Abstract
Organic producers have few certified organic options to meet crop nitrogen (N) demand. Poultry-based amendments, including manures and processed fertilizers from livestock waste (e.g., feather meal), are commonly used in these systems, but synchronizing nutrient release with plant demand is challenging. Cover crop residues are also used in organic systems and interact with amendments to affect soil health and nutrient cycling. We conducted a greenhouse study to quantify the effects of four cover crop residues (millet, sorghum sudangrass, cowpea, sunn hemp) and three amendments (heat-treated poultry manure, poultry manure biochar, organic fertilizer) on spinach. We measured spinach yield and nutrient uptake; soil inorganic N; total soil carbon (C) and N; and two soil health indicators: permanganate oxidizable C (POXC) and autoclaved citrate-extractable (ACE) protein. Legume residues released the greatest inorganic N, whereas all cover crop residues exhibited a higher soil ACE protein concentration compared to the control without residues. The organic fertilizer released more inorganic N but had a lower ACE protein concentration than manure-based amendments. Grass residues increased POXC relative to sunn hemp, but cover crop residues had no effect on total C. In contrast, manure-based amendments increased soil’s total C but did not affect its POXC. Spinach yield and nutrient uptake were highest with biochar, with no consistent effect of cover crop residues observed on nutrient uptake. Overall, cover crops had the greatest effect on soil health indicators (POXC and ACE protein), whereas manure-based amendments had a greater impact on crop productivity and nutrition (spinach nutrient uptake and yield).
1. Introduction
Intensive agriculture can degrade soils through practices that affect the chemical, physical, and biological properties and functions of soil [1,2]. Sustainable agricultural practices seek to prevent soil degradation and provide balanced nutrient inputs to improve soil health and agricultural economic viability [3,4], e.g., by using amendments to increase soil carbon (C) while improving crop nutrition. Certified organic systems rely on organic inputs like animal and green manure that can enhance soil properties like biological activity, water-holding capacity, and bulk density [5,6].
Nutrient management is inherently difficult in organic farming because producers must rely on animal manures, cover crops, crop rotations with legumes, and/or organic fertilizers made with livestock byproducts (e.g., feather meal) [7,8]. As organic nitrogen (N) dominates in these inputs, its mineralization into ammonium (NH4+) and subsequent nitrification to nitrate (NO3−) are necessary prior to plant uptake [9]. Hence, nutrient availability varies based on soil temperature and moisture, unlike synthetic fertilizers that are available to plants immediately after application [10,11]. Synchronizing N release with plant uptake is key to minimize nutrient losses [12], which is particularly challenging in organic systems because crop nutrient use efficiencies are lower when using organic inputs [13].
Animal manures are commonly used to meet N demands, especially poultry manure that can enhance crop nutrition and soil health [14]. The review of Lin et al. [15] found that the long-term application of poultry manure improved crop yields relative to inorganic fertilizer. However, the widespread application of manures, especially low N:P ratio poultry manure, can over-apply phosphorus (P) [16,17]. As poultry production continues to increase, waste products derived from the poultry industry must be reused efficiently [18]. Composting and heat treatment, in addition to pyrolysis-producing biochar, can densify manures, increase nutrient concentrations, and reduce transport costs.
Biochar is a high-C material that can increase soil C and add plant-available nutrients to soils [19], although agronomic benefits vary and depend heavily on biochar feedstock and production conditions [20,21]. Biochar effects also vary based on soil type and characteristics, which must be considered when biochar is commercially produced for large-scale applications. Because pyrolysis alters the concentration and speciation of C and nutrients, comparing charred vs. non-charred manure is important.
Another form of livestock waste consists of processed organic fertilizers made from byproducts of poultry processing, e.g., feather, meat, blood, and/or bone meal [22]. Processed fertilizers have high N concentrations and low C:N ratios, resulting in rapid N mineralization [23]. These N-rich compounds can also be blended with other nutrient sources to produce fertilizers with more balanced ratios of N, P, and other nutrients (e.g., potassium, K). However, these fertilizers are expensive, limiting grower adoption [24].
Cover crops are used to add organic matter and stimulate nutrient cycling for the following cash crops when residues are returned to the soil at termination [25]. Legumes like sunn hemp (Crotalaria juncea L.) and cowpea (Vigna unguiculata (L.) Walp) produce low C:N ratio residues that should rapidly decompose and release N after termination, while grasses like sorghum sudangrass (Sorghum bicolor (L.) Moench × Sorghum sudanense) and millet (Pennisetum glaucum (L.) R. Br.) produce higher C:N ratio residues that should decompose and release N more slowly [26]. Differences in the C:N ratio of residue material also has implications for C cycling, as slower decomposition should increase soil C [27].
Improving soil health in agricultural systems is a critical research focus [28], including in organic systems that have become increasingly popular [29]. Identifying management-responsive indicators is useful to finetune amendment applications and cover crop management. Indicators like permanganate oxidizable C (POXC) and autoclaved citrate-extractable (ACE) protein can act as proxies for C and N cycling, respectively, that may respond more rapidly to change than traditional variables like total C and N [30,31]. As there are many certified organic biobased amendments available [11], assessing their effectiveness for crop production and soil improvement across environments is critical.
Florida has a unique biophysical context that sets it apart from other crop production areas in the US. Most Florida soils are coarse-textured and inherently well drained with low water-holding capacity and low organic matter content [32], resulting in low fertility that requires substantial nutrient inputs for crop production [33]. These well-drained soils are also prone to nutrient leaching, which is a significant problem due to the heavy precipitation events experienced in the state and its proximity to vulnerable surface water and groundwater ecosystems (e.g., Everglades) [34]. Thus, determining how soil fertility, soil health, and crop production are affected simultaneously in this context is necessary.
We designed a greenhouse study to test how two legume (sunn hemp and cowpea) and two grass (sorghum sudangrass and millet) cover crop residues in combination with three organic amendments affected crop growth and nutrient uptake, soil nutrient availability, and soil health indicators. We expected that crop growth, nutrient uptake, and soil N would be the highest with inputs that release N most rapidly (i.e., processed fertilizer and legume residues), with greater benefits of grass residues and manure-based products observed in soil health indicators.
