Abstract
Arugula (Eruca sativa Mill.) is a nutritious vegetable, commonly used in salads, known for its high glucosinolate content and various health benefits and flavors. However, arugulas may contain -excessive nitrate levels, potentially harmful to human health. We aimed to examine the effect of substrate moisture levels on the growth and quality of arugula under controlled irrigation conditions to investigate a proper irrigation practice for the quality production of arugula. The plants were cultivated using a sensor-based automated irrigation system to maintain the substrate volumetric water content (VWC) levels at 0.20, 0.30, 0.40, and 0.50 m3·m−3 over three weeks (vegetative stage). The treatment with VWC of 0.20 m3·m−3 resulted in reduced shoot growth, primarily attributed to drought-induced constraints on leaf expansion. Despite the initial reductions in stomatal conductance in arugulas subjected to lower VWC treatments, they eventually recovered and exhibited similar stomatal conductance levels across all VWC treatments 15 days after treatment, indicating acclimation to drought stress. The VWC treatment did not affect the nitrate and total glucosinolate contents of arugula, except for a decrease in glucoerucin content observed in the lowest VWC treatment. Maintaining a VWC level at 0.20 m3·m−3 could impair both the growth and quality of arugula due to severe drought conditions. Alternatively, maintaining the VWC at 0.30 m3·m−3 would ensure a high water use efficiency while securing the growth and quality of arugula.
1. Introduction
Arugula is a leafy vegetable belonging to the Brassica family that is commonly used as a garnish in various dishes and salads. Arugula is a highly valued crop because of its abundance of nutrients, such as phytochemical constituents, vitamins, carotenoids, and nutritionally valuable substances [1,2]. Among its phytochemical compounds are potent antioxidants and beneficial components such as glucosinolates, which are associated with various health benefits including anti-inflammatory and anticancer properties [3,4]. Arugula is rich in glucoraphanin and glucoerucin, a specific glucosinolate known to induce phase II detoxifying enzymes including quinone reductase [5,6]. The levels of glucosinolates in vegetables are affected by various environmental conditions such as temperature, light period, quality, and drought [7,8,9,10]. Arugula can absorb and accumulate excessive amounts of nitrate during growth, and its leaves can contain excessive nitrate. While nitrate is vital for protein synthesis, encouraging plant growth, and activating enzymes in vegetables, excessive nitrate content can lower the quality of these products due to the health risks linked to consuming it in high amounts. The implementation of regulations in several European countries, therefore, restricted the maximum allowable nitrate content in arugula [11].
Numerous studies have been conducted to ensure the quality of arugula throughout cultivation and to implement effective production strategies that enhance their desirable qualities. A high light intensity [12] and high temperature [13] can increase the glucosinolate content in arugula. The increase in the red-light fraction in the light sources and the increase in nitrogen availability could enhance the quality of arugula in terms of the glucosinolate and nitrate content [14]. The effects of drought stress on arugula have been reported, which include a decreased leaf area [15] and decreased carotenoid content [16]. However, the appropriate irrigation threshold for securing arugula yield and quality remains unclear.
With the development of controlled environment agriculture using sensor technology, several substrate moisture monitoring and control systems have been deployed to ensure precise irrigation management. Among the soil moisture sensors, frequency domain reflectometry (FDR) sensors are considered the most appropriate for automated irrigation systems, as they measure practical soilless substrate moisture levels in volumetric water content (VWC) [17]. The FDR-sensor-based automated irrigation system provided extensive data on drought conditions to enhance irrigation efficiency for Petunia × hybrida [18], Cymbidium [19], and Ocimum basilicum [20], while ensuring proper growth and high quality.
In the current study, we utilized an FDR-sensor-based automated irrigation system to quantify the effects of various VWC levels on the production of arugula to investigate the optimum VWC level for its growth while enhancing its quality. The general growth and physiological parameters of arugula, as well as changes in the nitrate and glucosinolate content, were investigated to assess the growth and quality of arugula.
