A Comparison of Semi-Solid, Liquid, and Temporary Immersion Bioreactor Systems for Effective Plant Regeneration of Gerbera jamesonii “Shy Pink”
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
2.2. Collection of Data on the Morphological and Growth Parameters
2.3. Estimation of the Chlorophyll and Carotenoid Contents
2.4. Measurement of Chlorophyll Fluorescence
2.5. The Stomatal Index
2.6. Acclimatization
2.7. Statistical Analysis
3. Results
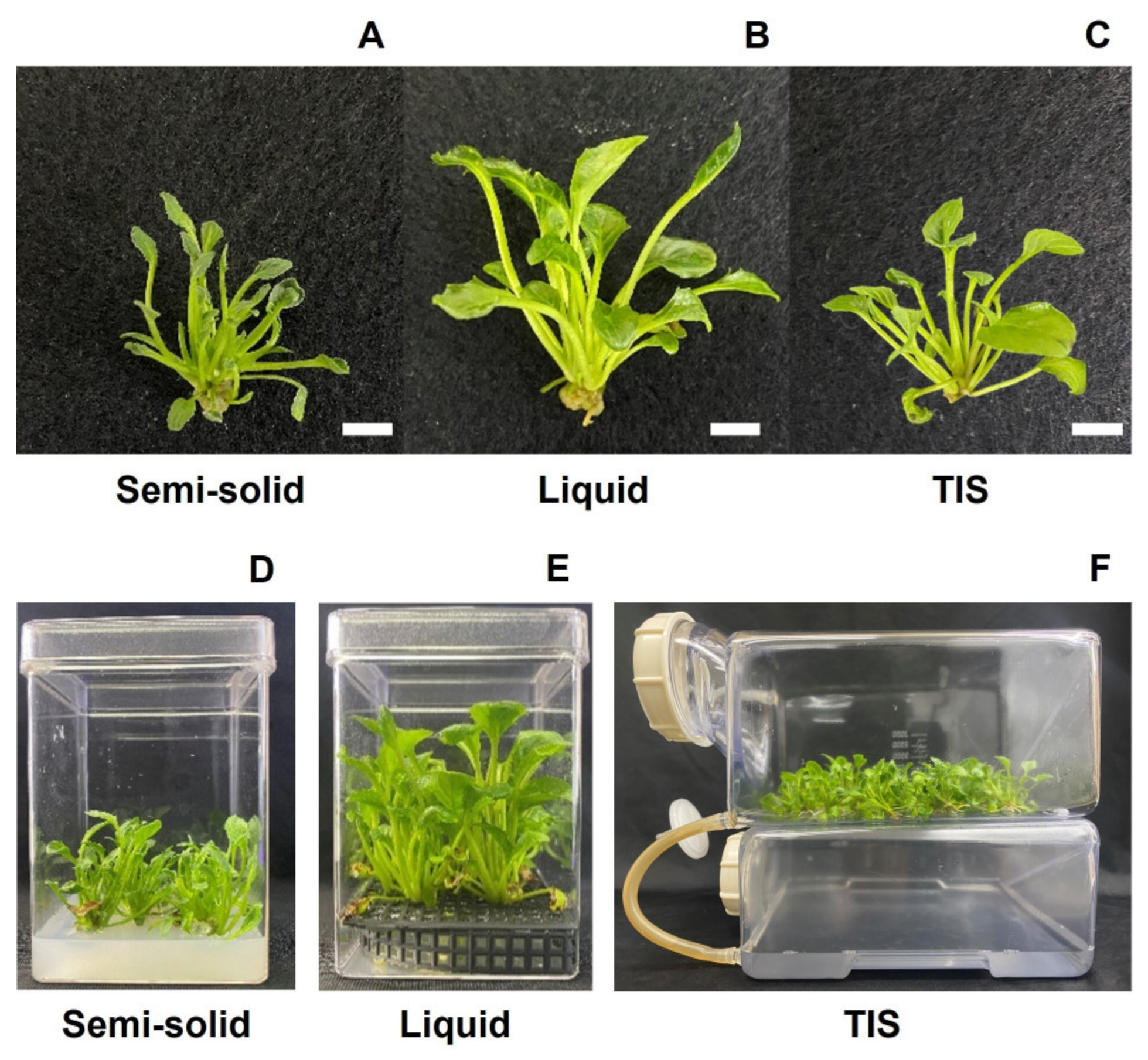
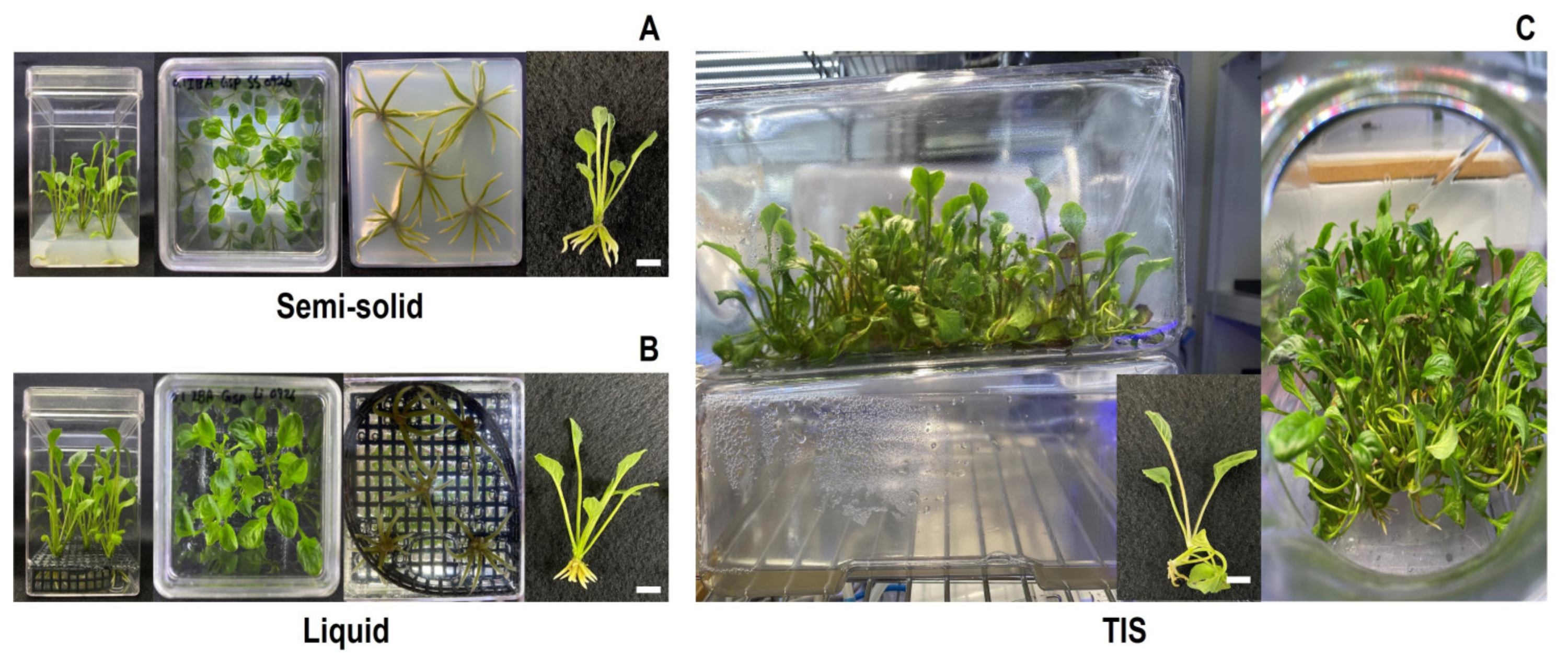
3.1. Morphological and Growth Parameters of Shoots and Plantlets Regenerated with Different Culture Systems
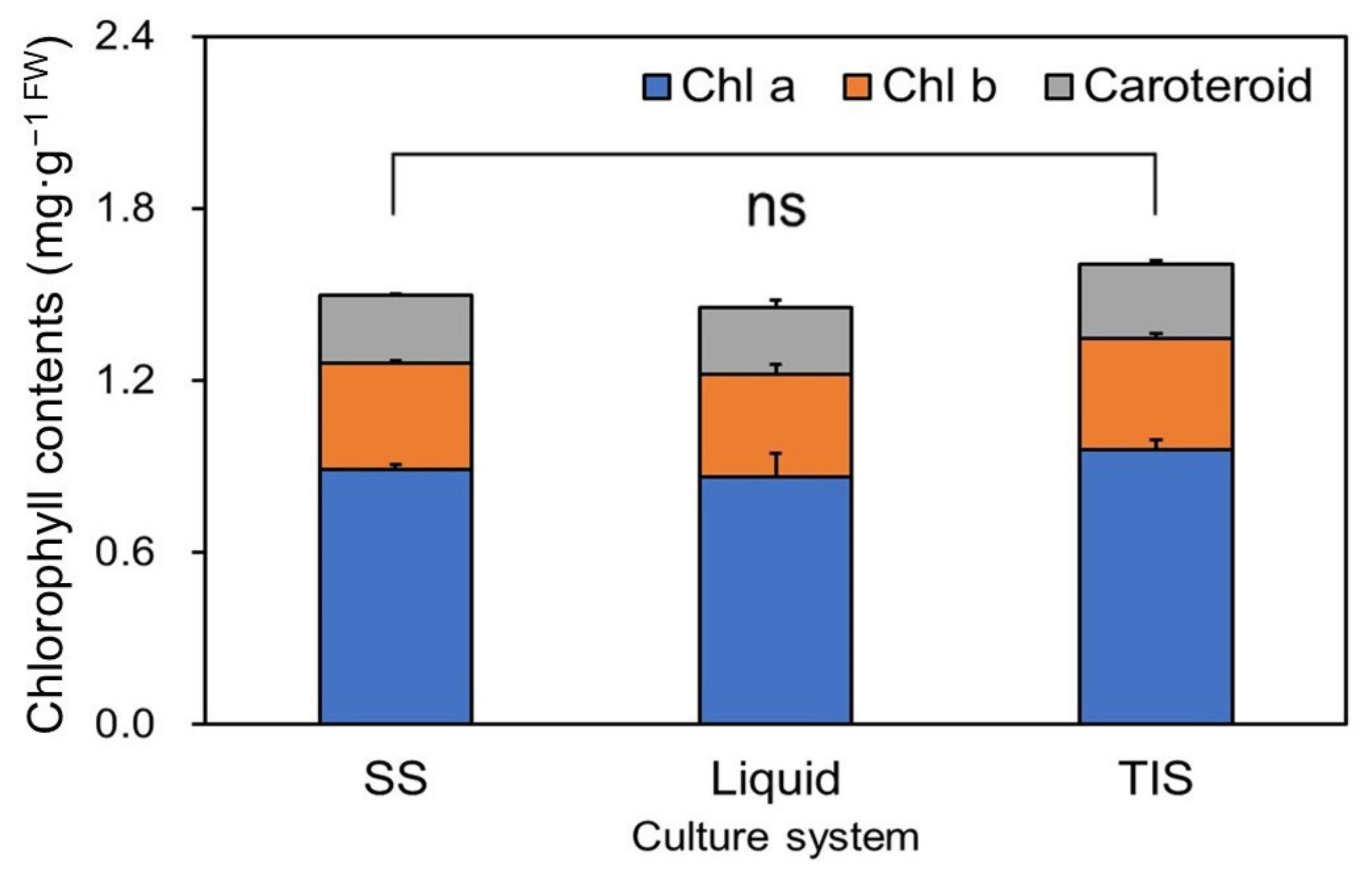
3.2. Chlorophyll and Carotenoid Contents of the Regenerated Plants Using Different Culture Systems
3.3. Chlorophyll Fluorescence Parameters
3.4. Analysis of the Stomatal Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Yin, M.; Abbas, F.; Sun, Y.; Gao, T.; Yan, F.; Li, X.; Yu, Y.; Yue, Y.; Yu, R.; et al. Classification and association analysis of gerbera (Gerbera hybrida) flower color traits. Font. Plant Sci. 2022, 12, 779288. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.C.; Teixeira da Silva, J.A. Gerbera micropropagation. Biotechnol. Adv. 2013, 31, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.J.; Murthy, H.N.; Song, H.Y.; Lee, S.Y.; Park, S.Y. Influence of white, red, blue and combination of LED lights on in vitro multiplication of shoots, rooting, and acclimatization of Gerbera jamesonii cv. ‘Shy Pink’ plants. Agronomy 2023, 13, 2216. [Google Scholar] [CrossRef]