2. Materials and Methods
2.1. Study Design and Experimental Setup
The experiment was conducted between 9 February 2021 and 5 May 2021 in a temperature-controlled greenhouse on the main campus of the University of Florida (UF; Gainesville, FL, USA). The soil used in this experiment was collected from the UF/IFAS (Institute of Food and Agricultural Sciences) Plant Sciences Research and Education Unit (Citra, FL, USA) on 26 January 2021 and then air-dried and sieved to 2 mm. The soil is a sandy Entisol, mapped as a Gainesville loamy sand (hyperthermic coated typic quartzipsamments) and containing >90% sand. Soil testing at the UF/IFAS soil testing laboratory (https://soilslab.ifas.ufl.edu/extension-soil-testing-laboratory/, accessed on 29 May 2024) reported a pH in water (2:1 water/soil ratio) of 6.5, and Mehlich-III extractable concentrations (measured via ICP-OES) of 95 mg P kg−1 (high fertility), 8 mg K kg−1 (low fertility), and 29 mg Mg kg−1 (medium fertility). For spinach (Spinacia oleracea L.), 101 kg N ha−1 (90 lb N ac−1), 112 kg K ha−1 (120 lb K2O ac−1), and 22 kg Mg ha−1 (20 lb Mg ac−1) were recommended by UF/IFAS, but no lime, P, Ca, or S inputs.
Three amendments were used to meet the N requirement for spinach: a heat-treated poultry manure (Everlizer, Live Oak, FL, USA), a poultry manure biochar (Frye, Wardensville, WV, USA), or NatureSafe 10-2-8 fertilizer (Cold Spring, KY, USA) derived from hydrolyzed feather, meat, and blood meal, in addition to bone meal and sulfate of potash (see the next paragraph for details on how rates were computed). The heat-treated manure and fertilizer are approved for use on certified organic farms through the Organic Materials Review Institute (OMRI), but not the biochar, which consisted of poultry manure and bedding material [35,36] processed by pyrolysis [37].
The input recommendation (101 kg N ha−1) made by the UF/IFAS soil testing laboratory was converted from an areal-based rate to a target concentration of 45 mg plant-available N (PAN) kg−1 soil, using a bulk density of 1.5 g cm−3 and a depth of mixing of 15 cm, which is typical of farms in Florida. This target N input was used to determine how much amendment to add to each treatment, using estimates of the total N in an amendment found as PAN. To do so, amendments were analyzed by Agrolabs (AgroLab Inc., Harrington, DE, USA) prior to the experiment (Table 1). Inputs were determined with the estimated availability of N in the first year for each amendment using a lab-based estimate provided by Agrolabs of 23% (heat-treated manure) or 24% (biochar); we used 75% for the NatureSafe fertilizer based on Allar and Maltais-Landry’s study [26]. These availability estimates were used to adjust the total N concentration of each amendment and determine an adequate input rate to reach the target of 45 mg PAN kg−1. A total of 15.3 g (heat-treated manure), 14.3 g (biochar), or 1.49 g (fertilizer) were added to each pot.

Table 1.
Carbon and nutrient concentration inputs for the amendments and cover crop residues used in this study.
Cover crops were grown as monocultures of sunn hemp (cultivar not specified), sorghum sudangrass (cv. BMR Sweet Forever), cowpea (cv. Iron and Clay), and pearl millet (cv. Tifleaf 3) in a field experiment located at the Field and Fork farm (Gainesville, FL, USA). The biomass was harvested after 53 days of growth (i.e., a typical cover cropping duration for organic vegetable systems in Florida [26]) by harvesting the aboveground biomass of cover crops and immediately drying it at 60 °C until constant weight.
Dried cover crop residues were chopped with scissors (pieces ca. 2 cm) and added based on a 7000 kg ha−1 dry weight production (14 g of dried residues per pot). We used a fixed input rate corresponding to the average biomass production measured at the Field and Fork farm in 2019 and 2020 to facilitate comparisons among cover crop types and to not be restricted to the specific biomass production of a given site or year. However, this value was more representative of biomass production for sunn hemp (4700–7500 kg ha−1), millet (4900–8600 kg ha−1), and sorghum (2700–12,000 kg ha−1) than cowpea (3200–6400 kg ha−1). Hence, we recognize that using a uniform residue input rate to standardize comparisons among cover crops led to higher inputs for cowpea than what would typically be observed, which could overestimate its effects. Differences in nutrient concentrations found in each cover crop type resulted in variable nutrient inputs (Table 1) and C:N ratios of 14.2 (cowpea), 18.6 (sunn hemp), 19.8 (millet), and 23.0 (sorghum sudangrass).
On 9 February 2021, 13 kg of soil was thoroughly mixed for several minutes with the appropriate amount of amendments and/or cover crop residues for each treatment. A control treatment was established for both the amendment factor and the cover crop residue factor, with an unamended control and a control with no cover crop residues. There were four amendment treatments (control, Everlizer, Frye, and NatureSafe), each receiving one of five cover crop residue treatments (control, sunn hemp, sorghum sudangrass, cowpea, and millet), for a total of 20 treatments. Five plastic pots (diameter: 15 cm, depth: 16 cm) per treatment were set up (100 pots in total) and arranged in a randomized complete block design. In each pot, we added 2.5 kg of mixed soil with amendments and/or cover crops and planted four seeds of spinach (cv. ‘Arrowhead’), and drip irrigation was started. As germination and growth were poor and uneven after the first seeding, pots were replanted on 4 March 2021 (four seeds per pot), and plants were thinned to two per pot on 18 March, after true leaves were visible. Pots were irrigated daily at a rate that ensured adequate moisture while minimizing leaching, with increasing irrigation duration as plants became larger. A harvest occurred on 5 May 2021.
2.2. Soil Analyses
Soil samples were collected every two weeks from each pot throughout the experiment (9 March, 25 March, 8 April, 22 April, and 5 May), using a custom-built soil sampler that allowed to collect a very narrow soil core (1 cm diameter, 15 cm length) from the bottom to the top of each pot. After each sampling, the coring hole was refilled with soil from the pot. A subsample was used to analyze inorganic N within 48 h of collection, and a second subsample was dried at 105 °C for 48 h to determine moisture content. For the final soil sampling (5 May), a third subsample was air-dried and sieved at 2 mm to remove as many residues and roots as possible, before processing for POXC, protein, and total C and N.
On each sampling day, fresh soils were used to analyze inorganic N. Briefly, 5 g of fresh soil was extracted using 25 mL of 2M KCl for 30 min on a reciprocal shaker, followed by centrifugation and filtration, and quantification was performed by colorimetry for ammonium [38] and nitrate [39].