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
Arugula (Eruca sativa Mill.) seeds (Asia Seed Co., Seoul, Republic of Korea) were sown in 128-cell plug trays filled with a germinating substrate (Sunshine Mix #5; SunGro Horticulture, Agawam, MA, USA) and grown in a glass greenhouse at Korea University, Seoul, Republic of Korea (37° N, 127° E), between March 28 and 19 April 2023. After 15 days, the seedlings were transplanted into round plastic pots (top diameter of 10 cm, height of 9 cm, volume of 440 mL) filled with a soilless substrate (Sunshine Mix #4; Sun Gro Horticulture, Agawam, MA, USA) mixed with a controlled-release fertilizer (Multicote 6, NPK 14-14-14, Haifa Chemicals, Haifa, Israel) at a rate of 4 g·L−1. The transplanted seedlings were grown for two weeks to acclimatize, and then 128 uniform plants were selected for the experiment. The substrate VWC of the pots was maintained at 0.55 m3·m−3 using an FDR-sensor-based automated irrigation system for three days before the irrigation treatments. Air temperature, relative humidity, and light intensity were monitored at 10 s intervals using VP-4 (Meter Group, Pullman, WA, USA) and SQ-110 (Apogee Instruments, Logan, UT, USA) sensors connected to CR1000 datalogger (Campbell Scientific, Logan, UT, USA), and their hourly and daily averages were logged. The daily average temperature and relative humidity were 21.37 ± 2.54 °C and 50.08 ± 10.67%, respectively, and the daily light integral (DLI) was 12.30 ± 5.04 mol·m−2·d−1 during the experiment period (mean ± SD).
2.2. Irrigation Treatment via FDR Sensor-Based Automated Irrigation System
A modified FDR-sensor-based automated irrigation system [20] was applied in the experiment. A total of 32 FDR-type soil moisture sensors (EC-5; Meter Group) were connected to a data logger (CR1000; Campbell Scientific) via a multiplexer (AM 16/32B; Campbell Scientific). The substrate VWCs of two pots in the middle of each experimental unit were averaged to monitor and control the VWCs. Each EC-5 sensor was powered at 2.5 V excitation and inserted on the substrate at a 45° angle toward the center of the pot. Substrate-specific sensor calibration was conducted to improve the precision substrate VWC control [21] and applied for the measurement of the VWC [VWC (m3·m−3) = 0.001352 × sensor output (mV) − 0.3373, r2 = 0.98]. Arrow-type drippers with pressure-compensated emitters (2 L/h; Netafim, Tel Aviv, Israel) were used for drip irrigation.
The irrigation VWC thresholds were assigned at 0.50 m3·m−3 (−2.8 kPa, Easily Available Water, EAW), 0.40 m3·m−3 (−5.1 kPa, boundary of EAW and water buffering capacity, WBC), 0.30 m3·m−3 (−9.8 kPa, boundary of WBC), and 0.20 m3·m−3 (−24.6 kPa, below WBC) as verified on the soilless substrate [22,23]. The moisture retention curve of the substrate was obtained using a soil moisture release curve measuring instrument (Hyprop, Meter Group; Figure 1A). The irrigation thresholds were checked for each experimental unit every 20 min. When the average VWC of the unit decreased to below the irrigation threshold, the solenoid valves connected to the data logger were opened for 10 s to provide irrigation (5.6 mL per application). The daily and cumulative irrigation amounts were recorded using the data logger.
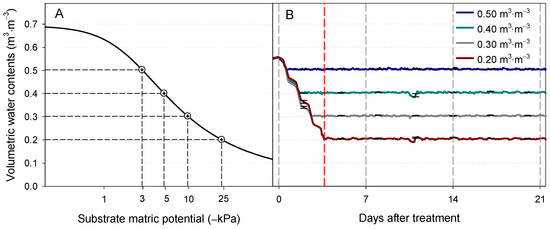
Figure 1.
(A) Moisture release curve of the soilless substrate used in the current study and (B) average substrate VWC (n = 4) of Eruca sativa during the experiment. The substrate VWCs of each experimental unit were monitored and controlled using a soil-moisture-sensor-based automated irrigation system. The vertical red dashed line indicates when all the treatments reached the target VWC levels (3.66 DAT), and the gray dashed lines indicate the harvest days (0, 7, 14, and 21 DAT). The error bars represent the standard errors (n = 4). DAT, days after the treatment started.
