of the Mediterranean basin [1]. Olive fruits are commonly processed for olive oil extraction, but a small amount of the 21,449,868 tons of olives produced in 2022 [2], around 2.5–3.0 million tons, are consumed as table olives [3]. Table olives are mainly produced in Spain, Turkey, Italy, Portugal, and Greece, but the market of table olives involves several countries worldwide. Olive and its processed products have become even more valuable in recent years since their health benefits have come to light.
Table olives are consumed as appetizers and/or highly healthy culinary ingredients in many recipes for their low sugar content, high unsaturated fatty acids content, and additional contribution of fibers, minerals, vitamins, and bioactive components useful to the diet [1].
Taking into account the wide olive germplasm, table olives show sensorial and nutritional characteristics depending on the genotype and the processing method [4]. Italy, and particularly the Puglia region, southeastern Italy, has many olive cultivars that are used for table olive production, although many of them can be considered dual purpose because they also produce excellent olive oils.
There are reports of various health benefits of consuming table olives, such as the prevention of coronary heart, cancer, and inflammation diseases. It has been claimed that consuming 5–10 table olives per day might cover the daily intake of antioxidants [5].
However, the olive fruit needs to be processed to transform it into an appetizing and edible food because of its bitterness. There is a wide range of production styles, depending on the cultivar, ripening degree, and type of fruit (whole or cracked), aimed at hydrolyzing and/or diffusing into the brine the bitter oleuropein glucoside [1]. The most widespread systems are those that use alkaline hydrolysis or slow acid and enzymatic hydrolysis. In general, the fermentation step also contributes to the sweetening of the olive fruits, but due to processing variability, the final table olives produced can vary in color, form, and other sensorial parameters [6].
Although the chemical constituents of the drupe can vary depending on several factors (geographical origin, cultivar, cultural practices), the average composition of the drupes includes 70–76% water, 12–20% oil (mainly oleic acid), 5% total carbohydrates (mainly dietary fibers), 1.1% proteins, and 4.3% ash (USDA food database). Olives are also an important source of antioxidants, namely polyphenols, which are classified as follows: phenolic acids, phenolic alcohols, phenylpropanoids, flavonoids, and secoiridoids [7,8]. Olives, including table olives, are rich in oleuropein and ligstroside, compounds responsible for their characteristic bitter taste [8]. During the ripening process, hydrolytic activity operated by endogenous enzymes inside the olive leads to the release of hydroxytyrosol (HT), tyrosol (Tyr), and elenolic acid, which represent the most abundant phenolic compounds recovered in table olives, and they are well recognized for their health-promoting characteristics [9]. Furthermore, cultivar, environmental (temperature, water availability), and technological factors, such as the fermentation process, can influence the qualitative and quantitative phenolic composition of table olives [10].
Among table olives, there are some cultivars defined as ‘sweet olives’ because their fruits can be eaten fresh without previous debittering treatments. The geographic distribution of such cultivars is limited to some Mediterranean areas with particular climate and soil characteristics, including the Puglia region in Italy. However, some olive cultivars grown in the Karaburun peninsula, in the western part of Turkey (locally called Hurma), are considered sweet olives and mostly come from the Erkence region. These cultivars go through a natural debittering phase during their ripening period and become ready to eat as a table olive while still on the tree [4]. Similar sweet olives were also reported in studies from Greece [11] and Tunisia [12]. Ref. [13] found that Hurma olive has a low phenolic content compared to other cultivars, thus explaining the sweet taste as the effect of a physiological low content of some compounds responsible for the bitterness. Major soluble sugars in olives are reported as glucose, fructose, sucrose, xylose, rhamnose, and mannitol [14,15]. Sugars not only provide energy for metabolic changes that take place in the fruit but are also related to the textural properties of the olive. Moreover, sugars are the precursor for fatty acid biosynthesis and they act as carbon sources for microorganisms during table olive processing [14]. In sweet Thassos olives, for example, glucose and mannitol were detected as the main sugar and sugar alcohol [14]. In naturally debittered Dhokar olives, glucose and mannitol reach their highest level of concentration at the last stage of ripening, and their concentrations in naturally debittered Dhokar olives are higher compared to regular Chemlali olives [12]. Organic acids, which are approximately 1.5% of the flesh part [16] of an olive, are produced during the metabolism and catabolism of the other fruit components, such as carbohydrates [16]. According to a study about Turkish olives, succinic, malic, and citric acids were reported to be the major organic acids in Memecik and Domat cultivars [17]. Citric, succinic, and galacturonic acids were also identified as the main organic acids in another study which determined the organic acid profile of olives during the ripening stage [18]. Despite these studies, knowledge about the natural debittering process is limited mostly to phenolic changes, and further studies are needed to identify the other chemical changes that take place in olive composition during ripening stages.
