Abstract
Cucumber is one of the top ten vegetables globally and is widely cultivated worldwide. However, Fusarium wilt, caused by Fusarium oxysporum f. sp. Cucumerinum, is one of the most serious soil-borne diseases in cucumber cultivation, causing significant economic losses. Biological control has great potential in the prevention of cucumber wilt disease, but the mechanism involved still needs further research. In this study, biocontrol isolate Bacillus subtilis 1JN2, which was isolated in our previous work, was evaluated in field conditions against Fusarium wilt, and the rhizosphere fungal diversity was analyzed. The results indicated that the biocontrol efficacy of B. subtilis 1JN2 reached 58.5% compared with the blank control, and the population density of F. oxysporum in the rhizosphere decreased from 495 copies/g of soil before inoculation to 20 copies/g 14 days after treatment. High-throughput sequencing demonstrated that after an inoculation of 1JN2, the populations that decreased significantly include the genera of Olpidium and Pseudallescheria, from more than 20% to less than 8%. And the most increased population belonged to the family Chaetomiaceae, from 6.82% to 18.77%, 12.39%, 44.41%, and 19.41% at the four sample time points after treatment. In addition, soil-related enzyme activities, including catalase, soil dehydrogenase, alkaline phosphatase, and polyphenol oxidase, were analyzed before and after treatment with 1JN2. The results indicated that all the enzyme activities showed an upward trend following inoculation. These findings demonstrate the potential of using B. subtilis 1JN2 as a biocontrol agent for controlling Fusarium wilt in cucumber.
1. Introduction
Cucumber (Cucumis sativus L.) is one of the top ten vegetables globally and is widely cultivated worldwide [1]. According to data from the Food and Agriculture Organization of the United Nations (FAO), the total production area of cucumbers worldwide in 2021 was 2.17 million hectares, with China reaching 1.29 million hectares, accounting for 59% of the global total. The total global cucumber production was 93.52 million tons in 2021, with China reaching 75.59 million tons, accounting for 80% of the global total [2]. However, while cucumber cultivation is growing rapidly, there are also some problems. Among them, cucumber wilt, caused by Fusarium oxysporum f. sp. cucumerinum J. H. Owen (FOC), is one of the most serious soil-borne diseases in cucumber cultivation [3]. The occurrence of wilt disease generally leads to a reduction in cucumber production by 10–30%, and in severe cases, it can reach 80–90% [4]. It is assumed that one of the reasons for the sustained occurrence of wilt disease is the imbalance of soil microbial diversity, such as the reduction of beneficial microorganisms and the enrichment of pathogens [3,4,5].
At present, the main methods for controlling cucumber wilt include cultural control, chemical control, disease resistance breeding, and biological control [6]. Cultural control measures include crop rotation and grafting, such as using black pumpkin as a rootstock for grafting, which has the advantages of resistance to wilt disease, the high survival of the grafted plants, and a high yield [7]. However, grafting is expensive and difficult to perform, and may result in decreased fruit quality. In addition, reasonable rotation can also alleviate the occurrence of wilt disease to a certain extent [8,9]. However, rotation involves economic challenges and is not suitable for large-scale promotion. Chemical control generally uses soil disinfestation, seed treatment, its application to transplants, or the treatment of cucumber plants in the field [10]. However, the long-term use of chemical pesticides may have adverse effects on the environment and result in pesticide residues and fungicide resistance [11].
Biological control is the use of antagonistic microorganisms and/or their metabolites to inhibit pathogens and weaken their pathogenicity, and thus reduce the occurrence of wilt disease [12]. Bacillus bacteria are widely used in plant disease control due to their wide environmental distribution and strong stress resistance. In the research on controlling cucumber wilt disease, a large number of Bacillus isolates have also been screened and isolated. Yang et al. [13] reported that B. velezensis VJH504 could produce proteases, amylases, β-1,3-glucanases, and cellulases, as well as siderophores and indole-3-acetic acid, and had a significant disease inhibition effect against Fusarium wilt on cucumber both in vitro and in vivo [13]. In another study, pot experiments with vermiculite and B. subtilis Z-14 achieved a biocontrol efficacy of 61.23% against Fusarium wilt on cucumber [14]. Furthermore, a metabolomics analysis revealed that Z-14 affected ABC transporters, amino acid synthesis, and the biosynthesis of plant secondary metabolites. The antifungal substances produced by Bacillus isolates have significant effects in directly inhibiting the pathogen of wilt disease; for example, Xue et al. [15] isolated B. atrophaeus NX-12, which secreted fengycin and not only inhibited the germination of FOC spores, but also induced the production of reactive oxygen species (ROS) in FOC cells, leading to oxidative stress and the accumulation of glycerol, and the accumulated glycerol further promoted the production of fengycin [15]. However, according to current research reports, there are problems in the biological control of cucumber wilt disease, such as a single mode of action of the biocontrol agent and insufficient field colonization, which are affected by environmental factors and lead to unstable field efficacy.
For soil-borne disease control, the use of biocontrol strains is generally limited to the rhizosphere of plants, and the first step in exercising their biocontrol potential is their own colonization, which has always been a problem that has plagued biological control research [16]. The factors that affect the colonization of biocontrol strains not only include the interaction between biocontrol strains and their host plants, but more importantly, the interaction between biocontrol strains and target and non-target pathogens, as well as other microorganisms in the plant root zone [17]. The application of a biocontrol agent may affect the microbial structure of the plant rhizo-microbiome, which may contribute to the suppression of the pathogen population, which is also the key to achieving sustainable disease prevention and control [18,19].
