Investigating the Impact of Various Growing Media on the Expansion of Green Wall Plant Coverage with Image Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Components of Growing Media
2.2. Installation of Green Wall System
2.3. Physical and Chemical Properties of Growing Media
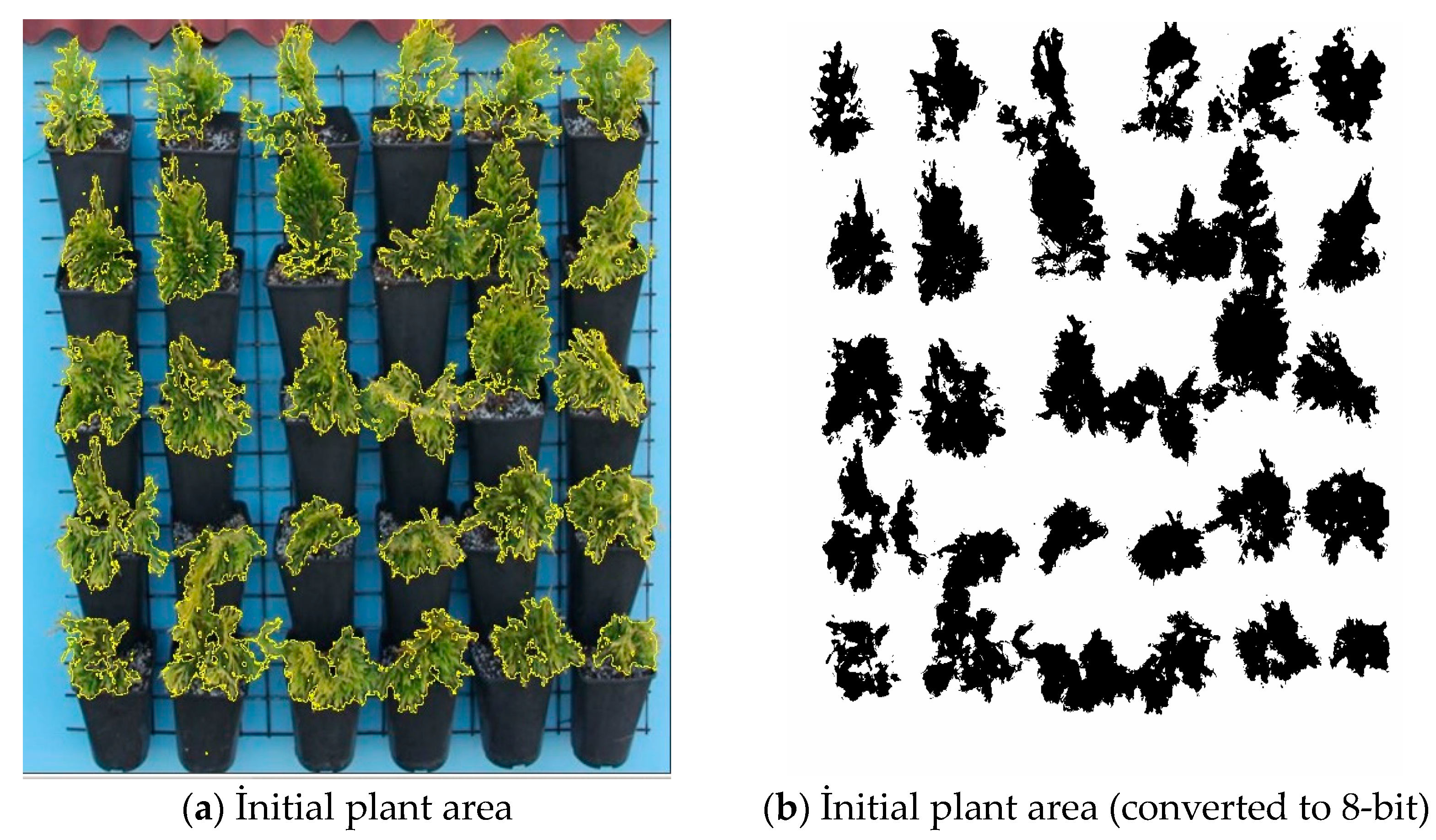
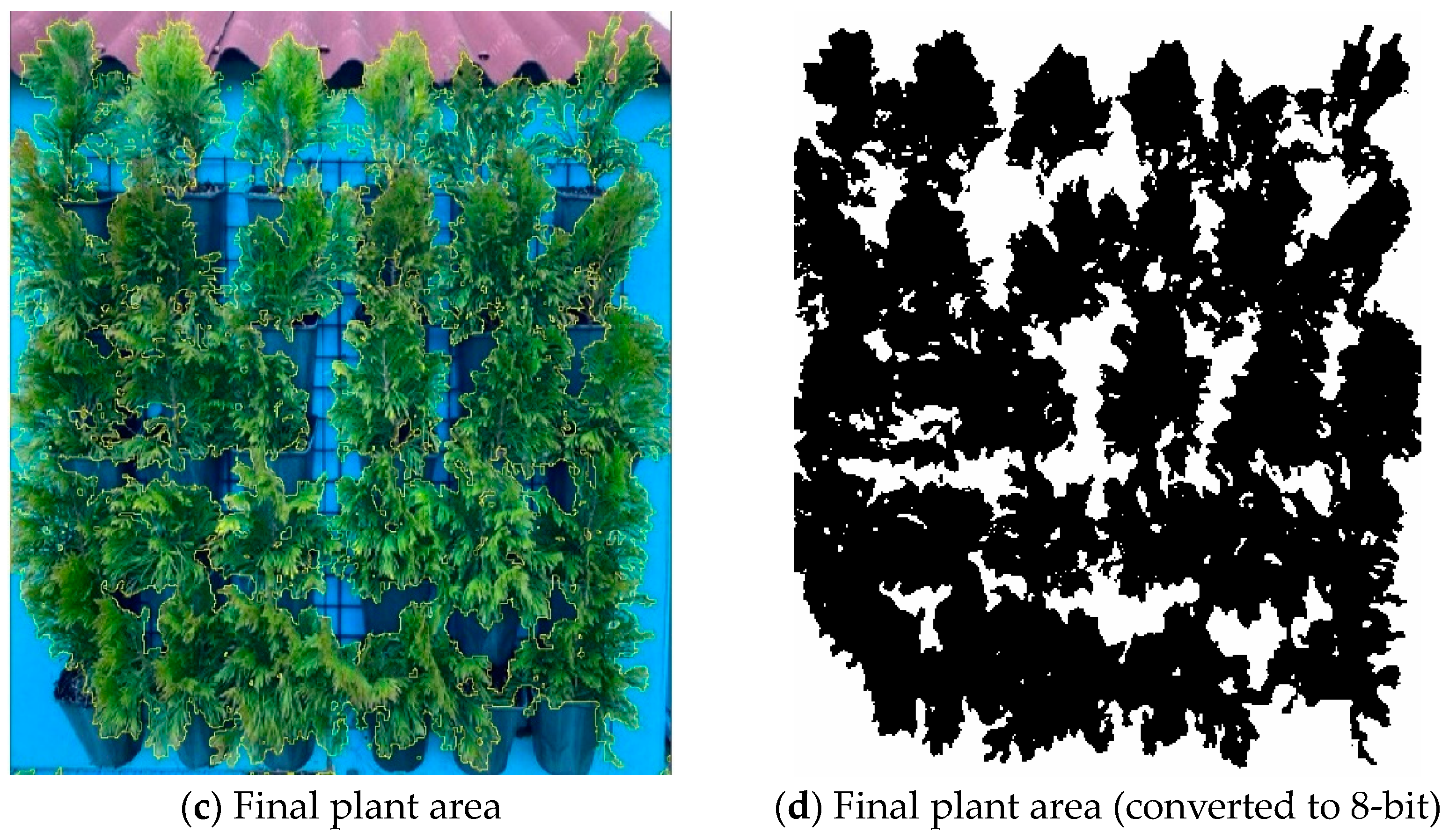
2.4. Plant Area of Green Wall
2.5. Statistical Analyses
3. Results and Discussions
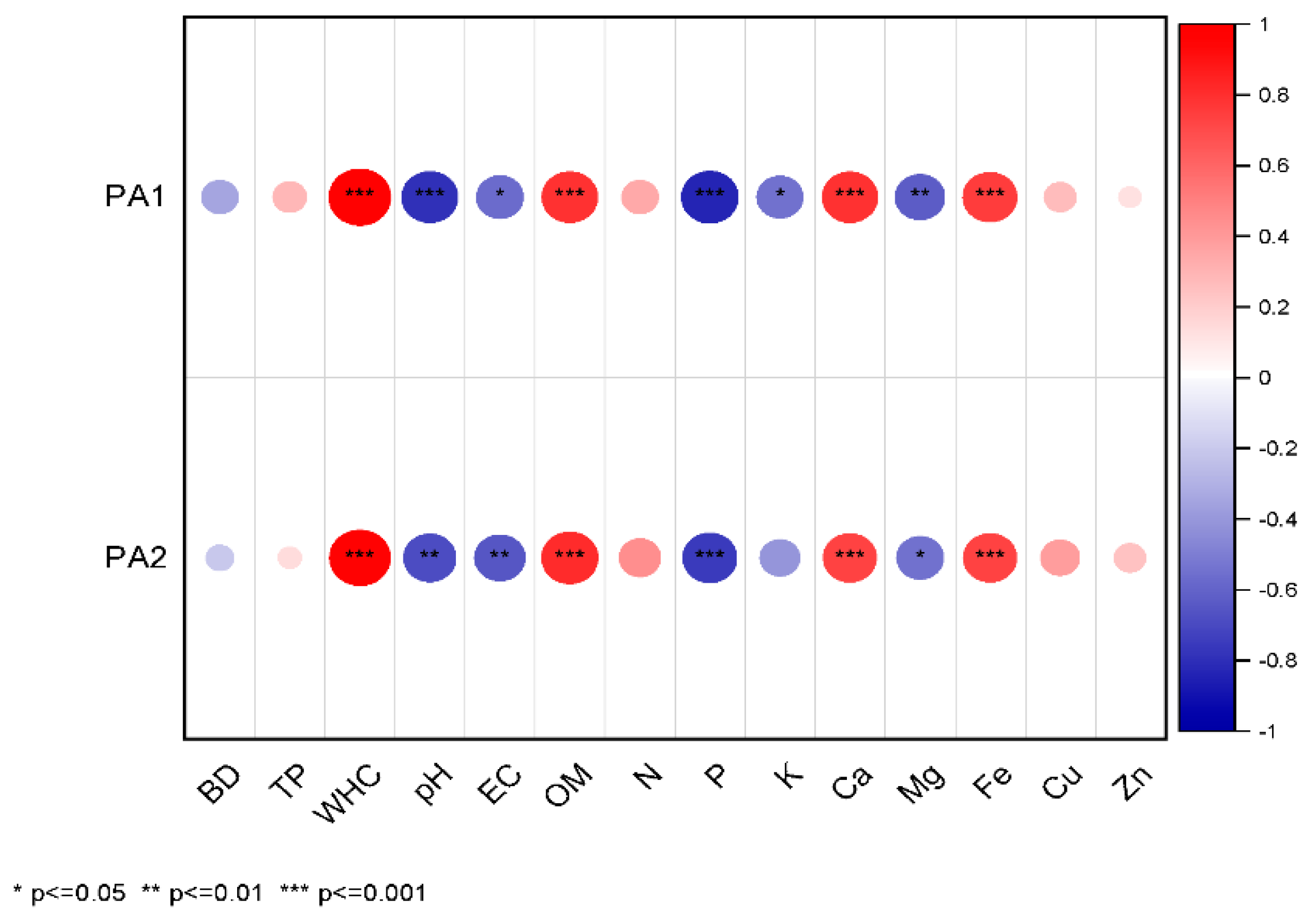
3.1. Evaluation of Growing Media Properties
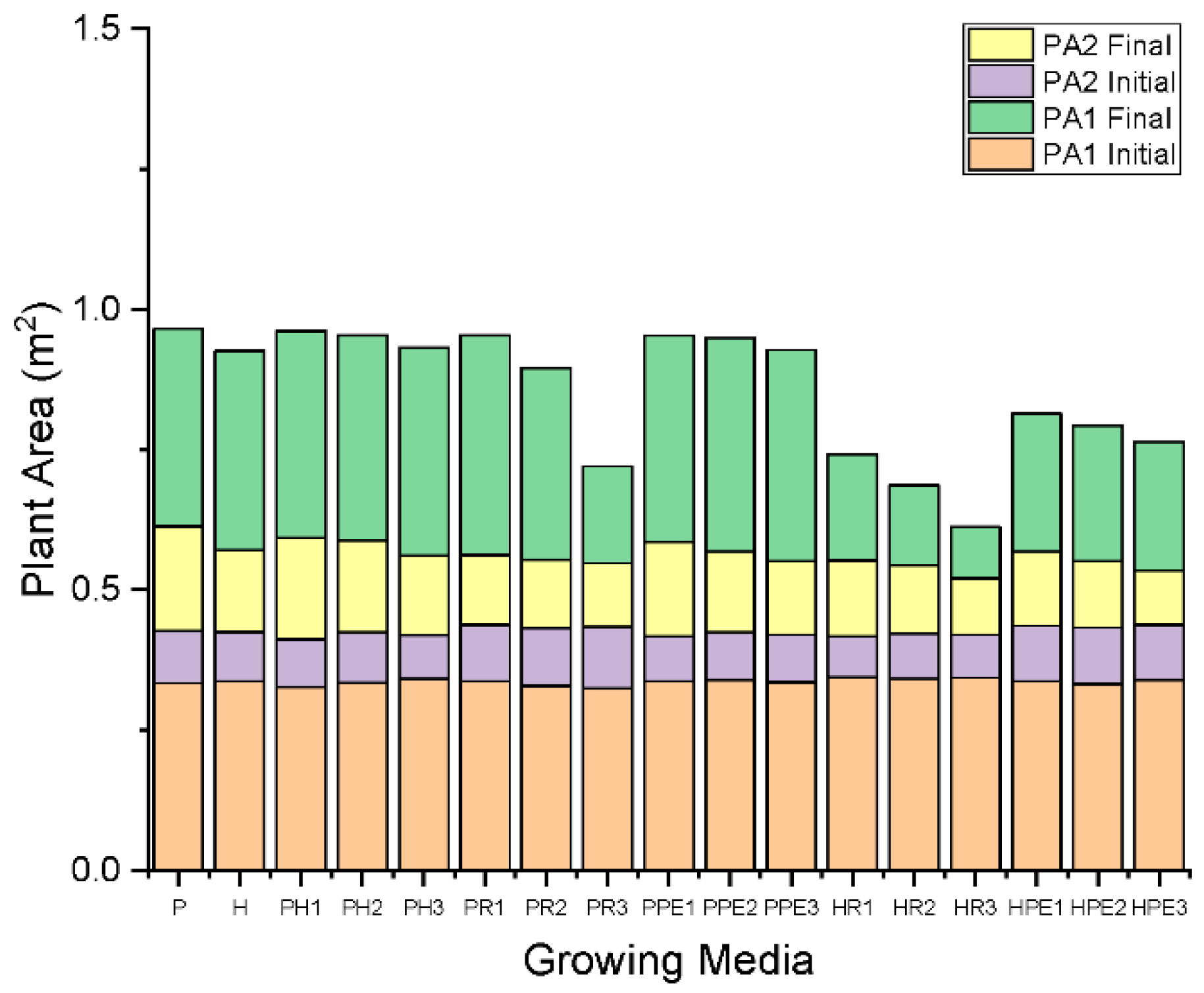
3.2. Evaluation of Plant Area of Green Wall Increase with Indirect Monitoring Technique
4. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dede, O.H.; Mercan, N.; Ozer, H.; Dede, G.; Pekarchuk, O.; Mercan, B. Thermal insulation characteristics of green wall systems using different growing media. Energy Build. 2021, 240, 110872. [Google Scholar] [CrossRef]
- Marchi, M.; Pulselli, R.M.; Marchettini, N.; Pulselli, F.M.; Bastianoni, S. Carbon dioxide sequestration model of a vertical greenery system. Ecol. Model. 2015, 306, 46–56. [Google Scholar] [CrossRef]
- Cheng, C.; Cheung, K.K.; Chu, L. Thermal performance of a vegetated cladding system on facade walls. Build. Environ. 2010, 45, 1779–1787. [Google Scholar] [CrossRef]
- Susca, T.; Gaffin, S.R.; Dell’Osso, G.R. Positive effects of vegetation: Urban heat island and green roofs. Environ. Pollut. 2011, 159, 2119–2126. [Google Scholar] [CrossRef]
- Pan, L.; Chu, L. Energy saving potential and life cycle environmental impacts of a vertical greenery system in Hong Kong: A case study. Build. Environ. 2016, 96, 293–300. [Google Scholar] [CrossRef]
- Mayrand, F.; Clergeau, P. Green Roofs and Green Walls for Biodiversity Conservation: A Contribution to Urban Connectivity? Sustainability 2018, 10, 985. [Google Scholar] [CrossRef]