- Maene, P.; Debergh, P. Liquid medium additions to established tissue cultures to improve elongation and rooting in vivo. Plant Cell Tiss. Organ. Cult. 1985, 67, 25–35. [Google Scholar] [CrossRef]
- Garcia-Ramirez, Y. Temporary immersion system for in vitro propagation via organogenesis of forest plant species. Trees 2023, 37, 611–626. [Google Scholar] [CrossRef]
- Paek, K.Y.; Chakrabarty, D.; Hahn, E.J. Application of bioreactor systems for large scale production of horticultural and medicinal plants. Plant Cell Tissue Organ. Cult. 2005, 81, 287–300. [Google Scholar] [CrossRef]
- Watt, M.P. The status of temporary immersion system (TIS) technology for plant micropropagation. Afr. J. Biotechnol. 2012, 11, 14025–14035. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Bioreactor systems for micropropagation of plants: Present scenario and future prospects. Front. Plant Sci. 2023, 14, 1159588. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, J.; Ji, H.; An, L.; Xia, X. Hyperhydricity-induced ultrastructural and physiological changes in blueberry (Vaccinium spp.). Plant Cell Tissue Organ. Cult. 2018, 133, 65–76. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Aragón, C.E.; Sánchez, C.; Gonzalez-Olmedo, J.; Escalona, M.; Carvalho, L.; Amâncio, S. Comparison of plantain plantlets propagated in temporary immersion bioreactors and gelled medium during in vitro growth and acclimatization. Biol. Plant 2014, 58, 29–38. [Google Scholar] [CrossRef]