In the Puglia region, there are different table olive cultivars, some of them defined as ‘sweet olives’, such as Cerasella, Mele, Pasola, Nolca, Dolce di Cassano, Termite di Bitetto, etc. These olives are generally consumed after fast and simple processing or cooking. In the past, cooking methods under ashes were often used, which involved the use of either braziers or fireplaces to heat houses (with the production of ashes). Other typical recipes of the Puglia tradition are fried olives with cherry tomatoes and served with the addition of oil, salt, and, in some cases, pepper; the olives keep their sweet taste with a little appreciated bitterness. These sweet olives were commonly consumed in the past and often associated with other minor and neglected fruits such as fig, either fresh or dried [19,20], during fall and winter time.
In this study, we compared some table olive cultivars, defined as sweet, cultivated in the Puglia region for sensory (panel test), qualitative, pomological, and physico-chemical parameters. The objective of this research was to discriminate the fruits of the considered cultivars in order to suggest some possible new uses of these olives without any processing but only for fresh consumption as other fruits.
2. Materials and Methods
2.1. Sample Collection
This study was carried out in the Puglia region, southeastern Italy. The region has a typical Mediterranean climate with an average temperature of 15–16 °C, and it is characterized by warm summers (average temperature 25–30 °C) and mild winters (average temperature 6–10 °C), with average annual rainfall ranging between 450 and 650 mm spread over the seasons, but mostly in autumn–winter, with occasional heavy rains often occurring in summer in recent years [21].
The investigated cultivars were as follows: Cazzinicchio, Cerasella, Cellina di Nardò, Mele, Nolca, Termite di Bitetto, and Triggiano Dolce (a local landrace). Samples were collected during two consecutive seasons (2022 and 2023); in particular, Cerasella, Mele, and Termite di Bitetto were obtained from the olive repository of the Department of Soil, Plant and Food Science (Di.S.S.P.A) located in the countryside of Valenzano (Bari province), Cazzinicchio and Triggiano Dolce form the countryside of Triggiano (Bari province), Cellina di Nardò from the countryside of Lecce, and Nolca from the countryside of Molfetta (Bari province). Healthy and ripe olives were handpicked at the four cardinal points and at different heights in the canopy of each tree, placed in paper bags, and successively transported to the laboratory for analysis. Approximately 1 kg of matured olives (as commonly harvested in the area for consumption) per tree from three sampled trees per cultivar was collected each season.
2.2. Sensory Evaluation
Ten volunteer panelists (aged 25–55 years, 6 males and 4 females), chosen for their usual consumption of sweet olives (hereinafter called the panel), from the Department of Soil, Plant and Food Science (Di.S.S.P.A) research group (University of Bari ‘Aldo Moro’, Bari, Italy), were selected to carry out the descriptive sensory analysis of the samples under study.
To each participant, an aliquot of about 20 olives per sample was presented in a white plastic dish reporting the sample name. The following attributes were evaluated: flavor intensity, sweetness, astringency, bitterness, destoning capacity, and overall acceptability.
All these attributes, out of the overall acceptability, were evaluated using an unstructured graphical scale of 10 points, with the left end representing the lowest intensity of perception and the right end representing the highest intensity. The overall acceptability was evaluated using a four-degree structured scale according to the following judgements: 1 = absolutely not; 2 = probably not; 3 = probably yes; 4 = surely yes. Each attribute term was then described and explained to avoid any doubt about the meaning. The samples of table olives used for tasting were kept between 20 and 25 °C, and the panel test was performed under diffused daylight. The sensory profile was adapted to the sweet olive cultivars analyzed in this study from our previous work on the sensory evaluation of fig products [22].
Destoning capacity is the parameter that evaluates how easily the stone separates from the flesh inside the oral cavity under the action of incisors and molars and how ’clean‘ from pulp residues the stone is after expelling it from the mouth [23].
2.3. Pomological, Physical, and Chemical Parameters
The pomological parameters were evaluated for each olive cultivar using weight (g), length (mm), and width (mm) as evaluation criteria. The average fresh weight of the fruits was calculated by weighing 100 fresh fruits per replicate, whereas the width and the length of the olives were measured using a caliper. The olives were destoned, and the stones and pulp were weighed separately for the determination of the pulp-to-stone (P/S) ratio, an important trait for table olives.