- Ysebaert, T.; Koch, K.; Samson, R.; Denys, S. Green walls for mitigating urban particulate matter pollution—A review. Urban For. Urban Green. 2021, 59, 127014. [Google Scholar] [CrossRef]
- De Bock, A.; Belmans, B.; Vanlanduit, S.; Blom, J.; Alvarado-Alvarado, A.; Audenaert, A. A review on the leaf area index (LAI) in vertical greening systems. Build. Environ. 2023, 229, 109926. [Google Scholar] [CrossRef]
- Van Renterghem, T.; Hornikx, M.; Forssen, J.; Botteldooren, D. The potential of building envelope greening to achieve quietness. Build. Environ. 2013, 61, 34–44. [Google Scholar] [CrossRef]
- Kontoleon, K.; Eumorfopoulou, E. The effect of the orientation and proportion of a plant-covered wall layer on the thermal performance of a building zone. Build. Environ. 2010, 45, 1287–1303. [Google Scholar] [CrossRef]
- Chianucci, F.; Puletti, N.; Giacomello, E.; Cutini, A.; Corona, P. Estimation of leaf area index in isolated trees with digital photography and its application to urban forestry. Urban For. Urban Green. 2015, 14, 377–382. [Google Scholar] [CrossRef]
- Peper, P.J.; McPherson, E.G. Evaluation of four methods for estimating leaf area of isolated trees. Urban For. Urban Green. 2003, 2, 19–29. [Google Scholar] [CrossRef]
- Pérez, G.; Coma, J.; Sol, S.; Cabeza, L.F. Green facade for energy savings in buildings: The influence of leaf area index and facade orientation on the shadow effect. Appl. Energy 2017, 187, 424–437. [Google Scholar] [CrossRef]
- Convertino, F.; Schettini, E.; Blanco, I.; Bibbiani, C.; Vox, G. Effect of Leaf Area Index on Green Facade Thermal Performance in Buildings. Sustainability 2022, 14, 2966. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Burés, S. National inventory of organic wastes for use as growing media for ornamental potted plant production: Case study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Puchades, R.; Maquieira, A.; Noguera, V. Physico-chemical and chemical properties of some coconut coir dusts for use as a peat substitute for containerised ornamental plants. Bioresour. Technol. 2002, 82, 241–245. [Google Scholar] [CrossRef]
- Martinez, F.X. Proposal of methodology for the determination of the physical properties of the substrata. Minutes Gard. 1992, 11, 5566. [Google Scholar]
- Dede, O.H.; Oztekin, M.H. Relationship between optical microscopic structure and physical characterization of organic wastes originated peat substitutes. Appl. Ecol. Environ. Res. 2018, 16, 1173–1184. [Google Scholar] [CrossRef]
- CEN 13650; Soil Improvers and Growing Media—Extraction of Aqua Regia Soluble Elements. Eurpean Union: Brussels, Belgium, 2001.
- Benito, M.; Masaguer, A.; Moliner, A.; De Antonio, R. Chemical and physical properties of pruning waste compost and their seasonal variability. Bioresour. Technol. 2006, 97, 2071–2076. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis, 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Orsini, F.; D’Urzo, M.P.; Inan, G.; Serra, S.; Oh, D.-H.; Mickelbart, M.V.; Consiglio, F.; Li, X.; Jeong, J.C.; Yun, D.-J.; et al. A comparative study of salt tolerance parameters in 11 wild relatives of Arabidopsis thaliana. J. Exp. Bot. 2010, 61, 3787–3798. [Google Scholar] [CrossRef]
- Warman, L.; Moles, A.T.; Edwards, W. Not so simple after all: Searching for ecological advantages of compound leaves. Oikos 2010, 120, 813–821. [Google Scholar] [CrossRef]
- Juneau, K.J.; Tarasoff, C.S. Leaf Area and Water Content Changes after Permanent and Temporary Storage. PLoS ONE 2012, 7, e42604. [Google Scholar] [CrossRef]