- Hwang, H.D.; Kwon, S.H.; Murthy, H.N.; Yun, S.W.; Pyo, S.S.; Park, S.Y. Temporary immersion bioreactor system as an efficient method for mass production of in vitro plant in horticulture and medicinal plants. Agronomy 2022, 12, 346. [Google Scholar] [CrossRef]
- Vervit SETISTM Bioreactor Temporary Immersion System in Plant Micropropagation. 2024. Available online: http://www.setissystems.be (accessed on 12 July 2024).
- Arano-Avalos, S.; Gómez-Merino, F.C.; Mancilla-Álvarez, E.; Sánchez-Páez, R.; Bello-Bello, J.J. An efficient protocol for commercial micropropagation of malanga (Colocasia esculenta L. Schott) using temporary immersion. Sci. Hortic. 2020, 261, 108998. [Google Scholar] [CrossRef]
- Kim, N.Y.; Hwang, H.D.; Kim, J.H.; Kwon, B.M.; Kim, D.; Park, S.Y. Efficient production of virus-free apple plantlets using the temporary immersion bioreactor system. Hortic. Environ. Biotechnol. 2020, 61, 779–785. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Cruz-Cruz, C.A.; Pérez-Guerra, J.C. A new temporary immersion system for commercial micropropagation of banana (Musa AAA cv. Grand Naine). Vitr. Cell Dev. Biol. Plant 2019, 55, 313–320. [Google Scholar] [CrossRef]
- Godoy, S.; Tapia, E.; Seit, P.; Andrade, D.; Sánchez, E.; Andrade, P.; Prieto, H. Temporary immersion systems for the mass propagation of sweet cherry cultivars and cherry rootstocks: Development of a micropropagation procedure and effect of culture conditions on plant quality. Vitr. Cell Dev. Biol. Plant 2017, 53, 494–504. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectrophotometry. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; Wiley: New York, NY, USA, 2001; pp. F4.3.1–F4.3.8. [Google Scholar]