In preliminary experiments, it was found that B. subtilis 1JN2 can effectively prevent cucumber wilt disease and affect the root microbiome of cucumber. This study aims to elucidate the mechanism of biocontrol of cucumber wilt by 1JN2 from the perspective of rhizosphere microecology. High-throughput sequencing technology and a quantitative PCR were used to detect changes in fungal diversity and pathogen population density in the cucumber rhizosphere after biocontrol treatment. The occurrence of cucumber wilt and soil-related enzyme activities were also analyzed in different treatment groups. The results provide a theoretical basis for the practical application of this strain and the screening of biocontrol strains for soilborne plant diseases.
2. Materials and Methods
2.1. Bacterial Strain and Plant Growth Conditions
B. subtilis strain 1JN2 (GU549436) was isolated in our previous work and identified as an effective biocontrol agent towards several plant diseases [20,21]. Luria–Bertani (LB) medium was used for bacterial growth.
Cucumber seeds (variety Jindian 303) were grown in a nursery site and transplanted to the field when the seedlings had 3–4 true leaves. The experiment was conducted in the vegetable experimental base of Huai’an Agricultural Technology Extension Center (E119.020292, N33.484059). The soil type of the experimental field is yellow brown soil, with an average organic matter content of 2.7% and a pH value between 6 and 8. The experimental field has been planted with cucumber for 8 years, and the average incidence rate of cucumber wilt is 50%. The transplanting time of cucumber seedlings in this experiment was 10 October 2022. After the first harvest of cucumbers (about 30 days after transplanting), compound fertilizer (N:P2O5:K2O = 15:15:15, total nutrients ≥ 45%) was applied once, with a dosage of 10 kg/acre. No chemical pesticides were used during the entire growth period of the cucumbers, and insect nets were used for pest control. The irrigation method was to irrigate moderately according to soil moisture.
2.2. Methods for the Field Experiment
Two groups were tested in the field experiment, B. subtilis 1JN2 vs. a blank control. Each treatment had three replicates, with 20 cucumber seedlings each. The plant spacing was 30 cm within rows and 60 cm across rows. After transplanting, the suspension of B. subtilis 1JN2 was adjusted to 107 CFU/mL, of which 20 mL was irrigated around each plant in the 1JN2 treatment group. Disease scores were recorded at 30 and 60 days after transplanting, based on National Technical Regulation NY/T 1857.3-2010:
Level 1: Both aboveground and underground areas are asymptomatic;
Level 2: Slight wilting of true leaves and browning of less than 10% of fibrous roots;
Level 3: Wilting of true leaves, browning of main roots, or browning of fibrous roots by 10 to 50%;
Level 4: True leaves wilting, main roots turning brown by 50%;
Level 5: More than 50% of the main roots turn brown; the aboveground parts wither or die.
A disease index was calculated as ∑ (number of diseased leaves/roots at each level × representative value at each level)/(total number of surveyed leaves/roots × highest representative value) × 100. Biocontrol efficacy was determined as the relative disease reduction (%) in the treatment compared with the control.
2.3. Soil Sampling and Enzyme Analysis
Three sites from each replicate in the treatment group, 5 m apart, were selected as the sampling sites, and each site consisted of three cucumber plants. Rhizosphere soil samples were collected at five time points, including 1 day before and 1 day, 3 days, 7 days, and 14 days after treatment with B. subtilis 1JN2. For sampling, the plants were taken out from the soil without disturbing the root system, then the plant roots were shaken softly to remove the root zone soil, and the remaining rhizosphere soil samples were collected using a small brush. All soil samples were stored at −80 °C.
Soil enzyme activities were determined using previously published and well-established protocols as described in Yang et al. [9]. For soil-related enzymes including catalase, soil dehydrogenase, alkaline phosphatase, and polyphenol oxidase, the detection at each time points consisted of three replicates.
2.4. Analysis of Rhizo-Fungal Diversity after Treatment by B. subtilis 1JN2
Total microbial genomic DNA of the soil samples was extracted with the E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) following the manufacturer’s instructions. The quality and concentration of the extracted DNA were determined by electrophoresis and a NanoDrop2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA), and the DNA was kept at −80 °C before further analysis.
Sequencing and further microbiota analysis were conducted at the platform of Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The primers ITS1F (5′-GGT TTC TGT AGG TGA ACC TGC-3′) and ITS2R (5′-CTC GGA CGA GGA TCC TCG CC-3′) were used to amplify the ITS1-ITS2 hypervariable region of the fungi. The PCR reaction system and PCR amplification cycling conditions were as described in our previous work [21]. The PCR product was extracted by electrophoresis and purified with a PCR Clean-Up Kit (YuHua, Shanghai, China). Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina PE300 platform (Illumina, San Diego, CA, USA) according to the standard protocols by Majorbio Bio-Pharm Technology.
A bioinformatic analysis of the soil microbiota was carried out using the Majorbio Cloud platform (https://cloud.majorbio.com (accessed on 23 December 2022)). The rarefaction curves and alpha diversity were calculated with Mothur v1.30.1 [22]. The similarity in different samples was determined by principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity, using Vegan v2.5-3. The PLS-DA (Partial Least Squares Discriminant Analysis) was conducted with R language as follows: firstly, construct an orthogonal score vector by maximizing the covariance between the independent variable data and the dependent variable data; then, use these score vectors to classify the samples; finally, the impact of each variable on the classification is measured by calculating the variable projection importance (VIP). The community composition analysis was conducted with R language, based on the species composition and relative abundance of each sample at different taxonomic levels.
2.5. Quantitative PCR Analysis of the Pathogen
The quantities of FOC in the rhizosphere soil of the cucumber were determined by real-time qPCR amplification. A PCR was performed with an Applied Biosystems 7500 Real-Time PCR system (Applied Biosystems, Waltham, MA, USA) with a Premix Ex Taq™ kit (Takara, Dalian, China). The primers FocF3 (5′-AAA CGA GCC CGC TAT TTG AG-3′) and FocR7 (5′-TAT TTC CTC CAC ATT GCC ATG-3′) were used for the detection of FOC [23]. The real-time PCR amplification system and reaction conditions followed Ye et al. [24]. Agarose gel electrophoresis and a melting curve analysis were used to indicate the specificity of the amplification products.