Air-dried and sieved soils were used to analyze POXC, ACE protein, and total C and N, according to Stott et al. [40]. For POXC, 2.5 g of air-dried soil was combined with 20 mL of 0.02 mol L−1 KMnO4, shaken for 2 min, incubated in the dark for 10 min, and quantified by colorimetry at 550 nm after dilution. For ACE protein, 3 g of air-dried soil was extracted with 24 mL of 0.02 mol L−1 sodium citrate for 5 min on a reciprocal shaker, autoclaved at 121 °C and 15 psi for 30 min, clarified by centrifuging 2 mL of each sample at 5000 rpm for 10 min, and quantified by colorimetry using the bicinchoninic acid (BCA) reagent, with readings taken at 562 nm. Soil total C and N were quantified by combustion on a CN analyzer (Costech ECS 4010 CHNS-O elemental analyzer; Valencia, CA, USA).
2.3. Plant Analyses
At harvest, the aboveground spinach biomass was sampled by cutting the biomass at the soil level. Six out of the hundred pots had no yield due to plant mortality (i.e., yield = 0 g), but each amendment–cover crop combination had at least four pots with harvestable biomass at the end of the experiment. Fresh biomass mass was collected at harvest, and dry mass was recorded after drying to a constant mass in a forced air dryer (65 °C). Dried spinach aboveground biomass was ground through a 20-mesh size and analyzed for total Kjeldahl N (TKN) and total P and K with acid digestion followed by ICP (UF/IFAS Analytical Services Laboratories, Analytical Research Laboratory, Gainesville, FL, USA). Nutrient uptake was computed for each pot by multiplying the nutrient concentration with the aboveground dry biomass.
2.4. Data Analysis
We analyzed experimental data using two-way ANOVAs with amendment and cover crop residues as fixed factors, followed by Tukey HSD tests. For inorganic N, an additional fixed factor (time) was added to the model, but as there was a significant effect of time and its interactions with amendments and cover crop factors (p < 0.001), the data for inorganic N were analyzed with a two-way ANOVA per sampling date.
In all cases, we ran the two-way ANOVAs first, and if the cover crop × amendment interaction was significant, amendments were compared for each cover crop treatment separately with one-way ANOVAs; cover crops were compared for each amendment separately with one-way ANOVAs. Pots with plant mortality were included in analyses, using 0 g for yield. For all analyses, we verified that residuals were normal (Shapiro–Wilk test), and variances were homogeneous (Levene test). When these conditions were not met, we transformed data using a square root transformation first and then a log transformation, if square root failed, or a rank transformation, if log failed. We only used the rank transformation for one-way ANOVAs. All statistical analyses were performed in R, version 3.5.3 [41].
3. Results
3.1. Soil Inorganic N
At 28 days after amendments were added (DAA) and five days after reseeding spinach, the amendment × cover crop interaction was significant for soil inorganic N (Table 2). NatureSafe had higher inorganic N than the unamended control (sorghum) or the unamended control, Everlizer and Frye treatments (millet, sunn hemp, and cowpea). In contrast, there were no differences among amendments for pots that received no cover crop residues. For unamended control pots, all cover crop treatments had higher inorganic N than the control, cowpea had higher inorganic N than millet and sorghum, and sunn hemp had higher N than millet. For all amended pots, cowpea had higher inorganic N compared to the control receiving no cover crops, millet, and sorghum; sunn hemp had higher inorganic N than the control in NatureSafe pots.

Table 2.
Mean (±standard error) of soil inorganic N (i.e., sum of NH4+-N and NO3−-N) from soil cores taken at five sampling times throughout the growing season (DAA = days after amendment).
At 44 DAA, the amendment × cover crop interaction was significant (Table 2). For pots with sorghum residues, NatureSafe, Everlizer, and Frye had higher inorganic N than the control, and NatureSafe also had higher inorganic N than Everlizer and Frye. In pots with millet, cowpea, and sunn hemp residues, NatureSafe had higher inorganic N compared to the unamended control and Frye pots; NatureSafe also had higher inorganic N than Everlizer in sunn hemp and cowpea pots. All amendments had higher inorganic N than the control in pots receiving no cover crops. In unamended pots, cowpea had higher inorganic N than all other cover crops and the control, sunn hemp had higher inorganic N than sorghum, millet and the control, and sorghum had higher inorganic N than millet and the control. For pots amended with Everlizer, cowpea had higher inorganic N than the control, millet, and sorghum, whereas for pots amended with Frye, cowpea had higher inorganic N than all other cover crops and the control. Lastly, in pots amended with NatureSafe, cowpea and sunn hemp were higher in inorganic N than the control, millet, and sorghum.
At 58 DAA, the main effects of amendments and cover crops were significant, but the amendment × cover crop interaction was not (Table 2). Cowpea (24.6 mg N kg−1; Tukey grouping = A) and sunn hemp (13.7 mg N kg−1; B) had more inorganic N than the control (7.2 mg N kg−1; C), sorghum (5.5 mg N kg−1; C), and millet (4.3 mg N kg−1; C). Inorganic N was highest in NatureSafe (23.2 mg N kg−1; A), intermediate in Everlizer (10.6 mg N kg−1; B), and lowest in Frye (5.8 mg N kg−1; C) and the control (4.6 mg N kg−1; C).
At 72 DAA, the amendment × cover crop interaction was significant (Table 2). For pots with cowpea, Frye had lower inorganic N than other treatments; there was no difference among amendments for sunn hemp pots. For pots with sorghum residues or no cover crops, Everlizer and NatureSafe had higher inorganic N than the unamended control and Frye. In pots with millet, NatureSafe had higher inorganic N than other treatments, and Everlizer had higher inorganic N than the unamended control. Cover crop residues did not affect inorganic N when pots were amended with Everlizer and NatureSafe, but there was more inorganic N with sunn hemp residues than without residues when pots were amended with Frye. In unamended pots, inorganic N was higher in cowpea than in all other residues, inorganic N was higher in sunn hemp than in millet and the control, and inorganic N in sorghum and millet was higher than in the control.
Lastly, at the time of spinach harvest (85 DAA), amendments and cover crops had significant main effects, with no significant interaction (Table 2). Everlizer (1.3 mg N kg−1; Tukey grouping = A) had higher inorganic N than control pots (0.5 mg N kg−1; B), and Frye (0.8 mg N kg−1; AB) and NatureSafe (0.9 mg N kg−1; AB) were not different compared to other treatments. Sunn hemp (1.4 mg N kg−1; A) and sorghum (1.0 mg N kg−1; A) had higher inorganic N when compared to control pots (0.6 mg N kg−1; B); cowpea (0.7 mg N kg−1; AB) and millet (0.8 mg N kg−1; AB) were not different in terms of inorganic N from other cover crop treatments.