Successively, for the physio-chemical parameters, the pulp fraction for each sample was homogenized using a blade blender and transferred in 50 mL centrifuge tubes. After centrifugation at 6000× g per 25 min, a clear water phase was obtained and used for the following analysis: pH using a pH meter XS Instrument model pH 510, equipped with pH sensor FoodTrode (Hamilton, Franklin, MA, USA); soluble solids content using a portable refractometer with automatic temperature control and Brix scaled (model HI 96801, Hanna Instruments, Woonsocket, RI, USA). The water content of the olives was determined gravimetrically using 30 g of homogenized olive flesh after oven-drying at 105 °C until constant weight; the total fat content was obtained using the Soxhlet apparatus, petroleum ether 40–60 °C, and the residue of the water determination. The total polyphenols content was performed according to [24], using 20 µL directly from the water phase after centrifugation and gallic acid as a reference standard for calibration. The results of the total polyphenols were expressed as a mg of gallic acid equivalent (GAE) per kg of olive pulp.
The ultra-high performance liquid chromatography system consisted of an LPG-3400 RS quaternary pump, WPS-3000 TRS autosampler, TCC-3000 RS column oven, and PDA-3000 Dionex, coupled with the HESI-II probe (Thermo Fischer Scientific, Waltham, MA, USA) and the LTQ Velos Pro ion trap mass spectrometer (Thermo Fischer Scientific, Waltham, MA, USA). The separation of phenolic compounds was performed on Hipersyl Gold aQ C18 1.9 μm 2.1 × 100 mm (WatersTM, Milford, MA, USA) maintained at 30 °C using a binary mobile phase consisting of (A) water/formic acid (99.9:0.1, v/v) and (B) methanol/acetonitrile/formic acid (94.9:5:0.1, v/v) at a constant flow rate of 0.3 mL min−1. The gradient program of solvent A was as follows: 0–1 min isocratic 95%; 1–18 min linear decrease to 55%; 18–20 min linear decrease to 65%. The UV absorbance was monitored at 280 nm. The MS conditions were capillary temperature 320 °C; source heater temperature 280 °C; nebulizer gas N2; sheath gas flow 30 psi; auxiliary gas flow 7 arbitrary units; capillary voltage −2800 V, S-Lens RF Level 60%. Data were acquired in negative ionization and data-dependent methods. The data-dependent settings were full scan from 250 to 1200 m/z, activation level 65,000 counts, isolation width 2 Da, default charge state 2, and CID energy 35. All data were acquired and processed using Xcalibur v.2 (Thermo Fischer Scientific). A tentative identification of the phenolic compounds was achieved by a comparison of the obtained experimental molecular ion [M − H]− and MS2 fragmentation patterns with data reported in the literature. Relative quantities were estimated using the external standard calibration method and gallic acid, analyzed in the same experimental condition, in a range of concentration between 0.01 and 10 µg/mL. All quantities were expressed as mg/kg of gallic acid equivalent and used for comparative purposes only.
2.4. Statistical Analyses
All results were analyzed using SPSS v22.0 (IBM). The results of continuous variables, like soluble solids (°Brix), pH, fat content, olive weight, pulp percentage, and total polyphenols, were compared among cultivars using an ANOVA and Tukey’s post hoc test. The results obtained by the organoleptic evaluation were processed as nonparametric variables by applying the Kruskal–Wallis test [25].
3. Results
3.1. Sensory Profiles
Sensory profiles were obtained considering all six parameters (sweetness, astringency, bitterness, destoning capacity, flavor intensity, and acceptability) assessed by the evaluation panel. The average values of the organoleptic parameters for the seven cultivars evaluated are reported in Figure 1.
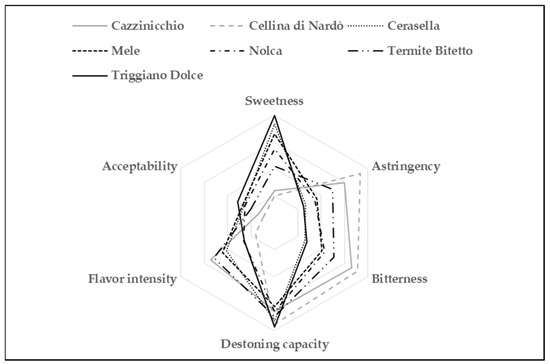
Figure 1.
Graphical representation of the organoleptic evaluation results of the olive samples.
In addition, the results of the organoleptic evaluation were reported as numerical data (statistically analyzed) in Table 1. The data represent the mean of the median values obtained in the two harvest years (2022 and 2023). The studied cultivars showed some significant differences between the two seasons.

Table 1.