2.6. Statistical Analysis
A statistical analysis was performed using a t-test to compare the treatment and control in the field experiment (SPSS 16.0). Statistical significance was determined at p < 0.05.
3. Results
3.1. Biological Control Efficacy
The biocontrol efficacy of B. subtilis 1JN2 was assessed at 30 days and 60 days after the transplanting of the cucumber seedings (Table 1). As the time after transplanting increased, the occurrence of Fusarium wilt in the blank control group gradually intensified. The disease index increased from 0.46 on the 30th day after transplantation to 0.69 on the 60th day. In contrast, the disease index of the biocontrol strain treatment group did not show marked changes over time. As the disease index of the blank control group increased, the efficacy of the biocontrol isolate treatment group also improved with the prolongation of transplantation time, reaching 58.5% on the 60th day.

Table 1.
Biocontrol efficacy of Bacillus subtilis 1JN2 against Fusarium wilt on cucumber in the field.
Consistent results were also observed for the population density of the pathogen in the soil, as determined by real-time qPCR amplification (Figure 1). The amount of FOC in the soil before biocontrol treatment was 495 copies/g of soil. With the treatment of B. subtilis 1JN2, the density of FOC decreased. Two weeks after treatment, the population density in the soil decreased to 20 copies/g. This indicated that the application of the biocontrol strain directly or indirectly inhibited the quantity of FOC in the soil.
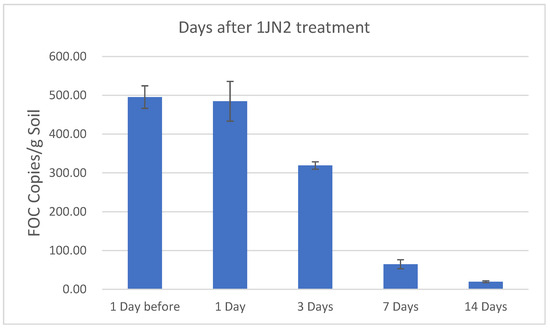
Figure 1.
The population density of Fusarium oxysporum f. sp. cucumerinum (FOC) in the rhizosphere soil of cucumber after treatment with Bacillus subtilis 1JN2. Values are means and standard deviations of three replicates.
3.2. Changes in Rhizo-Fungal Diversity
In order to elucidate the impact of B. subtilis 1JN2 on the diversity of cucumber rhizosphere fungi, high-throughput sequencing was used to analyze the trend of microbial changes in the soil at various sample time points after biocontrol isolate treatment. According to the results of the Partial Least Squares Discriminant Analysis (PLS-DA) (Figure 2), the community structure of cucumber rhizosphere fungi showed significant changes after the application of the biocontrol agent, with the most notable changes occurring on the first and third days after treatment, indicating that the application of the biocontrol agent led to a rapid change in the population structure of cucumber rhizosphere fungi. Among the soil samples collected across the five sampling time points, there were a total of 95 identical genera. With the treatment of B. subtilis 1JN2, the number of endemic (unique) genera at each sampling time point increased. Among them, the number of endemic genera reached 16 on the first and third days after treatment, which was the highest number of the endemic genera among the five time points. This result is consistent with the PLS-DA result, indicating that the application of biocontrol bacterial strain quickly adjusted the community structure of cucumber rhizosphere fungi.
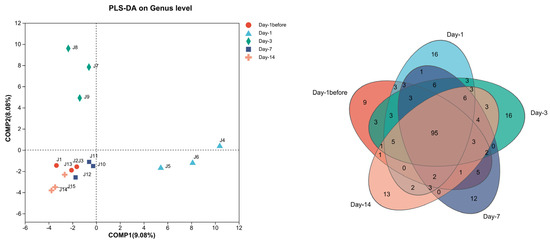
Figure 2.
Trend of changes at the genus level of cucumber rhizosphere fungi at different time points after the treatment of field soil with the biocontrol agent Bacillus subtilis 1JN2. PLS-DA = Partial Least Squares Discriminant Analysis.
3.3. Rhizo-Fungal Community Analysis at the Genus Level
After treatment with B. subtilis 1JN2, the changes in the cucumber rhizo-fungal community mainly included Ascomycota, Olpidiomycota, Basidiomycota, Blastocladiomycota, Mortierellomycota, Rozellomycota and Chytridiomycota at the phylum level (Figure 3A). Among them, the population of Olpidiomycota decreased with the prolongation of treatment with 1JN2. In contrast, the population of Basidiomycota increased over time. The populations of fungi in the other phyla did not show notable changes.