- Murphy, M.R.C.; Jordan, G.J.; Brodribb, T.J. Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant Cell Environ. 2012, 35, 1407–1418. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Easlon, H.M.; Nemali, K.S.; Richards, J.H.; Hanson, D.T.; Juenger, T.E.; Mckay, J.K. The physiological basis for genetic variation in water use effi ciency and carbon isotope composition in Arabidopsis thaliana. Photosynth. Res. 2014, 119, 119–129. [Google Scholar]
- Gruda, N.; Schnitzler, W.H. Suitability of wood fiber substrate for production of vegetable transplants: I. Physical properties of wood fiber substrates. Sci. Hortic. 2004, 100, 309–322. [Google Scholar] [CrossRef]
- Abad, M.; Fornes, F.; Carrión, C.; Noguera, V.; Noguera, P.; Maquieira, A.; Puchades, R. Physical Properties of Various Coconut Coir Dusts Compared to Peat. HortScience 2005, 40, 2138–2144. [Google Scholar] [CrossRef]
- Spiers, T.; Fietje, G. Green Waste Compost as a Component in Soilless Growing Media. Compos. Sci. Util. 2000, 8, 19–23. [Google Scholar] [CrossRef]
- Handreck, K.A. Rapid assessment of the rate of nitrogen immobilisation in organic components of potting media: II. Nitrogen drawdown index and plant growth. Commun. Soil Sci. Plant Anal. 1992, 23, 217–230. [Google Scholar] [CrossRef]
- Graceson, A.; Hare, M.; Hall, N.; Monaghan, J. Use of inorganic substrates and composted green waste in growing media for green roofs. Biosyst. Eng. 2014, 124, 1–7. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M.; Abad, M.; Noguera, P.; Puchades, R.; Maquieira, A.; Noguera, V. The microstructure of coconut coir dusts for use as alternatives to peat in soilless growing media. Aust. J. Exp. Agric. 2003, 43, 1171–1179. [Google Scholar] [CrossRef]