Analysis of variance (ANOVA) and comparison of 2-year means of organoleptic parameters of the olive samples of the seven olive cultivars 1.
Considering global appreciation, the cultivars Triggiano Dolce, Cerasella, and Mele, and, to a lesser extent Nolca, were the table olives preferred by the panelists. Furthermore, the cultivars Triggiano Dolce and Cerasella were significantly (p ≤ 0.01) the sweetest; moreover, they had the lowest levels of astringency and bitterness, significantly (p ≤ 0.01) lower compared to the other cultivars. The destoning capacity also showed some significant differences among the examined samples, with Triggiano Dolce, Cerasella, and Cellina di Nardò more appreciated when chewing.
When it comes to flavor intensity, Cazzinicchio and Termite di Bitetto had significantly (p ≤ 0.01) higher values. However, due to their characteristic bitterness and astringency, these cultivars received significantly (p ≤ 0.01) low scores for acceptability, whereas Triggiano Dolce ranked first also for this parameter (Table 1).
3.2. Pomological, Chemical, and Physical Parameters
3.2.1. Pomological Parameters
The morphological parameters of the studied olive cultivars are shown in Figure 2. The selected set of morphological parameters was adequate to successfully differentiate each of the drupe and endocarp traits of the seven studied cultivars. Four cultivars, namely, Cerasuola, Termite di Bitetto, Mele, and Nolca, were characterized by a medium–large olive size and rounded shape. The remaining Cellina di Nardò, Cazzinicchio, and Triggiano Dolce had a medium–small size and elongated shape.
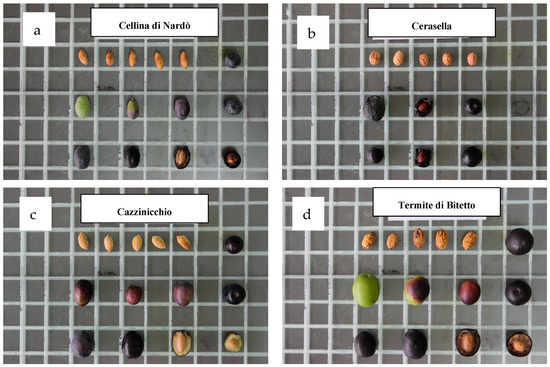
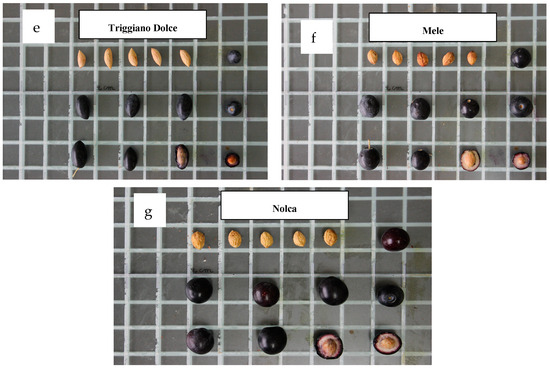
Figure 2.
Images show the morphological parameters of the different olive cultivars evaluated in the study. Each square is 2 × 2 cm.
The weight, length, and width data for drupes and endocarps show significant differences among cultivars and across years (Table 2). From Table 2, the ANOVA results indicated that the differences in weight (p ≤ 0.001), length (p ≤ 0.001), and width (p ≤ 0.001) of both drupes and endocarps were significant based on both cultivar and year. The only exception was the width of endocarp, where the year factor was less significant (p ≤ 0.05). The highest P/S values were noticed for Termite di Bitetto, Cerasella, and Cazzinicchio, which is a very important aspect when chewing table olives; Triggiano Dolce and Nolca presented the lowest P/S values.

Table 2.
Average values (2022 and 2023) of fresh weight and length and width of drupe and endocarp parameters of the seven olive cultivars 1.
The cultivar × year interaction was also significant (p ≤ 0.0001) for all three variables, suggesting that the considered cultivars have a different response to the drought conditions occurring during summer, which is one of the main factors of variability in the Puglia region for the agricultural sector.
The Termite di Bitetto cultivar had a significantly higher weight, length, and width of drupes and the weight and width of endocarps. Even if the olive size is strongly affected by pedoclimatic and agronomic conditions (rainfall, irrigation, fertilization, and pruning), Termite di Bitetto resulted in being the cultivar characterized by bigger fruit parameters even if the fruits’ average sizes were lower in 2023 with respect to the values observed in 2022. Similarly, the Triggiano Dolce cultivar was characterized by the smallest fruit size, followed by the Cellina di Nardò (Table 2).