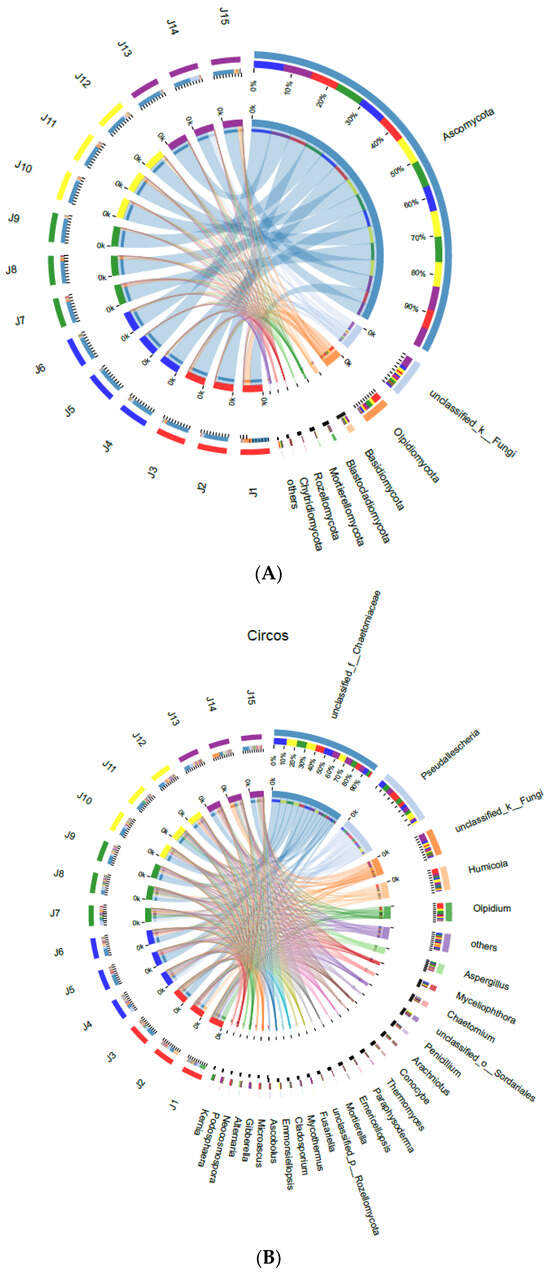
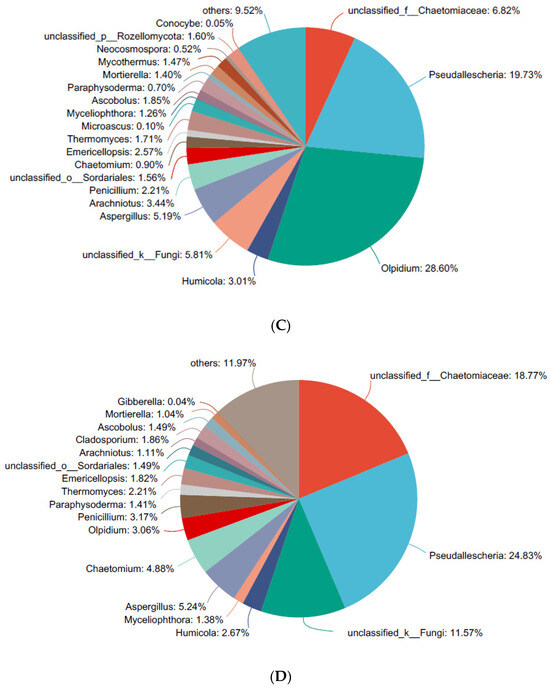
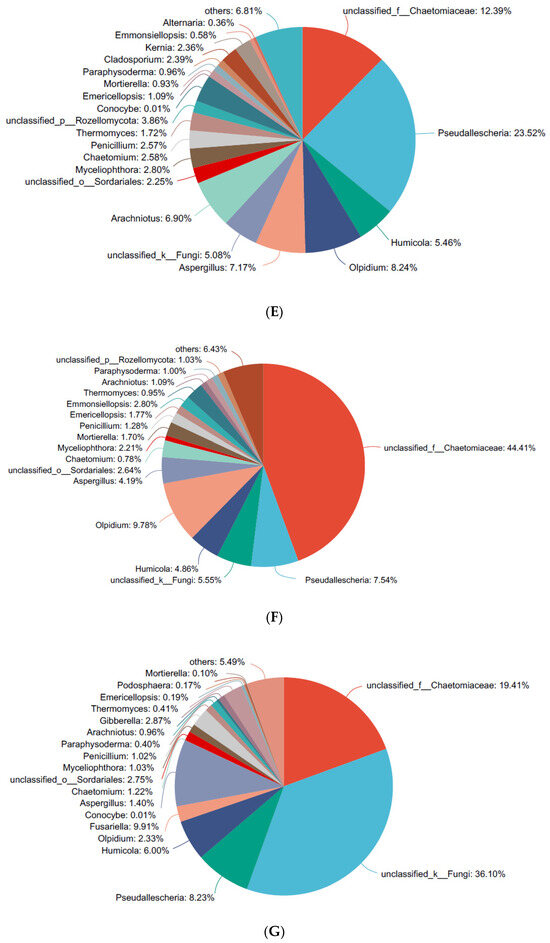
Figure 3.
Analysis of the rhizo-fungal community of cucumber after treatment with Bacillus subtilis 1JN2. (A) Circos analysis at the phyla level; (B) Circos analysis at the genus level; (C–G) analysis at the genus level by sampling time point. In (A,B), J1–J3, J4–6, J7–J9, J10–J12, and J13–J15 represent three repetitions of each sampling time point, respectively. The small semicircle (left half-circle) represents the composition of species in the sample. The color of the outer color band represents which group it comes from, the color of the inner color band represents the species, and the length represents the relative abundance of the species in the corresponding sample. The large semicircle (right half-circle) represents the distribution ratio of species in different samples at the taxonomic level, with the outer color band representing the species, the inner color band representing different groups, and the length representing the distribution ratio of the sample in a certain species.
At the genus level (Figure 3B), the populations of fungi that decreased after treatment with 1JN2 mainly included the genera Olpidium and Pseudallescheria, from more than 20% to less than 8%.
After treatment with 1JN2, the fungi in the rhizosphere of cucumber that increased included the family of Chaetomiaceae, from 6.82 to 18.77, 12.39, 44.41, and 19.41% at the four sample time points after treatment (Figure 3C–G). Except the unknown genera in the family of Chaetomiaceae, fungi from Chaetomium, which was the model genera of the Chaetomiaceae family, also increased at one day and three days after being treated with B. subtilis 1JN2.