3.2. Soil Health Indicators
Soil protein measured at harvest (Figure 1) was significantly affected by amendments (p < 0.001) and cover crops (p < 0.001), but their interaction was not significant (p = 0.45). Pots amended with Everlizer (3085 mg kg−1; Tukey grouping = A) and Frye (2988 mg kg−1; AB) but not NatureSafe (2907 mg kg−1; BC) had higher soil protein than the control (2843 mg kg−1; C); Everlizer also had higher soil protein than NatureSafe. Soil protein was higher in all cover crops—sorghum (3054 mg kg−1; A), cowpea (3016 mg kg−1; A), millet (2984 mg kg−1; A), and sunn hemp (2965 mg kg−1; A)—compared to the control pots (2760 mg kg−1; B) that received no cover crop residues, with no difference among cover crops.
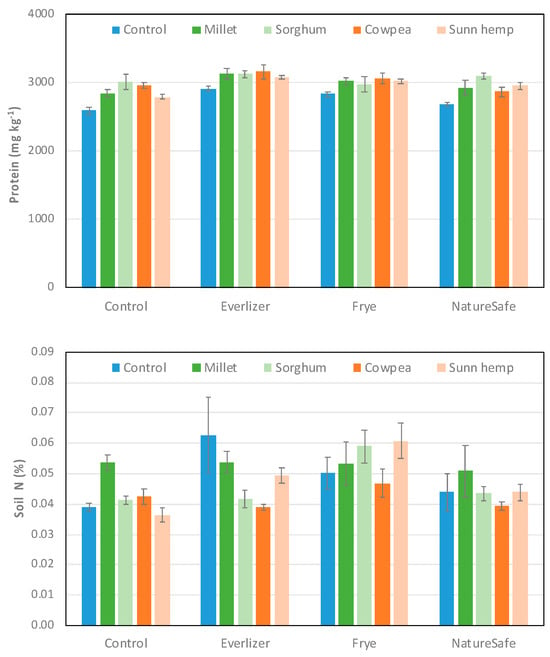
Figure 1.
Mean (±standard error) soil protein (mg kg−1) and soil total nitrogen (%) from soil cores taken at the end of the spinach growing season.
In contrast to soil protein, there was no clear and consistent pattern among treatments for soil total nitrogen, where the effects of amendments (p < 0.001), cover crops (p = 0.02) and their interaction (p = 0.046) were all significant (Figure 1); see the first paragraph of Section 4.2 for a comparison of TN and ACE protein. Frye had higher TN than the control, Everlizer, and NatureSafe (sorghum pots) or higher TN than the control and NatureSafe (sunn hemp); there was no difference in TN among amendments in pots that received control, cowpea, and millet. In the unamended control, millet had higher TN than all other cover crops, with no difference among these treatments. In Everlizer pots, the control and millet treatments had higher TN than cowpea and sorghum, and sunn hemp was higher than cowpea. There were no differences among cover crop residues in pots amended with Frye and NatureSafe.
Soil POXC was significantly affected by cover crops (p < 0.001) but not amendments (p = 0.13) or their interaction (p = 0.81, Figure 2). Millet (327 mg kg−1; Tukey grouping = A), sorghum (319 mg kg−1; A), and cowpea (303 mg kg−1; AB) had higher POXC than the control (251 mg kg−1; C), and millet and sorghum pots had more POXC than sunn hemp pots (275 mg kg−1; BC).
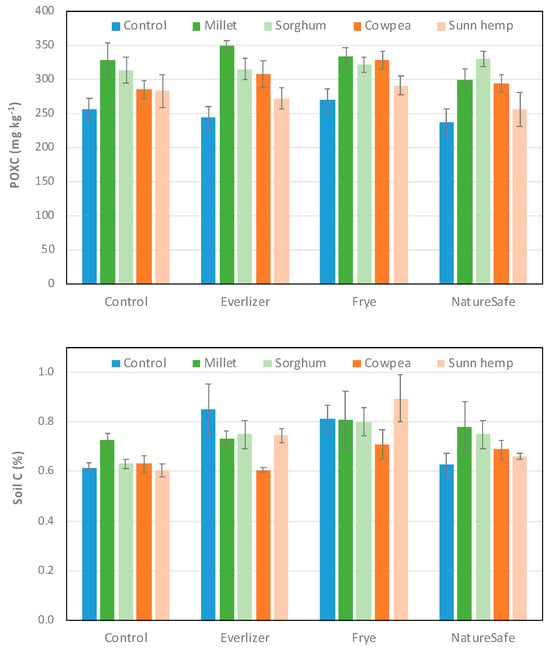
Figure 2.
Mean (±standard error) soil POXC (mg kg−1) and soil total carbon (%) from soil cores taken at the end of the spinach growing season.
In contrast, soil total C was not affected by cover crops (p = 0.11) or the amendment × cover crop interaction (p = 0.18), although amendments had a significant effect (p < 0.001, Figure 2). Soil total C was higher in Frye (0.81%; Tukey grouping = A) compared to the unamended control (0.64%; B), whereas NatureSafe (0.70%; AB) and Everlizer (0.74%; AB) were not different from other treatments. There were marginally significant trends (p < 0.1) of lower soil total C for NatureSafe relative to Frye and higher soil total C in Everlizer relative to the unamended control.
3.3. Plant Responses
Fresh spinach biomass harvested at the end of the growing season was significantly affected by amendments (p < 0.001), but not cover crops (p = 0.23) or their interaction (p = 0.33, Figure 3). Pots amended with Frye (1.62 g plant−1; Tukey grouping = A) and Everlizer (0.51 g plant−1; B) had higher yields than the control pots (0.29 g plant−1; C); pots amended with Frye also had higher yield than Everlizer and NatureSafe (0.31 g plant−1; BC). There was a marginally significant trend (p < 0.1) of lower biomass with NatureSafe than Everlizer.
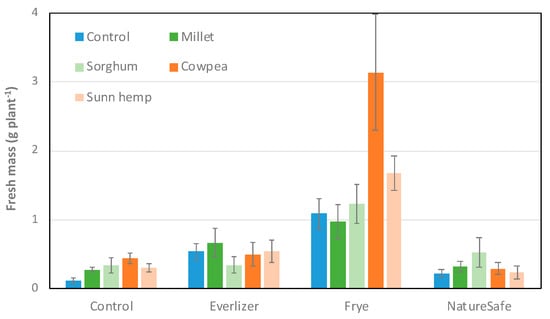
Figure 3.
Mean (±standard error) fresh spinach biomass (g plant−1) harvested at the end of the growing season.