- Alvard, D.; Cote, F.; Teisson, C. Comparison of methods of liquid medium culture for banana micropropagation. Plant Cell Tissue Organ. Cult. 1993, 32, 55–60. [Google Scholar] [CrossRef]
- Frometa, O.S.; Morgado, M.M.E.; Teixeira da Silva, J.A.; Morgado, D.T.P.; Gradaille, M.A.D. In vitro propagation Gerbera jamesonii Bolus ex Hooker f. In a temporary immersion bioreactor. Plant Cell Tissue Organ. Cult. 2017, 129, 543–551. [Google Scholar] [CrossRef]
- Marchant, M.J.; Molina, P.; Montecinos, M.; Guzman, L.; Balada, C.; Fassio, C.; Castro, M. In vitro propagation of Ester Island Curcuma longa from rhizome explants using temporary immersion system. Agronomy 2021, 11, 2121. [Google Scholar] [CrossRef]
- Martinez-Estrada, E.; Islas-Luna, B.; Perez-Sato, A.; Bello-Bello, J.J. Temporary immersion improves in vitro multiplication and acclimatization of Anthurium andreanum Lind. Sci. Hortic. 2019, 249, 185–191. [Google Scholar] [CrossRef]
- Arigundam, U.; Variyath, A.M.; Siow, Y.L.; Marshall, D.; Debnath, S.C. Liquid culture for efficient in vitro propagation of adventitious shoots in wild Vaccinium vitis-idea ssp. minus (lingonberry) using temporary immersion and stationary bioreactors. Sci. Hortic. 2020, 264, 109199. [Google Scholar] [CrossRef]