3.4. Analysis of Cucumber Rhizosphere Soil Enzyme Activities
Soil enzyme activity can be a proxy for the metabolic activity of rhizosphere microbes, along with being an important criterion for judging the health of soil. Four enzyme activities were analyzed after treatment with B. subtilis 1JN2, including catalase, soil dehydrogenase, alkaline phosphatase, and polyphenol oxidase.
With the extension of time after biocontrol treatment, the above soil enzyme activities all showed an upward trend (Figure 4). From the third day after treatment, the upward trend became more apparent. On day 14 after biocontrol treatment, all enzyme activities reached their highest values.
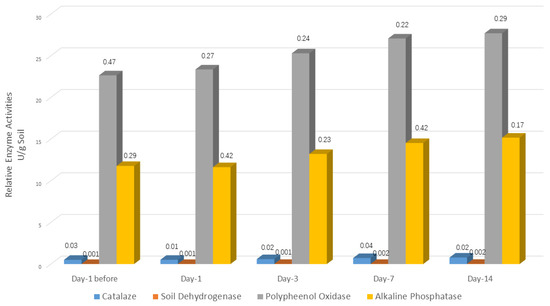
Figure 4.
Rhizosphere soil enzyme activities of cucumber after treatment with Bacillus subtilis 1JN2. Values are means of standard deviations of three replicates.
4. Discussion
Biological control is currently a promising method for controlling cucumber wilt disease [12,25]. A large number of biocontrol strains that have inhibitory effects against F. oxysporum have been screened in the laboratory [26,27,28]. For example, B. subtilis FJ3 has the potential to produce lipopeptides such as fengycin, surfactin, mycosubtilin, and pilpastatin that could inhibit several plant diseases, including the Fusarium wilt of cucumber [29]. The endophytic isolate Paenibacillus polymyxa hg18, with significant biocontrol efficacy against Fusarium wilt on cucumber, secreted IAA, a siderophore; hydrolytic enzymes, including protease, cellulase and glucanase; and antimicrobial compounds, such as iturin, fengycin, and non-ribosomal polypeptide synthase, as well as fixed nitrogen [30]. However, in actual field cultivation, the disease prevention efficacy of biocontrol agents tends to be uneven, with the main reason being the influence of environmental factors. This includes, among others, soil type, plant species and growth period, and indigenous microorganisms [31], among which the latter are often the main factors affecting the effectiveness of biological control [32]. The reason for this is that when screening for biocontrol activity in the laboratory, the direct inhibitory effect against the pathogen is often taken for granted [20]. However, in practical application, it is not a one-on-one relationship between the biocontrol agent and pathogen, but rather an interaction between the entire microbial community in the rhizosphere of the host plant [17]. This is also why many biocontrol strains that do not directly inhibit pathogens exhibit high disease prevention effects when applied in the field [33,34]. The addition of biocontrol bacteria may result in a dynamic balancing of the entire rhizosphere microbial community, thus keeping the pathogen in a suppressed condition, which is the key to achieving long-term effective biocontrol [17].
The adjustment of the root microbiome involves multiple methods, such as the application of disease-suppressive soil and crop rotation between different crops [35,36]. Research has shown that compared with susceptible soil, inhibitory soil has a higher diversity of rhizosphere microbial communities, and the rhizosphere is enriched with more beneficial microorganisms, such as Bacillus, Pseudomonas, and Streptomyces [37,38]. In the rotation system of corn and soybean, soil microorganisms can also serve as predictive indicators of yield [39]. Similar results were also found in our preliminary research that crop rotation between cucumber and Volvariella volvacea (straw mushroom) can effectively shift the cucumber rhizosphere microbial community structure, thereby inhibiting the occurrence of Fusarium wilt [9]. Therefore, regardless of the measures taken, the restoration of the root microbiome is the key to achieving disease prevention.
In addition, the addition of beneficial microorganisms can also achieve the restoration of the root microbiome of host plants. This includes two roles: one is the direct interaction between the biocontrol agent and indigenous microbial population, and the other is the indirect influence of the biocontrol agent through changes in the root exudates of host plants. For example, Wang et al. [40] reported that after the inoculation of a biocontrol agent, beneficial bacteria such as Sphingomonas, Bacillus, Nocardioides, Rhizobium, Streptomyces, Pseudomonas, and Microbacterium were more abundant in the treatment groups than in the control group. And beneficial fungi such as Chaetomium, Penicillium, and Humicola were also more abundant in the treatment groups than in the control group. In our previous study, we found that after inoculation with B. subtilis 1JN2, the content of amino acid in the root exudates of cucumber was significantly increased; thus, bacteria of Hydrogenispora and Vicinamibacteria in the rhizosphere were significantly increased after treatment [21].
In this study, the rhizosphere fungal diversity of cucumber was analyzed after inoculation with 1JN2. The populations that decreased included the genera Olpidium and Pseudallescheria, and the most increased population belonged to the family Chaetomiaceae. Among them, the members of the Olpidium genus include various plant pathogens, such as Olpidium brassicae, which is a ubiquitous obligate root-infecting fungal pathogen and an important vector of a wide range of plant viruses [41]. Olpidium bornovanus, previously regarded as a nonpathogenic organism, was proved to be a root pathogen by Stanghellini et al. [42], which can significantly brown the roots and reduce the shoot and root growth of melons.
Pseudallescheria, also, is considered the cause of one of the “clinically significant emerging mycoses” [43], but has been typically associated with animals rather than plants. The authors suggested that Pseudallescheria species prefer human-impacted environments, such as agricultural and garden soil, sewers, polluted ponds, and sediments.
Most thermophilic species in the fungal kingdom belong to the family of Chaetomiaceae, and the enzymes produced by these species exhibit the highest activity at high temperatures ranging from 50 to 70 °C, making them an important reserve for new industrial heat-stable enzymes [44,45]. Ibrahim et al. [46] summarized the enzymes and secondary metabolites with diverse biotechnological and industrial applications of the Chaetomiaceae species.