The amendment × cover crop interaction was significant for spinach N, P, and K uptake measured during harvest (Table 3). Frye pots had higher N uptake than the unamended control in all cover crop treatments except for sorghum, and Frye also had higher N uptake than NatureSafe in sunn hemp and cowpea pots. Everlizer had higher N uptake than the unamended control in millet and control pots without cover crops. Cowpea pots had higher N uptake than other cover crop treatments in pots amended with Frye, and they also had higher N uptake when compared to millet and the no cover crop control in unamended pots. There was no effect of cover crops on pots amended with Everlizer and NatureSafe.

Table 3.
Mean (±standard error) of N, P, and K uptake measured from spinach biomass (mg plant−1) harvested at the end of the experiment.
Frye-amended pots had consistently higher P uptake than the unamended control and NatureSafe across cover crop treatments, and Frye also had higher P uptake than Everlizer in the cowpea, sunn hemp, and sorghum pots (Table 3). Pots amended with Everlizer had higher P uptake than the unamended control in sunn hemp, millet, and control pots and higher P uptake than NatureSafe in cowpea and sunn hemp pots. In pots amended with Frye, cowpea had higher P uptake than millet and sorghum, whereas in unamended pots, both cowpea and sorghum had higher P uptake than the no cover crop control. There was no difference among cover crops for Everlizer or NatureSafe pots.
Pots amended with Frye had the highest K uptake for all cover crop treatments (Table 3). In the no cover crop control and cowpea pots, Everlizer had higher K uptake than NatureSafe. Potassium uptake was only significantly affected by cover crops in the unamended control pots, where millet and sorghum had higher K uptake than the control and sunn hemp; millet also had higher K uptake than cowpea.
4. Discussion
4.1. Effects of Cover Crops and Amendments on N Cycling and Uptake
Soil inorganic N was consistently higher in pots with legume residues compared to grasses and the control without cover crop residues. Legumes typically have lower C:N ratios that stimulate the rapid release of plant-available N, especially at high temperature and moisture [42], while grass residues with higher C:N ratios decompose slower [43]. In this study, cowpea residues had the lowest C:N ratio (14.2), followed by sunn hemp (18.6), millet (19.8), and sorghum (23.0), consistent with the inorganic N release observed among residues. In the first three sampling periods, cowpea (especially) and sunn hemp released the most N, and legumes had the highest inorganic N in the non-amended control in the fourth sampling period, although cover crops had a lesser impact on inorganic N than amendments by then. By the end of the experiment, sunn hemp and sorghum had the highest inorganic N, although low soil inorganic N by this timepoint suggests that lignified material dominated in the remaining biomass, resulting in slow mineralization and N release [44].
Amendments had a significant influence on soil inorganic N, with higher soil inorganic N with NatureSafe fertilizer relative to other amendments early in this study. Everlizer became similar to NatureSafe (fourth sampling) and then had the highest inorganic N (final sampling), although concentrations were low at harvest. NatureSafe acted as an effective slow-release fertilizer, consistent with expectations and with manufacturer specifications, which indicated that 90% of total N is water insoluble that should release slowly over 7–8 weeks. In contrast, Everlizer took more time to release plant-available N, after a quick and large release of inorganic N early in the experiment (i.e., between the first and second seeding). This is consistent with the meta-analysis of Geisseler et al. [45] who found that, within 100 days of application, 60–75% of N is available from feather meal fertilizers and 30–40% of N is available from poultry manure. Poultry manure may take more time to fully decompose and mineralize than the relatively short duration of this experiment. The Frye biochar generally had lower soil inorganic N than other amendments throughout the experiment.
In contrast to soil inorganic N for which it had significantly lower concentrations, Frye biochar had the highest spinach N uptake, especially combined with legume residues. Biochar can reduce N leaching through NO3− adsorption and increase N2 fixation from legumes, leading to higher availability for plant uptake [46,47]. Palanivell et al. [48] found that poultry manure biochar decreases N desorption, resulting in a slow release of plant-available N. Because of poor germination from the initial planting in this study, pots were reseeded 23 days after the initial input of amendments and residues, with regular watering to encourage germination in this coarse-textured soil (>90% sand). This might have resulted in N leaching before spinach plants were well established and took up N at an appreciable rate, at least for pots without biochar. This may have resulted in lower N uptake and spinach yields from some treatments (e.g., Everlizer) than if the first seeding had been successful. When combining legume residues with biochar, N mineralized from residues might be retained by biochar and subsequently released gradually, leading to higher N availability over the growing season. Thus, reduced leaching and higher buffering capacity in the pots treated with Frye biochar, especially when combined with legume residues, could explain why spinach N uptake was higher compared to other amendments. However, given that this experiment was conducted only once, this interpretation is speculative and would need to be confirmed in future experiments.
4.2. Effects of Cover Crops and Amendments on Soil Health Indicators
Soil ACE protein quantifies a large pool of amino acids that account for N cycling compounds and can positively correlate with N mineralization rates [45], making it a potential alternative to laboratory incubation studies [30]. Soil ACE protein is also a comprehensive soil health indicator that correlates with soil aggregation and is responsive to management [49,50]. At the end of this study, all cover crop treatments had higher ACE protein content than the no cover crop control, consistent with the review of Marshall et al. [51]. Amendments derived from poultry manure (Everlizer and Frye) had higher ACE protein concentrations than the control and NatureSafe fertilizer made from feather meal and blood meal. This difference was likely driven by the lower availability of N in manure-based amendments (23–24% in manure-based amendments vs. 75% for NatureSafe fertilizer), which resulted in higher total N and organic N inputs required with Everlizer (431 kg TN ha−1) and Frye (428 kg TN ha−1) relative to NatureSafe (134 kg TN ha−1) to provide similar inputs of estimated PAN (101 kg PAN ha−1) among amendments. The difference in ACE protein could partially explain the higher spinach N uptake with poultry manure products, if they encouraged higher microbial stimulation that would enhance plant N uptake and soil nutrient retention [52,53]. In contrast to soil protein, soil TN exhibited no clear pattern, consistent with the perception that it is an indicator that is slowly impacted by management [30]. In addition, given the short duration of this study and the relatively small inputs of TN relative to the soil TN pool, it is not surprising that we did not observe significant differences among treatments.