3.2.2. Chemical and Physical Parameters
The mean values of the chemical and physical parameters are reported in Table 3. Significant differences among cultivars were observed for all considered parameters. The harvest year showed significant effects on pulp, water, fat percentage, and polyphenols. The cultivar × year interaction was significant only for the fat content, showing important changes in the same cultivars probably because of the different climatic conditions that strongly affected the ripening process and oil accumulation (drought, high temperatures, etc.). Among the studied olive cultivars, Cazzinicchio had the highest content of polyphenols, followed by Cellina di Nardò, both with a value of about 300 mg/kg. In contrast, the lowest polyphenol values were detected in the cultivars Cerasella, Mele, and Triggiano Dolce, the ones which also showed the highest sweetness. The pH values showed no significant differences, and in both years, Nolca and Triggiano Dolce were characterized by the lowest pH values. In the same manner of pH, soluble solids (°Brix) resulted in no significant difference in the two seasons, but Nolca and Triggiano Dolce reported the highest values among the studied cultivars.

Table 3.
Average values (2022 and 2023) of chemical and physical parameters of the olive samples of the seven olive cultivars 1.
The identification data obtained by LC/MS are reported in Table 4. Eleven phenolic compounds were identified in the water phase of the olive samples. In two cases, at retention times of 9.78 and 13.42, there was a coelution of two compounds in the same UV peak at 280 nm and they were quantified together in the successive Table 5. Six out of the eleven identified compounds belong to the class of secoiridoid, rutin and luteolin rutinoside belong to the class of flavonoid, p-coumaroyl-hexoside and verbascoside belong to the class of phenylpropanoid, and hydroxytyrosol belongs to the class of phenylethanoid.

Table 4.
Identification data of compounds found in the water phase of olive samples. MH− = Negative molecular ions m/z, MS2 = Second stage fragmentation pattern. The number in brackets represents the relative intensity.

Table 5.
Quantitative data were obtained by LC/MS of polyphenol compounds identified in the water phase of the olive samples. Values are in mg/kg gallic acid equivalent 1.
The quantitative data related to the identified compounds are reported in Table 5. There were important differences among the analyzed samples. Cerasella showed only trace amounts (not quantified) for almost all target compounds, with the exception of 1-D-glucopyranosyl acyclodihydroelenolic acid. The cultivars with the highest number of detected compounds were Cellina di Nardò, Cazzinicchio, and Triggiano Dolce with seven compounds detected in a quantifiable amount.
4. Discussion
In the literature, the topics regarding the importance of pomological and sensory properties in food quality evaluations [31,32] have been extensively discussed. On the other hand, few studies are available relating to the chemical, pomological, and sensory analysis of the so-called sweet table olives.
In the present study, we provided valuable insights into important characteristics of different sweet olive cultivars in the Puglia region and ways that these cultivars could receive more attention for their economic and health values.
Despite its importance as a commercial parameter, both the determination of the pulp-to-stone ratio (P/S) and the percentage of pulp contributed to the comprehensive chemical analysis of the olive samples. A recent paper [33] indicated, for maintaining the commercial value of table olives, that the size and pulp-to-stone ratio are very important parameters in the table olive market. In this study, the Termite di Bitetto cultivar has a pulp of 82.6%, which was significantly higher compared to other cultivars. With a high pulp-to-stone ratio (7.55), Termite di Bitetto can be considered a cultivar with a noteworthy commercial value. Ref. [34] indicated that the commercial value increases with the increase in the pulp-to-stone ratio.
Size does matter, but there are also other important factors attracting consumers to the table olive market. From the organoleptic evaluation’s point of view, the sensory profiles of the seven olive cultivars indicated that olive sweetness appeared to be the most influencing factor in the panel’s overall acceptability. This is the case of the cultivars Cerasella, Triggiano Dolce, and Mele. Theoretically, the sweet taste should be correlated with the soluble solids content. The sweetness was more appreciated by younger panelists with respect to the more expert (older) ones who appreciated the bitterness of some olive cultivars. However, this difference in the organoleptic characteristics of the examined cultivars could be used to target each cultivar to different consumers, i.e., consumers appreciating more the sweet vs. consumers appreciating the bitter. However, in partial disagreement with our results, ref. [35] found that olive flavor is the influencing factor due to qualitative and quantitative compositions of volatiles, and the fragrance transmitted derivates from an equilibrium of several chemical classes of volatile compounds. In our study, the cultivars Cazzinicchio and Termite di Bitetto had higher flavor scores; however, the lowest acceptability scores recorded (1.3 and 2, respectively, out of 4) suggested that, for market orientation, more organoleptic parameters of table olives should be considered instead of flavor. Maybe consumers first buy with their eyes and nose, but they continued to keep buying with their mouth. Moreover, the Cellina di Nardò samples showed significantly lower values for most of the organoleptic parameters considered. This can be understood, given the small size of the olives, as the green-to-black ripening stage and the transformation process [7]. It should be kept in mind that this cultivar is mainly used for olive oil production and in baking (puccia salentina, a bread cooked with Cellina di Nardò olives).