Although the three cucumber rhizosphere fungi mentioned above showed notable changes after 1JN2 treatment, the patterns of changes in each fungus over time were not consistent. Among them, fungi belonging to the Olpidium genus rapidly decreased after 1JN2 treatment, directly decreasing from 28.6% on the day before treatment to 3.06% on the day after treatment. Then there was a slight increase in the two following sampling times, and it only accounted for 2.33% at 14 days after treatment. In contrast, fungi from Pseudolescheria showed a slight increase after being treated with 1JN2, this being followed by a significant decrease on the seventh day after treatment. On the 14th day after treatment, it only accounted for 8.23%. According to previous reports, whether in response to drought stress or to host plants and exogenous nitrogen fertilizers, the response speed of plant rhizosphere bacteria is generally faster than that of plant rhizosphere fungi, and the magnitude of changes is greater [47,48]. However, in this study, two genera of fungi, especially the Olpidium genus, showed a fast response to the inoculation of the biocontrol agent. The reason for this may be due to the long-term planting of a single crop in the experimental plot, which resulted in relatively low microbial diversity in the soil, making the rhizo-microbiome more susceptible to the influence of the exogenous inoculation of microorganisms or host plants. For fungi from the family of Chaetomiaceae that show an increasing trend, their population density did not show a significant correlation with 1JN2 inoculation time. The reason may be due to the fact that the family contains a large number of genera, and the fungal changes in each genus are not consistent. In future research, the focus should be on specific genera of this family, and a longer research period should be set up.
At the same time, we found that the population density of the pathogen causing cucumber wilt disease also decreased after biocontrol inoculation. This suggests that the inoculation of B. subtilis 1JN2 can effectively alter the microbial community structure of the cucumber rhizosphere, thereby achieving the prevention and control of wilt disease. Inoculation with the biocontrol agent also enhances soil-related enzyme activity, which is part of a vital soil environment. Based on previous results [21], we speculate that the change in the root microbiome is the result of the interaction between the biocontrol bacterial strain and the host plant after inoculation, but further work is needed to confirm this hypothesis.
Soil enzymes are known for a substantial role in energy transfer, catalyzing reactions necessary for all life processes, and are also used as an indicator of soil health. Soil microbes and enzyme are co-dependent with one another, enhancing soil fertility by increasing nutrient availability for plant growth [49]. In this study, four soil enzyme activities were detected, involving organic matter conversion, organic phosphorus mineralization, and reactive oxygen elimination. According to the results, the activity of all four enzymes showed a slow increasing trend during this study period. Compared with the notable shifts in the population structure of cucumber rhizosphere fungi, the changes in soil-related enzyme activity were not significant. Analyzing the reasons, this may be due to the short research period and the low soil microbial diversity caused by long-term monoculture in the experimental field. The microbiome of the cucumber rhizosphere after inoculation with the biocontrol strain 1JN2 is not yet stable, so the changes reflected in soil enzyme activity are not notable. In future research, multiple inoculations of biocontrol strains and longer testing periods can be used to elucidate the mechanisms of changes in the soil microecology.
5. Conclusions
It was found that inoculation with the biocontrol agent B. subtilis 1JN2 caused notable changes in the fungal diversity of the cucumber rhizosphere, with a decrease in Olpidium and Pseudolescheria and an increase in Chaetomiaceae. In addition, the population density of the FOC decreased. The above results can provide a theoretical basis for the further application of this biocontrol strain and the screening of new soil-borne disease biocontrol strains. In future research, more attention should be paid to changes in the root microbiome of the host plants during the prevention and control of soil-borne diseases by different biocontrol strains—for example, by establishing a network relationship map between biocontrol strains, pathogens, and changing functional microbial communities. In this manner, effective multi-microbial ecological regulators can be constructed for different diseases, fundamentally playing a role in preventing and controlling soil-borne diseases.
Author Contributions
W.Y.: Conceptualization, Original draft, Funding acquisition, Formal analysis. L.W.: Investigation, Validation, Formal analysis. X.L.: Investigation, Formal analysis. H.Y.: Methodology, Resources. B.Z.: Writing—review and editing. X.D.: Writing—review and editing. Q.G.: Writing—review and editing. T.H.: Writing—review and editing. Y.L.: Supervision, Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation for Higher Education Institutions of Jiangsu Province (21KJA210005).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, J.; Cao, J.; Wang, C.; Hao, N.; Zhang, X.; Liu, M.; Wu, T. Research progress on the leaf morphology, fruit development and plant architecture of the cucumber. Plants 2022, 11, 2128. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Nie, W.; Qian, T.; He, L.; Zhang, H.; Jin, H.; Cui, J.; Wang, H.; Zhou, Q.; Yu, J. Low plant density improves fruit quality without affecting yield of cucumber in different cultivation periods in greenhouse. Agronomy 2022, 12, 1441. [Google Scholar] [CrossRef]