Similar to ACE protein, POXC is used as a management-responsive indicator of labile C [54], given that it relates to the biologically active C pool that has a quick turnover time in the soil [55]. In this study, POXC was not affected by amendments, despite much larger C inputs with manure-based amendments relative to NatureSafe, suggesting a much lower concentration of bioavailable C in manure-based amendments. In contrast, POXC was significantly impacted by cover crop residues, with all cover crop treatments having higher POXC concentrations than the control, and grass cover crops having higher POXC than sunn hemp. Although C inputs were similar among cover crops, millet and sorghum residues both had higher C:N ratios than legumes, which affect C and N mineralization. However, beyond C:N ratios, plant functional groups differ in their C speciation, which influences how cover crops alter soil C. For example, grass cover crops add higher amounts of particulate organic C (quickly decomposed) and lower amounts of mineral-associated organic C (slowly decomposed) when compared to legumes [56,57], and this addition of particulate organic C from grass cover crops likely increased soil POXC relative to legumes [58]. Thus, while the C:N ratio is a useful predictor of broad C cycling dynamics, the C speciation of different cover crop types also plays an important role through its impacts on different soil C fractions.
In contrast to POXC, soil total C was significantly impacted by amendments but not cover crops, with Frye having the highest soil total C. The amount of C added by each amendment varied based on C concentration, with NatureSafe, Frye, and Everlizer adding 536 kg C ha−1, 4100 kg C ha−1, and 4700 kg C ha−1, respectively. Thus, even though Frye biochar added 13% less C than heat-treated manure (Everlizer), it had the greatest soil total C at the end of the experiment. This could be due to the higher recalcitrance of biochar C relative to manure C (e.g., via the higher abundance of aromatic C), in addition to other biochar properties, such as high surface area [35] relative to manure that would improve C stabilization in the soil after application. Our results are consistent with Lentz and Ippolito [59] who found that soils treated with manure-based biochar lost less organic C than soils treated with solid dairy manure. Because NatureSafe had much higher N concentrations and availability than manure-based products, it was added at a much lower rate, resulting in C inputs roughly one order of magnitude lower. Furthermore, due to a low C:N ratio, a large fraction of C inputs from NatureSafe was expected to be respired during mineralization [60], highlighting the tradeoff between increasing N availability and building up soil C with organic amendments and fertilizers.
4.3. Effects of Cover Crops and Amendments on Cash Crop Yield and P and K Uptake
Spinach biomass at harvest was not impacted by cover crops but was significantly affected by amendments, with higher yields with Frye biochar than Everlizer, NatureSafe, and the unamended control and marginally higher yield with Everlizer than NatureSafe. This contrasts with the inorganic N results for which NatureSafe provided the most plant-available N for most of the study. However, this is consistent with the meta-analysis of Xiang et al. [61] that found leafy greens to be the type of vegetable to benefit the most from fertilization with manure products. Ultimately, spinach yield was proportional to soil ACE protein concentrations, indicating that Frye and Everlizer amendments created a more conducive environment for N uptake than NatureSafe. As such, ACE protein may be a better indicator of yield response, at least in conditions similar to this greenhouse study (e.g., coarse-textured soils where inorganic N is very prone to leaching).
Spinach P and K uptake followed a similar pattern in which Frye-amended spinach had significantly higher uptake than other amendments and the unamended control. Spinach P and K uptake exhibited differences in inputs to some extent, as P was added at a rate of 12 kg P ha−1 (NatureSafe), 317 kg P ha−1 (Frye), and 238 kg P ha−1 (Everlizer), whereas K was added at a rate of 89 kg K ha−1 (NatureSafe), 700 kg K ha−1 (Frye), and 594 kg K ha−1 (Everlizer). For spinach grown in this soil, the UF/IFAS lab recommended K inputs of 112 kg K ha−1 and no P inputs. As interactions among N, P, and K can help with regulatory functions, root system development, and biomass production [62], P and K could help increase yield and/or efficient N use in spinach. As Frye and Everlizer treatments over-fertilized for K whereas NatureSafe under-supplied K, this could partially explain why spinach yields were low with NatureSafe, although this would not explain why Frye produced more biomass than Everlizer.
Ultimately, multiple factors likely impacted spinach yield in this study. Poultry manure products added the highest amounts of P and K and had higher concentrations of soil ACE protein and soil total C, which may have stimulated nutrient cycling and plant nutrient uptake. This is consistent with previous studies reporting benefits of manures on greenhouse vegetable production [61], although results obtained in this single-season greenhouse experiment should be confirmed in future greenhouse and/or field studies.
5. Conclusions
This greenhouse study highlighted that legume cover crop residues increased soil inorganic N and N uptake but did not have impacts on yields, whereas all cover crops had a significant impact on two commonly measured soil health indicators. Amendments did not impact POXC, but heat-treated manure and manure biochar had a positive effect on soil ACE protein, nutrient uptake, and spinach yield. In contrast, and similar to legume cover crops, the processed organic fertilizer increased soil inorganic N concentrations, although this did not translate into higher yields. These results suggest that, for a direct-seeded crop grown in coarse-textured soils, practices that maximize soil inorganic N availability may not lead to higher spinach N uptake and yield, while potentially contributing less to soil health. However, given that these results were obtained in a single-season experiment, they should be confirmed in future greenhouse and/or field studies.
Given the positive effects of poultry manure biochar, better optimizing the use of this amendment for specialty vegetable crop production is a critical research focus for future studies, as poultry manure biochar can vary based on feedstock (e.g., bedding material, feed used) and pyrolysis conditions. As biochar can help reduce leaching, a leachate study could determine the potential of poultry biochar to increase plant uptake and yield via reduced leaching and higher soil nutrient retention, especially when applied to coarse-textured soils and/or in combination with inputs that have a higher N availability. Given the potential of poultry manure biochar to improve soil health and increase soil C, future studies should determine the impacts of different manure biochars on crop productivity, nutrient cycling, and soil health under field conditions, to identify whether the observations made under greenhouse conditions can be replicated at the field scale.