Similarly to other fruits, sweet table olives could be consumed fresh as appetizers or cooked as side dishes for main courses of meat and fish. Cultivars with a general lower bitterness should be preferred for fresh consumption, whereas for cooked dishes, either sweet or bitter cultivars could be used.
In Table 6, the results of the r Pearson correlation coefficients are reported. As expected, and well known by the scientific literature, there is a negative correlation between the pH of fruit and soluble solids due to the progressive reduction in organic acid and accumulation of sugars during ripening. In the results of the present work, the bitterness and astringency of the olive are strongly positively correlated (r > 0.7), and both are positively correlated with the total polyphenols content. On the other hand, the total polyphenols content is strongly and negatively correlated with sweetness.

Table 6.
Pearson correlation among the organoleptic descriptors and chemical parameters.
The Pearson correlation analysis was also performed on the results of the LC/MS data and the organoleptic parameters, with the results reported in Table 7. Almost all target compounds have a negative correlation with the sweetness sensation. Two compounds, at retention times of 7.16 and 9.78, have a strong positive correlation with both astringency and bitterness. The chromatographic peak at 7.16 was identified as a glucoside of p-coumaric acid, whereas at a retention time of 9.78, there were two identified compounds, namely luteolin rutinoside and oleuropein aglycon. Bitterness and astringency were also positively correlated with the concentration of the oleanolic acid derivate (RT 2.12) and the oleuropein derivate (RT 11.39). It is worth mentioning that there was a weak negative correlation between the verbascoside content and both astringency and bitterness. The sweet olive cultivars with a sweet taste were characterized by a lower content of polyphenols such as luteolin rutinoside and oleuropein aglycon.

Table 7.
Pearson correlation analysis among the compound identified by LC/MS and the organoleptic evaluation.
5. Conclusions
This work is the first report on the identification and characterization of important organoleptic, pomological, qualitative, and physico-chemical parameters of seven sweet olive cultivars in the Puglia region (southeastern Italy).
Termite di Bitetto and Cerasella resulted in being the cultivars characterized by bigger fruit dimensions and, with Mele, a high pulp-to-stone ratio.
The highest value of total polyphenols was observed in the Cazzinicchio and Cellina di Nardò samples with a value close to 300 mg/kg. Triggiano Dolce and Cerasella were characterized by the sweetness in the panel evaluation and good acceptability, thus suggesting a better use for fresh consumption.
The polyphenols detected in some olive cultivars partly explained the more astringent and bitter taste of such cultivars.
In comparison with the existing literature, this work provides new information about the sensory, physico-chemical, and pomological data already available about sweet olive cultivars. However, there has been limited research conducted on table olives (and even less for sweet olives), and there is still not a real focus on the sweet olive market possibilities.
In conclusion, despite their economic value, as they could be eaten fresh without previous debittering treatment or cooked for many dishes, this study revealed important information regarding the chemical compositions of different sweet olive cultivars. Further studies on the effects of environmental factors and growing conditions on the studied parameters are required to promote ‘sweet olives’ for wider fresh consumption.
Author Contributions
Conceptualization, G.F., A.M. and A.T.; methodology, G.F., A.M. and A.T.; formal analysis, A.M. and A.T.; investigation, A.T., A.M. and G.F.; data curation, A.T., S.A.A., S.P. and S.B.; writing—original draft preparation, A.T., S.A.A., S.P., S.B. and G.F.; writing—review and editing, S.A.A., A.T., A.M. and G.F.; supervision, G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partly carried out within the Agritech National Research Center and received funding from the European Union Next-Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022). This activity was also conducted within the DAJS (Distretto Agroalimentare di qualità Jonico Salentino) (CUP: J89J21013750001).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors wish to thank the people participating in the panel test, in particular Giuseppe Gambacorta.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gandul-Rojas, B.; Gallardo-Guerrero, L. Characterization and Processing of Table Olives: A Special Issue. Foods 2020, 9, 1469. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#data (accessed on 10 July 2024).
- International Olive Oil Council (IOC). Production Data for Table Olives. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures (accessed on 10 July 2024).