- Sharma, D.; Shukla, A. Fusarium wilt of cucumber—A Review. Int. J. Econ. Plants 2021, 8, 193–200. [Google Scholar] [CrossRef]
- Guo, J.; Hu, X.; Li, Y. Effect of Fusarium oxysporum f. sp. cucumerinum on photosynthesis and water physiological characteristics in cucumber. J. Nanjing Agric. Univ. 2011, 34, 79–80. [Google Scholar]
- Yang, F.; Jiang, H.; Chang, G.; Liang, S.; Ma, K.; Cai, Y.; Tian, B.; Shi, X. Effects of rhizosphere microbial communities on cucumber Fusarium wilt disease suppression. Microorganisms 2023, 11, 1576. [Google Scholar] [CrossRef]
- Hiddink, G.A.; Termorshuizen, A.J.; van Bruggen, A.H. Mixed cropping and suppression of soilborne diseases. In Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming; Springer: Berlin/Heidelberg, Germany, 2010; pp. 119–146. [Google Scholar]
- Fu, H.; Fu, J.; Zhou, B.; Wu, H.; Liao, D.; Liu, Z. Biochemical mechanisms preventing wilting under grafting: A case study on pumpkin rootstock grafting to wax gourd. Front. Plant Sci. 2024, 15, 1331698. [Google Scholar] [CrossRef] [PubMed]
- Gong, X. The Effect of Crop Rotation of Celery on the Yield of Continuous Cropping Cucumber and Soil Microorganisms. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2020. [Google Scholar]
- Yang, W.; Yan, H.; Zhang, J.; Meng, Y.; Wang, X.; Ji, L.; Luo, Y. Response of rhizosphere microbial diversity and soil physico-chemical properties in a rotation of cucumber with Volvariella volvacea. Biocontrol Sci. Technol. 2017, 27, 311–323. [Google Scholar] [CrossRef]
- Zhao, S.; Du, C.; Tian, C. Current advances in integrated management of Cucumber Fusarium Wilt. Chin. Agric. Sci. Bull. 2014, 30, 254–259. [Google Scholar]
- Lecomte, C.; Alabouvette, C.; Edel-Hermann, V.; Robert, F.; Steinberg, C. Biological control of ornamental plant diseases caused by Fusarium oxysporum: A review. Biol. Control 2016, 101, 17–30. [Google Scholar] [CrossRef]
- Tariq, M.; Khan, A.; Asif, M.; Khan, F.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Biological control: A sustainable and practical approach for plant disease management. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2020, 70, 507–524. [Google Scholar] [CrossRef]
- Yang, F.; Jiang, H.; Ma, K.; Wang, X.; Liang, S.; Cai, Y.; Jing, Y.; Tian, B.; Shi, X. Genome sequencing and analysis of Bacillus velezensis VJH504 reveal biocontrol mechanism against cucumber Fusarium wilt. Front. Microbiol. 2023, 14, 1279695. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, J.; Fan, C.; Sun, S.; An, X.; Sun, Y.; Gao, T.; Zhang, D. Influence of Bacillus subtilis strain Z-14 on microbial ecology of cucumber rhizospheric vermiculite infested with Fusarium oxysporum f. sp. cucumerinum. Pestic. Biochem. Physiol. 2024, 201, 105875. [Google Scholar] [CrossRef]
- Xue, J.; Sun, L.; Xu, H.; Gu, Y.; Lei, P. Bacillus atrophaeus NX-12 Utilizes Exosmotic Glycerol from Fusarium oxysporum f. sp. cucumerinum for Fengycin Production. J. Agric. Food Chem. 2023, 71, 10565–10574. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, J.; Suo, M.; Wu, H.; Zhao, M.; Yang, H. Biocontrol mechanisms of Bacillus velezensis against Fusarium oxysporum from Panax ginseng. Biol. Control 2023, 182, 105222. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, Y.; Gan, G.; Li, W.; Wan, W.; Jiang, Y.; Yang, T.; Zhang, Y.; Xu, Y.; Wang, Y.; et al. Exploring rhizo-microbiome transplants as a tool for protective plant-microbiome manipulation. ISME Commun. 2022, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Modi, D.; Picot, A. Soil and phytomicrobiome for plant disease suppression and management under climate change: A review. Plants 2023, 12, 2736. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xu, Q.; Liu, H.X.; Wang, Y.P.; Wang, Y.M.; Yang, H.T.; Guo, J.H. Evaluation of biological control agents against Ralstonia wilt on ginger. Biol. Control 2012, 62, 144–151. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Yan, H.; Sun, Y.; Wu, D.; Du, Y.; Luo, Y. Recruitment of beneficial cucumber rhizosphere microbes mediated by amino acid secretion induced by biocontrol Bacillus subtilis isolate 1JN2. Front. Microbiol. 2024, 15, 1379566. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Qiu, M.; Zhang, R.; Xue, C.; Zhang, S.; Li, S.; Zhang, N.; Shen, Q. Application of bio-organic fertilizer can control Fusarium wilt of cucumber plants by regulating microbial community of rhizosphere soil. Biol. Fertil. Soils 2012, 48, 807–816. [Google Scholar] [CrossRef]
- Ye, X.; Li, Z.; Luo, X.; Wang, W.; Li, Y.; Li, R.; Zhang, B.; Qiao, Y.; Zhou, J.; Fan, J.; et al. A predatory myxobacterium controls cucumber Fusarium wilt by regulating the soil microbial community. Microbiome 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Boulahouat, S.; Cherif-Silini, H.; Silini, A.; Bouket, A.C.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Biocontrol efficiency of rhizospheric Bacillus against the plant pathogen Fusarium oxysporum: A promising approach for sustainable agriculture. Microbiol. Res. 2023, 14, 892–908. [Google Scholar] [CrossRef]