Author Contributions
Conceptualization, methodology, investigation: G.P., S.V. and G.M.-L.; validation, formal analysis, data curation, visualization, writing—original draft preparation, writing—review and editing: A.F. and G.M.-L.; resources, supervision, project administration, funding acquisition: G.M.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by a University of Florida Institute of Food and Agricultural Sciences Early Career Seed Grant and a U.S. Department of Agriculture Hatch grant FLA-SWS-005733 to GML.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Angelique Bochnak for her assistance with laboratory protocols. Virginia Jin and Ahmad Ali provided valuable comments on an earlier version of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Chen, J.; Chen, J.-Z.; Tan, M.-Z.; Gong, Z.-T. Soil degradation: A global problem endangering sustainable development. J. Geogr. Sci. 2002, 12, 243–252. [Google Scholar]
- Purwanto, B.H.; Alam, S. Impact of intensive agricultural management on carbon and nitrogen dynamics in the humid tropics. Soil Sci. Plant Nutr. 2020, 66, 50–59. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Is there a need for a more sustainable agriculture? CRC Cr. Rev. Plant Sci. 2011, 30, 6–23. [Google Scholar] [CrossRef]
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M.A. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Soil Sci. Plant Nutr. 2015, 15, 333–352. [Google Scholar] [CrossRef]
- Gopinath, K.A.; Saha, S.; Mina, B.L.; Pande, H.; Kundu, S.; Gupta, H.S. Influence of organic amendments on growth, yield and quality of wheat and on soil properties during transition to organic production. Nutr. Cycl. Agroecosyst. 2008, 82, 51–60. [Google Scholar] [CrossRef]
- Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Jannoura, R.; Joergensen, R.G.; Bruns, C. Organic fertilizer effects on growth, crop yield, and soil microbial biomass indices in sole and intercropped peas and oats under organic farming conditions. Eur. J. Agron. 2014, 52, 259–270. [Google Scholar] [CrossRef]
- Watson, C.A.; Atkinson, D.; Gosling, P.; Jackson, L.R.; Rayns, F.W. Managing soil fertility in organic farming systems. Soil Use Manag. 2002, 18, 239–247. [Google Scholar] [CrossRef]
- Hadas, A.; Kautsky, L. Feather meal, a semi-slow-release nitrogen fertilizer for organic farming. Fert. Res. 1994, 38, 165–170. [Google Scholar] [CrossRef]
- Kelley, A.; Wilkie, A.C.; Maltais-Landry, G. Food-based composts provide more soil fertility benefits than cow manure-based composts in sandy soils. Agriculture 2020, 10, 69. [Google Scholar] [CrossRef]
- Quilty, J.R.; Cattle, S.R. Use and understanding of organic amendments in Australian agriculture: A review. Soil Res. 2011, 49, 1–26. [Google Scholar] [CrossRef]
- Bergström, L.; Kirchmann, H.; Aronsson, H.; Torstensson, G.; Mattsson, L. Use efficiency and leaching of nutrients in organic and conventional cropping systems in Sweden. Org. Crop Prod.-Ambitions Limit. 2008, 143–159. [Google Scholar] [CrossRef]
- Stockdale, E.A.; Shepherd, M.A.; Fortune, S.; Cuttle, S.P. Soil fertility in organic farming systems–fundamentally different? Soil Use Manag. 2002, 18, 301–308. [Google Scholar] [CrossRef]
- Watts, D.B.; Torbert, H.A.; Prior, S.A.; Huluka, G. Long-term tillage and poultry litter impacts soil carbon and nitrogen mineralization and fertility. Soil Sci. Soc. Am. J. 2010, 74, 1239–1247. [Google Scholar] [CrossRef]
- Lin, Y.; Watts, D.B.; Van Santen, E.; Cao, G. Influence of poultry litter on crop productivity under different field conditions: A meta-analysis. Agron. J. 2018, 110, 807–818. [Google Scholar] [CrossRef]
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Ma, K.K.; Fang, K.M.; Cheung, C. Utilization of a manure compost for organic farming in Hong Kong. Bioresour. Technol. 1999, 67, 43–46. [Google Scholar] [CrossRef]
- Hoover, N.L.; Law, J.Y.; Long, L.A.M.; Kanwar, R.S.; Soupir, M.L. Long-term impact of poultry manure on crop yield, soil and water quality, and crop revenue. J. Environ. Manag. 2019, 252, 109582. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Sohi, S.; Lopez-Capel, E.; Krull, E.; Bol, R. Biochar, climate change and soil: A review to guide future research. CSIRO Land Water Sci. Rep. 2009, 5, 17–31. [Google Scholar]
- Wang, Y.; Lin, Y.; Chiu, P.C.; Imhoff, P.T.; Guo, M. Phosphorus release behaviors of poultry litter biochar as a soil amendment. Sci. Total Environ. 2015, 512, 454–463. [Google Scholar] [CrossRef]
- Brandelli, A.; Sala, L.; Kalil, S.J. Microbial enzymes for bioconversion of poultry waste into added-value products. Food Res. Int. 2015, 73, 3–12. [Google Scholar] [CrossRef]
- Delin, S.; Stenberg, B.; Nyberg, A.; Brohede, L. Potential methods for estimating nitrogen fertilizer value of organic residues. Soil Use Manag. 2012, 28, 283–291. [Google Scholar] [CrossRef]
- Maltais-Landry, G.; Buchanan, C.; Longanecker, J. Using processed fertilizers or composted poultry manure results in similar yields but contrasting nutrient budgets in organic cabbage production. J. Plant Nutr. 2022, 46, 2462–2472. [Google Scholar] [CrossRef]
- Weil, R.; Kremen, A. Thinking across and beyond disciplines to make cover crops pay. J. Sci. Food Agric. 2007, 87, 551–557. [Google Scholar] [CrossRef]
- Allar, J.; Maltais-Landry, G. Limited benefits of summer cover crops on nitrogen cycling in organic vegetable production. Nutr. Cycl. Agroecosyst. 2022, 122, 119–138. [Google Scholar] [CrossRef]
- Truong, T.H.H.; Marschner, P. Respiration, available N and microbial biomass N in soil amended with mixes of organic materials differing in C/N ratio and decomposition stage. Geoderma 2018, 319, 167–174. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Kaufman, P.R. Natural foods supermarkets gaining in popularity. Food Rev./Natl. Food Rev. 1998, 21, 25–27. [Google Scholar]
- Hurisso, T.T.; Moebius-Clune, D.J.; Culman, S.W.; Moebius-Clune, B.N.; Thies, J.E.; van Es, H.M. Soil protein as a rapid soil health indicator of potentially available organic nitrogen. Agric. Environ. Lett. 2018, 3, 180006. [Google Scholar] [CrossRef]
- Lucas, S.T.; Weil, R.R. Can a labile carbon test be used to predict crop responses to improve soil organic matter management? Agron. J. 2012, 104, 1160–1170. [Google Scholar] [CrossRef]