- Perpetuini, G.; Caruso, G.; Urbani, S.; Schirone, M.; Esposto, S.; Ciarrocchi, A.; Prete, R.; Garcia-Gonzalez, N.; Battistelli, N.; Gucci, R.; et al. Changes in Polyphenolic Concentrations of Table Olives (Cv. Itrana) Produced Under Different Irrigation Regimes During Spontaneous or Inoculated Fermentation. Front. Microbiol. 2018, 9, 1287. [Google Scholar] [CrossRef]
- Boskou, D. Olive Oil: Chemistry and Technology, 2nd ed.; AOCS Publishing: Champaign, IL, USA, 2006; ISBN 978-1-893997-88-2. [Google Scholar]
- Rocha, J.; Borges, N.; Pinho, O. Table Olives and Health: A Review. J. Nutr. Sci. 2020, 9, e57. [Google Scholar] [CrossRef]
- Lanza, B. Nutritional and Sensory Quality of Table Olives. In Olive Germplasm—The Olive Cultivation, Table Olive and Olive Oil Industry in Italy; Muzzalupo, I., Ed.; InTech: Takasago City, Japan, 2012; ISBN 978-953-51-0883-2. [Google Scholar]
- Ryan, D.; Robards, K. Critical Review. Phenolic Compounds in Olives. Analyst 1998, 123, 31R–44R. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive Oil Phenols Are Absorbed in Humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef]
- Romero, C.; Brenes, M.; Yousfi, K.; García, P.; García, A.; Garrido, A. Effect of Cultivar and Processing Method on the Contents of Polyphenols in Table Olives. J. Agric. Food Chem. 2004, 52, 479–484. [Google Scholar] [CrossRef]
- Zoidou, E.; Melliou, E.; Gikas, E.; Tsarbopoulos, A.; Magiatis, P.; Skaltsounis, A.-L. Identification of Throuba Thassos, a Traditional Greek Table Olive Variety, as a Nutritional Rich Source of Oleuropein. J. Agric. Food Chem. 2010, 58, 46–50. [Google Scholar] [CrossRef]
- Jemai, H.; Bouaziz, M.; Sayadi, S. Phenolic Composition, Sugar Contents and Antioxidant Activity of Tunisian Sweet Olive Cultivar with Regard to Fruit Ripening. J. Agric. Food Chem. 2009, 57, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Aktas, A.B.; Ozen, B.; Tokatli, F.; Sen, İ. Comparison of Some Chemical Parameters of a Naturally Debittered Olive (Olea europaea L.) Type with Regular Olive Varieties. Food Chem. 2014, 161, 104–111. [Google Scholar] [CrossRef]
- Marsilio, V.; Campestre, C.; Lanza, B.; De Angelis, M. Sugar and Polyol Compositions of Some European Olive Fruit Varieties (Olea europaea L.) Suitable for Table Olive Purposes. Food Chem. 2001, 72, 485–490. [Google Scholar] [CrossRef]
- López-López, A.; Jiménez-Araujo, A.; García-García, P.; Garrido-Fernández, A. Multivariate Analysis for the Evaluation of Fiber, Sugars, and Organic Acids in Commercial Presentations of Table Olives. J. Agric. Food Chem. 2007, 55, 10803–10811. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Ferreira, I.M.P.L.V.O.; Fernandes, J.O.; Faria, M.A.; Beatriz, M.; Oliveira, P.P.; Ferreira, M.A. Determination of lactic, acetic, succinic, and citric acids in table olives by HPLC/UV. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 1029–1038. [Google Scholar] [CrossRef]
- Ergönül, P.G.; Nergiz, C. Determination of Organic Acids in Olive Fruit by HPLC. Czech J. Food Sci. 2010, 28, 202–205. [Google Scholar] [CrossRef]
- Arslan, D.; Özcan, M.M. Phenolic Profile and Antioxidant Activity of Olive Fruits of the Turkish Variety “Sarıulak” from Different Locations. Grasas Aceites 2011, 62, 453–461. [Google Scholar] [CrossRef]
- Ferrara, G.; Mazzeo, A.; Pacuci, C.; Matarrese, A.M.S.; Tarantino, A.; Crisosto, C.; Incerti, O.; Marcotuli, I.; Nigro, D.; Blanco, A.; et al. Characterization of edible fig germplasm from Puglia, Southern Italy: Is the distinction of three fig types (Smyrna, San Pedro and Common) still valid? Sci. Hortic. 2016, 205, 52–58. [Google Scholar] [CrossRef]