- Kunakhonnuruk, B.; Inthima, P.; Kongbangkerd, A. In vitro propagation of rheophytic orchid, Epipactis flava Seidenf.—A comparison of semi-solid, continuous immersion and temporary immersion systems. Biology 2019, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Fraser, N.; Hashimoto, H.; Cogdell, R. Carotenoids and bacterial photosynthesis: The story so far. Photosynth. Res. 2001, 70, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Zobayed, S.M.A. Ventilation in micropropagation. In Photoautotrophic (Sugar-Free Medium) Micropropagation as a New Micropropagation and Transplant Production System; Kozai, T., Afreen, F., Zobayed, S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 147–186. [Google Scholar] [CrossRef]
- Vieira, L.; de Freitas Fraga, H.P.; dos Anjos, K.G.; Puttkammer, C.C.; Scherer, R.F.; da Silva, D.A.; Guerra, M.P. Light-emitting diodes (LED) increase the stomata formation and chlorophyll content in Musa acuminata (AAA) ‘Nanicão Corupá’ in vitro plantlets. Theor. Exp. Plant Physiol. 2015, 27, 91–98. [Google Scholar] [CrossRef]
- Martre, P.; Lacan, D.; Just, D.; Teisson, C. Physiological effects of temporary immersion on Hevea brasiliensis callus. Plant Cell Tissue Organ. Cult. 2001, 67, 25–35. [Google Scholar] [CrossRef]
- Aragón, C.E.; Escalona, M.; Rodriguez, R.; Cañal, M.J.; Capote, I.; Pina, D.; González-Olmedo, J. Effect of sucrose, light, and carbon dioxide on plantain micropropagation in temporary immersion bioreactors. Vitr. Cell Dev. Biol. Plant 2010, 46, 89–94. [Google Scholar] [CrossRef]
- Regueira, M.; Rial, E.; Blanco, B.; Bogo, B.; Aldrey, A.; Correa, B.; Varas, E.; Sánchez, C.; Vidal, N. Micropropagation of axillary shoots of Salix viminalis using a temporary immersion system. Trees 2018, 32, 61–71. [Google Scholar] [CrossRef]