- Dede, O.H.; Dede, G.; Ozdemir, S.; Abad, M. Physicochemical characterization of hazelnut husk residues with different decomposition degrees for soilless growing media preparation. J. Plant Nutr. 2011, 34, 1973–1984. [Google Scholar] [CrossRef]
- Farrell, C.; Ang, X.Q.; Rayner, J.P. Water-retention additives increase plant available water in green roof substrates. Ecol. Eng. 2013, 52, 112–118. [Google Scholar] [CrossRef]
- Paradelo, R.; Basanta, R.; Barral, M. Water-holding capacity and plant growth in compost-based substrates modified with polyacrylamide, guar gum or bentonite. Sci. Hortic. 2018, 243, 344–349. [Google Scholar] [CrossRef]
- Young, T.; Cameron, D.D.; Sorrill, J.; Edwards, T.; Phoenix, G.K. Importance of different components of green roof substrate on plant growth and physiological performance. Urban For. Urban Green. 2014, 13, 507–516. [Google Scholar] [CrossRef]
- Bunt, A.C. Media and Mixes for Container Grown Plants: A Manual on the Preparation and the Use of Growing Media for Growing Pot Plants, 2nd ed.; Unwin Hyman Ltd.: London, UK, 1988. [Google Scholar]
- Ogidi, E.G.O.; Okore, I.K.; Dike, J.C. Correlation analysis of nutrient soil-plant content and bud take success in hevea brasiliensis muell. arg. in acidic soil of South Eastern Nigeria. J. Exp. Biol. Agric. Sci. 2018, 6, 116–123. [Google Scholar] [CrossRef]


| Growing Media | Mixing Proportions of Materials |
|---|---|
| P | 100% Peat |
| H | 100% Hazelnut Husk |
| R | 100% Rice Hull |
| PE | 100% Perlite |
| PH1 | 87.5% Peat + 12.5% Hazelnut Husk |
| PH2 | 75% Peat + 25% Hazelnut Husk |
| PH3 | 50% Peat + 50% Hazelnut Husk |
| PR1 | 87.5% Peat + 12.5% Rice Hull |
| PR2 | 75% Peat + 25% Rice Hull |
| PR3 | 50% Peat + 50% Rice Hull |
| PPE1 | 87.5% Peat + 12.5% Perlite |
| PPE2 | 75% Peat + 25% Perlite |
| PPE3 | 50% Peat + 50% Perlite |
| HR1 | 87.5% Hazelnut Husk + 12.5% Rice Hull |
| HR2 | 75% Hazelnut Husk + 25% Rice Hull |