- Marcotuli, I.; Mazzeo, A.; Colasuonno, P.; Terzano, R.; Nigro, D.; Porfido, C.; Tarantino, A.; Aiese Cigliano, R.; Sanseverino, W.; Gadaleta, A.; et al. Fruit development in Ficus carica L.: Morphological and genetic approaches to fig buds for an evolution from monoecy toward dioecy. Front. Plant Sci. 2020, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Lombardini, L.; Mazzeo, A.; Bruno, G.L. Evaluation of pecan [Carya illinoinensis (Wangenh.) K. Koch] cultivars for a possible cultivation for both fruit and truffle production in the Puglia Region, Southeastern Italy. Horticulturae 2023, 9, 261. [Google Scholar] [CrossRef]
- Ferrara, G.; Magarelli, A.; Mazzeo, A.; Coletta, A.; Crupi, P.; Loperfido, F.; Maggi, G.; Venerito, P. Underutilized fig (Ficus carica L.) cultivars from Puglia region, Southeastern Italy, for an innovative product: Dried fig disks. Processes 2023, 11, 1485. [Google Scholar] [CrossRef]
- Bacceli, M.; Simone, N.; Lanza, B.; Cichelli, A. A Novel Approach for the Characterization of the Textural Properties of Table Olives: Acoustic Compression Related to Sensory Analysis. Foods 2023, 12, 241. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Acree, T.E.; Decker, E.A.; Penner, M.H.; Reid, D.S.; Schwartz, S.J.; Shoemaker, C.F.; Smith, D.; Sporns, P. Handbook of Food Analytical Chemistry, 1st ed.; Wiley: Hoboken, NJ, USA, 2004; ISBN 978-0-471-72187-1. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Chemical Screening of Olive Biophenol Extracts by Hyphenated Liquid Chromatography. Anal. Chim. Acta 2007, 603, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Arráez-Roman, D.; Segura-Carretero, A.; Menéndez, J.A.; Menéndez-Gutiérrez, M.P.; Micol, V.; Fernández-Gutiérrez, A. Qualitative Screening of Phenolic Compounds in Olive Leaf Extracts by Hyphenated Liquid Chromatography and Preliminary Evaluation of Cytotoxic Activity against Human Breast Cancer Cells. Anal. Bioanal. Chem. 2010, 397, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Malapert, A.; Reboul, E.; Loonis, M.; Dangles, O.; Tomao, V. Direct and Rapid Profiling of Biophenols in Olive Pomace by UHPLC-DAD-MS. Food Anal. Methods 2018, 11, 1001–1010. [Google Scholar] [CrossRef]
- Ammar, S.; Contreras, M.D.M.; Gargouri, B.; Segura-Carretero, A.; Bouaziz, M. RP-HPLC-DAD-ESI-QTOF-MS Based Metabolic Profiling of the Potential Olea europaea By-Product “Wood” and Its Comparison with Leaf Counterpart. Phytochem. Anal. 2017, 28, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Jerman Klen, T.; Golc Wondra, A.; Vrhovšek, U.; Mozetič Vodopivec, B. Phenolic Profiling of Olives and Olive Oil Process-Derived Matrices Using UPLC-DAD-ESI-QTOF-HRMS Analysis. J. Agric. Food Chem. 2015, 63, 3859–3872. [Google Scholar] [CrossRef] [PubMed]
- Szczesniak, A.S. Texture Is a Sensory Property. Food Qual. Prefer. 2002, 13, 215–225. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Food Science Text Series; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-6487-8. [Google Scholar]
- Oueslati, A.; Dabbou, S.; Methneni, N.; Montevecchi, G.; Nava, V.; Rando, R.; Bartolomeo, G.; Antonelli, A.; Di Bella, G.; Ben Mansour, H. Pomological and Olive Oil Quality Characteristics Evaluation under Short Time Irrigation of Olive Trees cv. Chemlali with Untreated Industrial Poultry Wastewater. Sustainability 2023, 15, 4198. [Google Scholar] [CrossRef]
- Mele, M.A.; Islam, M.Z.; Kang, H.M.; Giuffrè, A.M. Pre-and Post-Harvest Factors and Their Impact on Oil Composition and Quality of Olive Fruit. Emir. J. Food Agric. 2018, 30, 592–603. [Google Scholar] [CrossRef]
- Sabatini, N.; Mucciarella, M.R.; Marsilio, V. Volatile Compounds in Uninoculated and Inoculated Table Olives with Lactobacillus Plantarum (Olea europaea L., cv. Moresca and Kalamata). LWT—Food Sci. Technol. 2008, 41, 2017–2022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).