- Ahmadian, M.; Babaei, A.; Shokri, S.; Hessami, S. Micropropagation of carnation (Dianthus caryophyllus L.) in liquid medium by temporary immersion bioreactor in comparison with solid culture. J. Genet. Eng. Biotechnol. 2017, 15, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Yeh, D.M. In vitro leaf anatomy, ex vitro photosynthetic behaviors and growth of Calathea orbifolia (Linden) Kennedy plants obtained from semi-solid medium and temporary immersion systems. Plant Cell Tissue Organ. Cult. 2008, 93, 201–207. [Google Scholar] [CrossRef]

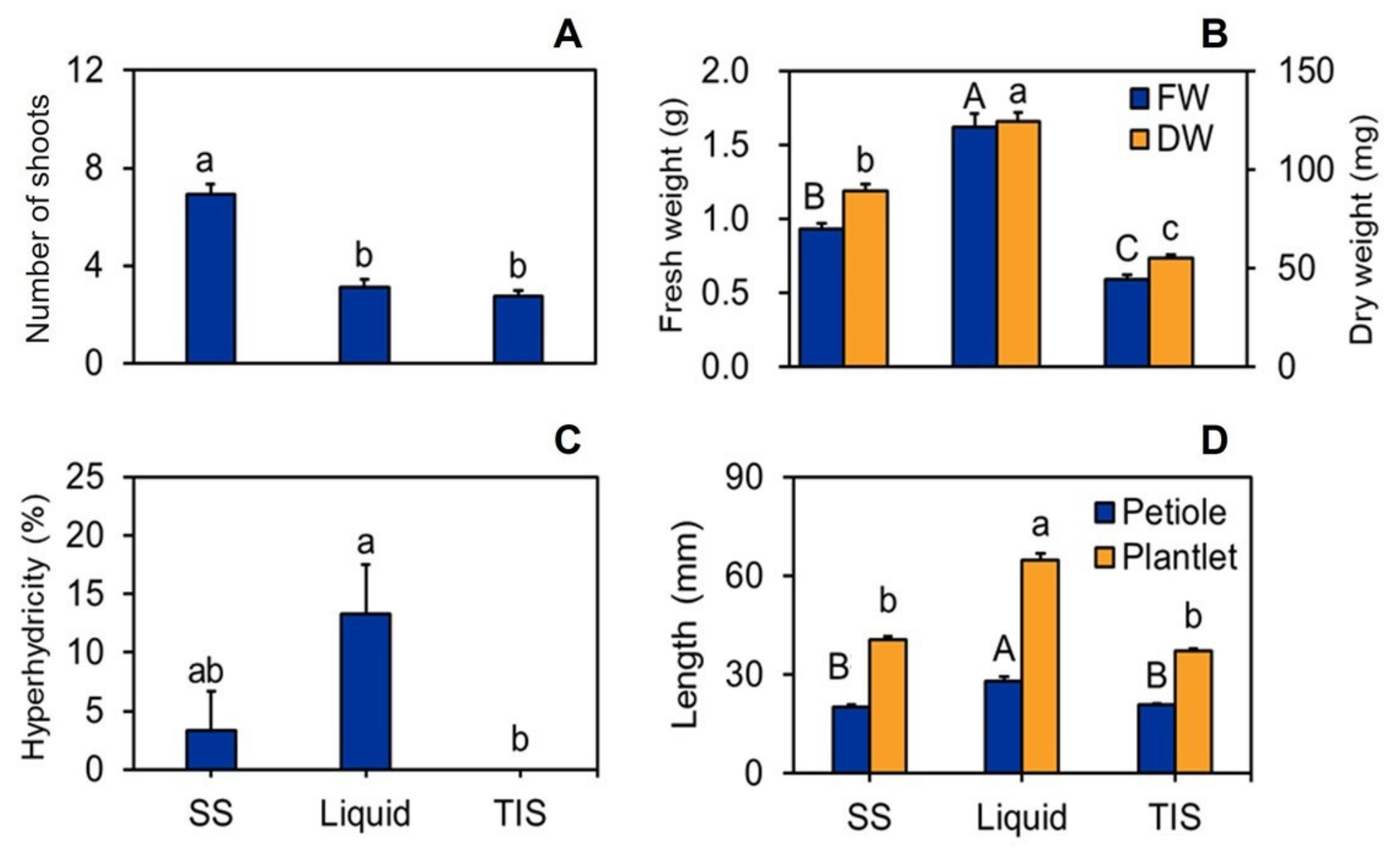



| Culture System | Total FW (g) | Total DW (mg) | Plantlet Height (mm) | Shoot Height (mm) | No of Roots | Root Length (mm) | Petiole Length (mm) | Leaf Length (mm) | Leaf Width (mm) | Leaf Area (mm2) | Leaf Index |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | 0.62 ± 0.0 c | 60.90 ± 1.0 c | 71.74 ± 0.8 c | 54.92 ± 0 c | 6.60 ± 0.4 b | 16.40 ± 0.7 b | 28.92 ± 1.2 c | 14.81 ± 0.4 c | 9.32 ± 0.2 c | 91.30 ± 3.9 c | 1.59 ± 0.0 a |
| LQ | 0.71 ± 0.0 b | 70.45 ± 1.1 b | 80.13 ± 1.5 b | 65.85 ± 1.3 b | 7.80 ± 0.2 a | 14.12 ± 0.8 c | 34.56 ± 1.5 b | 17.84 ± 0.5 b | 11.42 ± 0.4 b | 136.78 ± 8.7 b | 1.59 ± 0.0 a |
| TIS | 0.87 ± 0.0 a | 75.80 ± 2.3 a | 88.87 ± 1.5 a | 74.88 ± 0.8 a | 7.85 ± 0.2 a | 18.80 ± 0.6 a | 42.85 ± 0.9 a | 22.78 ± 0.3 a | 14.21 ± 0.3 a | 206.94 ± 6.8 a | 1.62 ± 0.0 a |
| Culture System | F0 | Fm | Fv | Fv/Fm |
|---|---|---|---|---|