| HR3 | 50% Hazelnut Husk + 50% Rice Hull |
| HPE1 | 87.5% Hazelnut Husk + 12.5% Perlite |
| HPE2 | 75% Hazelnut Husk + 25% Perlite |
| HPE3 | 50% Hazelnut Husk + 50% Perlite |
| Growing Media | Bulk Density (g/cm3) | Porosity (% v/v) | Water Holding Capacity (mg/L) |
|---|---|---|---|
| P | 0.21 efg * | 86.04 fg | 770.38 a |
| H | 0.28 a | 81.43 k | 619.29 ef |
| R | 0.11 k | 93.32 b | 114.88 j |
| PE | 0.15 j | 94.39 a | 229.26 i |
| PH1 | 0.22 de | 85.55 gh | 763.50 ab |
| PH2 | 0.22 de | 85.43 gh | 736.37 b |
| PH3 | 0.23 d | 84.81 hi | 651.41 d |
| PR1 | 0.2 gh | 87.00 e | 743.88 ab |
| PR2 | 0.18 i | 88.27 d | 698.91 c |
| PR3 | 0.15 j | 90.01 c | 640.06 de |
| PPE1 | 0.2 fgh | 86.53 ef | 748.45 ab |
| PPE2 | 0.19 hi | 88.09 d | 704.99 c |
| PPE3 | 0.18 i | 90. 24 c | 620.79 ef |
| HR1 | 0.26 b | 82.41 j | 616.33 ef |
| HR2 | 0.24 c | 82.76 j | 602.54 fg |
| HR3 | 0.18 i | 86.00 fg | 566.95 h |
| HPE1 | 0.27 b | 82.4 j | 619.53 ef |
| HPE2 | 0.26 bc | 84.09 i | 601.57 fg |
| HPE3 | 0.21 def | 88.39 d | 580.51 gh |
| Growing Media | pH | EC (µS/cm) | Organic Matter (%) |
|---|---|---|---|
| P | 5.63 f * | 209.03 l | 92.31 a |
| H | 7.54 a | 256.09 j | 89.2 de |
| R | 6.25 b | 496.96 b | 83.87 hi |
| PE | 6.33 b | 693.24 a | 0 n |
| PH1 | 5.78 def | 223.9 k | 91.69 ab |
| PH2 | 5.96 cd | 226.1 k | 90.64 bc |
| PH3 | 6.26 b | 228.24 k | 90.09 cd |
| PR1 | 5.71 ef | 355.84 h | 90.13 cd |
| PR2 | 5.93 cde | 366.72 h | 88.44 ef |
| PR3 | 6.12 bc | 468.63 d | 84.81 gh |
| PPE1 | 5.75 def | 287.51 i | 90.563 bc |
| PPE2 | 5.97 cd | 354.14 h | 79.97 j |
| PPE3 | 6.16 bc | 447.16 e | 56.25 l |
| HR1 | 7.52 a | 257.48 j | 87.56 f |
| HR2 | 7.48 a | 265.99 j | 85.38 g |
| HR3 | 7.46 a | 416.14 f | 83.07 i |
| HPE1 | 7.53 a | 292.99 i | 87.83 f |
| HPE2 | 7.51 a | 383.37 g | 73.1 k |