- Lian, H.; Li, R.; Ma, G.; Zhao, Z.; Zhang, T.; Li, M. The effect of Trichoderma harzianum agents on physiological-biochemical characteristics of cucumber and the control effect against Fusarium wilt. Sci. Rep. 2023, 13, 17606. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, X.; Yin, Y.; Norvienyeku, J.; Khan RA, A.; Zhang, M.; Ren, S.; Chen, J.; Liu, T. Biocontrol of cucumber Fusarium wilt by Trichoderma asperellum FJ035 dependent on antagonism and spatiotemporal competition with Fusarium oxysporum. Biol. Control 2023, 186, 105334. [Google Scholar] [CrossRef]
- Chu, R.; Li, Z.X.; Zhang, X.Q.; Yang, D.Y.; Cao, H.H.; Zhang, X.Y. Screening and identification of antagonistic Bacillus spp. against cucumber Fusarium wilt and its biocontrol effect. Biotechnol. Bull. 2023, 39, 262. [Google Scholar]
- Jan, F.; Arshad, H.; Ahad, M.; Jamal, A.; Smith, D.L. In vitro assessment of Bacillus subtilis FJ3 affirms its biocontrol and plant growth promoting potential. Front. Plant Sci. 2023, 14, 1205894. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Yang, C.; Ma, T.; Richard, O.; Jin, M.; Zhang, C.; Wang, Y. An endophytic Paenibacillus polymyxa hg18 and its biocontrol potential against Fusarium oxysporum f. sp. cucumerinum. Biol. Control 2023, 188, 105380. [Google Scholar] [CrossRef]
- Chiarini, L.; Bevivino, A.; Dalmastri, C.; Nacamulli, C.; Tabacchioni, S. Influence of plant development, cultivar and soil type on microbial colonization of maize roots. Appl. Soil Ecol. 1998, 8, 11–18. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Winding, A.; Binnerup, S.J.; Pritchard, H. Non-target effects of bacterial biological control agents suppressing root pathogenic fungi. FEMS Microbiol. Ecol. 2004, 47, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kaur, J.; Singh, R.S. Nonpathogenic Fusarium as a biological control agent. Plant Pathol. J. 2011, 9, 79–91. [Google Scholar] [CrossRef]
- Wu, H.; Chen, S.; Zhu, L.; Xu, Y.; Li, J.; Ling, N.; Yuan, J.; Xue, C.; Shen, Q. Identification of antibiotic resistant bacteria communities and a GeoChip based study of resistome in Fusarium wilt diseased and healthy soil. Appl. Soil Ecol. 2024, 193, 105103. [Google Scholar] [CrossRef]
- Duan, P.; Liu, X.; Niu, G.; Jia, N.; Wen, T.; Zeng, J.; Chen, Q.; Zhang, J.; Xue, C.; Shen, Q.; et al. Application of coronarin enhances maize drought tolerance by affecting interactions between rhizosphere fungal community and metabolites. Comput. Struct. Biotechnol. J. 2023, 21, 5273–5284. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Jiang, Q.; Bai, Y.; Shen, G.; Li, S.; Ding, W. Using community analysis to explore bacterial indicators for disease suppression of tobacco bacterial wilt. Sci. Rep. 2016, 6, 36773. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Shen, M.; Sun, F.; Wang, X.; Liu, J.; Li, J. Characteristics of Rhizosphere Microbial Communities in a Disease suppressive Soil of Tomato Bacterial Wilt and Its Disease-suppressive Transmission Mechanism. Acta Pedol. Sin. 2021, 59, 1125–1135. [Google Scholar]
- Benitez, M.S.; Ewing, P.M.; Osborne, S.L.; Lehman, R.M. Rhizosphere microbial communities explain positive effects of diverse crop rotations on maize and soybean performance. Soil Biol. Biochem. 2021, 159, 108309. [Google Scholar] [CrossRef]
- Wang, X.; Ji, C.; Song, X.; Liu, Z.; Liu, Y.; Li, H.; Gao, Q.; Li, C.; Zheng, R.; Han, X.; et al. Biocontrol of Two Bacterial Inoculant Strains and Their Effects on the Rhizosphere Microbial Community of Field-Grown Wheat. BioMed. Res. Int. 2021, 2021, 8835275. [Google Scholar] [CrossRef] [PubMed]
- Hartwright, L.M.; Hunter, P.J.; Walsh, J.A. A comparison of Olpidium isolates from a range of host plants using internal transcribed spacer sequence analysis and host range studies. Fungal Biol. 2010, 114, 26–33. [Google Scholar] [CrossRef]
- Stanghellini, M.E.; Mathews, D.M.; Misaghi, I.J. Pathogenicity and management of Olpidium bornovanus, a root pathogen of melons. Plant Dis. 2010, 94, 163–166. [Google Scholar] [CrossRef][Green Version]
- Walts, A.E. Pseudallescheria: An underdiagnosed fungus? Diagn. Cytopathol. 2001, 25, 153–157. [Google Scholar] [CrossRef]
- Wang, X.W.; Yang, F.Y.; Meijer, M.; Kraak, B.; Sun, B.D.; Jiang, Y.L.; Wu, Y.M.; Bai, F.Y.; Seifert, P.W.; Samson, R.A.; et al. Redefining Humicola sensu stricto and related genera in the Chaetomiaceae. Stud. Mycol. 2019, 93, 65–153. [Google Scholar] [CrossRef]
- Wang, X.W.; Han, P.J.; Bai, F.Y.; Luo, A.; Bensch, K.; Meijer, M.; Kraak, B.; Han, D.Y.; Sun, B.D.; Crous, P.W.; et al. Taxonomy, phylogeny and identification of Chaetomiaceae with emphasis on thermophilic species. Stud. Mycol. 2022, 101, 121. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, S.G.; Sindi, I.A.; Mohamed, G.A. Biologically active secondary metabolites and biotechnological applications of species of the family Chaetomiaceae (Sordariales): An updated review from 2016 to 2021. Mycol. Prog. 2021, 20, 595–639. [Google Scholar] [CrossRef]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 2019, 7, 136. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Jamir, E.; Kangabam, R.D.; Borah, K.; Tamuly, A.; Deka Boruah, H.P.; Silla, Y. Role of soil microbiome and enzyme activities in plant growth nutrition and ecological restoration of soil health. In Microbes and Enzymes in Soil Health and Bioremediation; Springer: Singapore, 2019; pp. 99–132. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).