| SS | 8070.88 ± 71.9 b | 44,926.13 ± 54.5 b | 36,855.25 ± 487.0 b | 0.82 ± 0.0 a |
| LQ | 8095.00 ± 190.2 b | 46,620.75 ± 1073.8 ab | 38,525.75 ± 904.3 ab | 0.83 ± 0.0 a |
| TIS | 8627.80 ± 147.2 a | 48,778.63 ± 1494.5 a | 40,150.88 ± 1386.4 a | 0.82 ± 0.0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, M.-J.; Han, J.-E.; Murthy, H.N.; Song, H.-Y.; Lee, S.-Y.; Park, S.-Y. A Comparison of Semi-Solid, Liquid, and Temporary Immersion Bioreactor Systems for Effective Plant Regeneration of Gerbera jamesonii “Shy Pink”. Horticulturae 2024, 10, 836. https://doi.org/10.3390/horticulturae10080836
Lim M-J, Han J-E, Murthy HN, Song H-Y, Lee S-Y, Park S-Y. A Comparison of Semi-Solid, Liquid, and Temporary Immersion Bioreactor Systems for Effective Plant Regeneration of Gerbera jamesonii “Shy Pink”. Horticulturae. 2024; 10(8):836. https://doi.org/10.3390/horticulturae10080836
Chicago/Turabian StyleLim, Myeong-Jin, Jong-Eun Han, Hosakatte Niranjana Murthy, Hyun-Young Song, Su-Young Lee, and So-Young Park. 2024. "A Comparison of Semi-Solid, Liquid, and Temporary Immersion Bioreactor Systems for Effective Plant Regeneration of Gerbera jamesonii “Shy Pink”" Horticulturae 10, no. 8: 836. https://doi.org/10.3390/horticulturae10080836
APA StyleLim, M.-J., Han, J.-E., Murthy, H. N., Song, H.-Y., Lee, S.-Y., & Park, S.-Y. (2024). A Comparison of Semi-Solid, Liquid, and Temporary Immersion Bioreactor Systems for Effective Plant Regeneration of Gerbera jamesonii “Shy Pink”. Horticulturae, 10(8), 836. https://doi.org/10.3390/horticulturae10080836