| HPE3 | 7.46 a | 482.78 c | 50.74 m |
| Growing Media | N (%) | P (mg/kg) | K (mg/kg) | Ca (mg/kg) | Mg (mg/kg) | Fe (mg/kg) | Cu (mg/kg) | Zn (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| P | 1.34 cd * | 1284.83 m | 1039.32 m | 2587.28 a | 1183.74 j | 192.39 a | 5.34 e | 28.38 de |
| H | 1.48 a | 2156.82 g | 2442.67 a | 1432 n | 1262.22 e | 134.25 i | 5.87 a | 36.35 a |
| R | 0.42 k | 3231.26 a | 1186.15 i | 1674 j | 1346.06 a | 168.2 e | 1.80 m | 8.44 j |
| PE | 0 l | 0.73 s | 36.43 r | 108.3 s | 42.53 p | 1.62 m | 0.11 n | 0.19 k |
| PH1 | 1.38 bc | 1316.01 l | 1464.75 h | 2183.94 f | 1206.36 hi | 190.48 ab | 5.73 c | 32.02 c |
| PH2 | 1.41 b | 1381.9 k | 1634.10 g | 1992.23 h | 1212.07 gh | 185.91 bc | 5.75 c | 33.55 bc |
| PH3 | 1.43 ab | 1489.26 j | 2193.96 d | 1787.7 i | 1220.92 g | 152.93 g | 5.81 abc | 37.06 a |
| PR1 | 1.15 e | 1380.06 k | 1052 l | 2491.89 b | 1197.87 i | 161.73 f | 5.16 f | 24.09 g |
| PR2 | 0.97 f | 2461.93 e | 1087.37 k | 2245.72 d | 1246.16 f | 164.04 ef | 2.91 k | 15.21 i |
| PR3 | 0.52 j | 2518.58 c | 1119.84 j | 2037.95 g | 1289.39 c | 176.61 d | 2.76 l | 13.33 i |
| PPE1 | 0.96 f | 1175.67 n | 992 n | 2425.5 c | 1150.38 k | 191.01 ab | 5.35 e | 27.43 ef |
| PPE2 | 0.68 h | 986.12 p | 953.87 o | 2218.84 e | 983.78 m | 182.48 cd | 5.28 e | 26.12 f |
| PPE3 | 0.44 k | 725.51 r | 745.44 p | 1482.24 m | 821.93 o | 113.12 k | 4.05 i | 19.07 h |
| HR1 | 1.32 d | 2284.46 f | 2426.26 b | 1484.67 m | 1278.43 d | 142.16 h | 5.83 ab | 35.13 ab |
| HR2 | 1.14 e | 2486.62 d | 2163 e | 1504.65 l | 1296.18 c | 148.64 g | 4.95 g | 28.53 de |
| HR3 | 0.82 g | 2921.58 b | 1948.95 f | 1544.77 k | 1318.47 b | 159.42 f | 3.64 j | 20.25 h |
| HPE1 | 1.39 bc | 1986.22 h | 2382.56 c | 1356.15 o | 1203.31 hi | 128.58 ij | 5.78 bc | 32.63 c |