- Bhadha, J.H.; Capasso, J.M.; Khatiwada, R.; Swanson, S.; LaBorde, C. Raising soil organic matter content to improve water holding capacity. EDIS 2017, SL447. [Google Scholar] [CrossRef]
- Mylavarapu, R.; Harris, W.; Hochmuth, G. Agricultural soils of Florida. EDIS 2016, SL441. [Google Scholar] [CrossRef]
- Harris, W.G.; Chrysostome, M.; Obreza, T.A.; Nair, V.D. Soil properties pertinent to horticulture in Florida. HortTechnology 2010, 20, 10–18. [Google Scholar] [CrossRef]
- Lima, I.M.; Boykin, D.L.; Klasson, K.T.; Uchimiya, M. Influence of post-treatment strategies on the properties of activated chars from broiler manure. Chemosphere 2014, 95, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.M.; Marshall, W.E. Adsorption of selected environmentally important metals by poultry manure-based granular activated carbons. J. Chem. Technol. Biotechnol. 2005, 80, 1054–1061. [Google Scholar] [CrossRef]
- Freitas, A.M.; Nair, V.D.; Harris, W.G. Biochar as influenced by feedstock variability: Implications and opportunities for phosphorus management. Front. Sustain. Food Syst. 2020, 4, 510982. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-Hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Doane, T.A.; Horwáth, W.R. Spectrophotometric determination of nitrate with a single reagent. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- Stott, D.E. Recommended Soil Health Indicators and Associated Laboratory Procedures; Soil Health Technical Note No. 450-03; U.S. Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2019.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Lynch, M.J.; Mulvaney, M.J.; Hodges, S.C.; Thompson, T.L.; Thomason, W.E. Decomposition, nitrogen and carbon mineralization from food and cover crop residues in the central plateau of Haiti. Springerplus 2016, 5, 973. [Google Scholar] [CrossRef]
- Teixeira, R.A.; Soares, T.G.; Fernandes, A.R.; Braz, A.M.d.S. Grasses and legumes as cover crop in no-tillage system in northeastern Pará Brazil. Acta Amaz. 2014, 44, 411–418. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Maltais-Landry, G.; Paudel, B.R. Dynamics of soil nitrogen availability following sunn hemp residue incorporation in organic strawberry production systems. HortScience 2021, 56, 138–146. [Google Scholar] [CrossRef]
- Geisseler, D.; Smith, R.; Cahn, M.; Muramoto, J. Nitrogen mineralization from organic fertilizers and composts: Literature survey and model fitting. J. Environ. Qual. 2021, 50, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fert. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Palanivell, P.; Ahmed, O.H.; Latifah, O.; Abdul Majid, N.M. Adsorption and desorption of nitrogen, phosphorus, potassium, and soil buffering capacity following application of chicken litter biochar to an acid soil. Appl. Sci. 2019, 10, 295. [Google Scholar] [CrossRef]
- Fine, A.K.; van Es, H.M.; Schindelbeck, R.R. Statistics, scoring functions, and regional analysis of a comprehensive soil health database. Soil Sci. Soc. Am. J. 2017, 81, 589–601. [Google Scholar] [CrossRef]
- Wright, S.F.; Starr, J.L.; Paltineanu, I.C. Changes in aggregate stability and concentration of glomalin, a glycoprotein produced by arbuscular mycorrhizal fungi, during transition from plow-to no-till management. Soil Sci. Soc. Am. J. 1999, 63, 1825–1829. [Google Scholar] [CrossRef]
- Marshall, C.B.; Burton, D.L.; Lynch, D.H. Cover crops improve some, but not all, soil health indicators in horticultural rotations. Can. J. Plant Sci. 2021, 102, 1–10. [Google Scholar] [CrossRef]
- Kobierski, M.; Bartkowiak, A.; Lemanowicz, J.; Piekarczyk, M. Impact of poultry manure fertilization on chemical and biochemical properties of soils. Plant Soil Environ. 2017, 63, 558–563. [Google Scholar] [CrossRef]
- Tejada, M.; Garcia, C.; Gonzalez, J.L.; Hernandez, M.T. Use of organic amendment as a strategy for saline soil remediation: Influence on the physical, chemical and biological properties of soil. Soil Biol. Biochem. 2006, 38, 1413–1421. [Google Scholar] [CrossRef]
- Calderón, F.J.; Culman, S.; Six, J.; Franzluebbers, A.J.; Schipanski, M.; Beniston, J.; Grandy, S.; Kong, A.Y. Quantification of soil permanganate oxidizable C (POXC) using infrared spectroscopy. Soil Sci. Soc. Am. J. 2017, 81, 277–288. [Google Scholar] [CrossRef]
- Wade, J.; Maltais-Landry, G.; Lucas, D.E.; Bongiorno, G.; Bowles, T.M.; Calderón, F.J.; Culman, S.W.; Daughtridge, R.; Ernakovich, J.G.; Fonte, S.J. Assessing the sensitivity and repeatability of permanganate oxidizable carbon as a soil health metric: An interlab comparison across soils. Geoderma 2020, 366, 114235. [Google Scholar] [CrossRef]
- Wooliver, R.; Jagadamma, S. Response of soil organic carbon fractions to cover cropping: A meta-analysis of agroecosystems. Agric. Ecosyst. Environ. 2023, 351, 108497. [Google Scholar] [CrossRef]
- Zhang, Z.; Kaye, J.P.; Bradley, B.A.; Amsili, J.P.; Suseela, V. Cover crop functional types differentially alter the content and composition of soil organic carbon in particulate and mineral-associated fractions. Glob. Chang. Biol. 2022, 28, 5831–5848. [Google Scholar] [CrossRef] [PubMed]
- Webster, E.; Gaudin, A.C.; Pulleman, M.; Siles, P.; Fonte, S.J. Improved pastures support early indicators of soil restoration in low-input agroecosystems of Nicaragua. Environ. Manag. 2019, 64, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Lentz, R.D.; Ippolito, J.A. Biochar and manure affect calcareous soil and corn silage nutrient concentrations and uptake. J. Environ. Qual. 2012, 41, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Marschner, P. Soil respiration, microbial biomass and nutrient availability in soil after repeated addition of low and high C/N plant residues. Biol. Fertil. Soils 2016, 52, 165–176. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, Y.; Luo, X.; Liu, Y.; Yue, X.; Yao, B.; Xue, J.; Zhang, L.; Fan, J.; Xu, X.; et al. Manure properties, soil conditions and managerial factors regulate greenhouse vegetable yield with organic fertilizer application across China. Front. Plant Sci. 2022, 13, 1009631. [Google Scholar] [CrossRef]
- Usherwood, N.R.; Segars, W.I. Nitrogen interactions with phosphorus and potassium for optimum crop yield, nitrogen use effectiveness, and environmental stewardship. Sci. World J. 2001, 11, 57–60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).