| HPE2 | 1.17 e | 1823.92 i | 2163.19 e | 1173.82 p | 1092.05 l | 122.72 j | 5.53 d | 29.69 d |
| HPE3 | 0.62 i | 1147.56 o | 1942.08 f | 895.36 r | 872.18 n | 96.55 l | 4.24 h | 23.61 g |
| Parameters | PC1 | PC2 | PC2 |
|---|---|---|---|
| BD | −0.09443 | 0.37949 * | −0.10411 |
| TP | 0.00595 | −0.39858 * | −0.00744 |
| WHC | 0.38277 * | −0.07975 | −0.01044 |
| pH | −0.32026 * | 0.23017 | −0.00949 |
| EC | −0.26822 | −0.28162 | 0.00564 |
| OM | 0.26756 | 0.17847 | 0.34917 * |
| N | 0.19003 | 0.32829 * | 0.01918 |
| P | −0.16911 | 0.13091 | 0.50752 * |
| K | −0.18691 | 0.34799 * | 0.00519 |
| Ca | 0.34645 * | −0.13559 | 0.1897 |
| Mg | 0.08005 | 0.20685 | 0.50307 * |
| Fe | 0.32535 * | −0.0518 | 0.27748 |
| Cu | 0.16026 | 0.29751 | −0.29747 |
| Zn | 0.1098 | 0.34872* | −0.22818 |
| Percentage Variation % | 39.29 | 38.10 | 16.78 |
| Cumulative % | 39.29 | 77.39 | 94.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dede, O.H.; Ozer, H. Investigating the Impact of Various Growing Media on the Expansion of Green Wall Plant Coverage with Image Analysis. Horticulturae 2024, 10, 654. https://doi.org/10.3390/horticulturae10060654
Dede OH, Ozer H. Investigating the Impact of Various Growing Media on the Expansion of Green Wall Plant Coverage with Image Analysis. Horticulturae. 2024; 10(6):654. https://doi.org/10.3390/horticulturae10060654
Chicago/Turabian StyleDede, Omer Hulusi, and Hasan Ozer. 2024. "Investigating the Impact of Various Growing Media on the Expansion of Green Wall Plant Coverage with Image Analysis" Horticulturae 10, no. 6: 654. https://doi.org/10.3390/horticulturae10060654
APA StyleDede, O. H., & Ozer, H. (2024). Investigating the Impact of Various Growing Media on the Expansion of Green Wall Plant Coverage with Image Analysis. Horticulturae, 10(6), 654. https://doi.org/10.3390/horticulturae10060654