2.3. Growth and Physiological Measurements
A total of two to three plants per experimental unit were harvested at 0, 7, 14, and 21 days after the treatment started (DAT) to analyze temporal changes. Here, one plant was used for growth and physiological measurements, and the other was used for the analysis of the glucosinolate content. The innermost to third fully expanded leaves were collected and the leaf physiological parameters were measured at noon on each harvest day. Plant height, width, and shoot and root fresh/dry weights were measured as general growth parameters. The size of the three outermost leaves and the total leaf area per plant sample were measured using a leaf area meter (LI-3100, Li-COR, Lincoln, NE, USA). The specific leaf area was calculated by dividing the leaf size by the dry weight of the three outermost leaves. At midday, the stomatal conductance of the innermost to third fully expanded leaves was measured using a leaf porometer (SC-1; Meter Group) in the same experimental units equipped with sensors. The water use efficiency was calculated by dividing the leaf dry weight by the cumulative irrigation amount.
2.4. Nitrate Content Measurement
The nitrate content of the plant shoots was measured using a compact nitrate ion meter (LAQUAtwin-NO3-11C; Horiba Ltd., Kyoto, Japan). The outermost three fresh leaves with petioles were squeezed using a garlic press to gather the leaf sap, which was then diluted in a 1:1 ratio with deionized water and vortexed. The collected samples (0.3 mL) were placed directly on the sensor of the nitrate ion meter. The nitrate ion meter was calibrated using two-point calibrations at 300 and 5000 ppm using nitrate standard solutions.
2.5. Glucosinolate Content Analysis
For the glucosinolate analysis, the shoot parts of the samples were collected, frozen immediately with liquid nitrogen, and then dry-frozen for seven days. The samples were ground thoroughly after freezing and drying. The glucosinolate levels were examined using the desulfo-glucosinolate method described by Chae et al. [24].
The glucosinolate compounds were identified based on the positive full-scan mode range of m/z 100.0−900.0. The ion spray voltage was 3.5 kV with 320 °C of capillary temperature. The compounds were identified based on characteristic fragmentation and their mass and compared with previously reported data [5]. The retention time of each glucosinolate was compared and identified using HPLC.
2.6. Experimental Design and Statistical Analysis
The experiment was conducted using a randomized complete block design with four treatments (TRTs) and four blocks, with eight sub-replicate plants in each experimental unit. Two sub-replicates in each experimental unit were harvested using different DATs for temporal analysis. Statistical analysis software (SAS 9.4, SAS Institute, Cary, NC, USA) was used to analyze the variance among treatments. All the measured parameters were analyzed using two-way ANOVA with TRT and DAT using block as a random variable, followed by the least significant difference test at α = 0.05.
3. Results
3.1. VWC Changes with the Automated Irrigation System
The VWC of each treatment gradually decreased from 0.55 m3·m−3 after treatment initiation and reached their irrigation thresholds (0.50, 0.40, 0.30, and 0.20 m3·m−3) at DATs of 0.58, 1.70, 2.63 and 3.66, respectively (Figure 1B). After all the experimental unit reached the irrigation threshold levels, the automated irrigation system with FDR sensors successfully maintained the VWC of arugula just above the specific irrigation thresholds (0.50, 0.40, 0.30, and 0.20 m3·m−3) during the experimental periods as programmed.
3.2. Growth and Leaf Physiological Parameters
At the final harvest, only the lowest VWC treatment (0.20 m3·m−3) was observed to significantly decrease the shoot growth of arugula (Table 1). The plant height, leaf size, total leaf area, and shoot fresh and dry weights of arugula grown under the 0.20 m3·m−3 treatment demonstrated the lowest values among the treatments, whereas the plant width and specific leaf area were not affected by the treatments. Excluding the root fresh weight for the 0.50 m3·m−3 treatment, plants irrigated with more than 0.30 m3·m−3 displayed better growth parameters than those irrigated with only 0.20 m3·m−3. Only the 0.20 m3·m−3 treatment displayed reduced growth, indicating that 0.20 m3·m−3 VWC exerted considerable drought stress on arugula.

Table 1.
General growth parameters of Eruca sativa with maintaining different substrate volumetric water content (VWC) levels (0.50, 0.40, 0.30, and 0.20 m3·m−3). Mean separation among the VWC treatments followed analysis of variance with the least significant difference at α = 0.05. Means followed by the same letter are not significantly different.
As plants grow with time, the plant height and leaf size of arugula grown under a VWC higher than 0.20 m3·m−3 gradually increased with the increase in DAT (Figure 2). A significant leaf size expansion was observed in arugula grown under higher VWC treatments from the first week after treatment, whereas plants in the 0.20 m3·m−3 treatment exhibited no significant leaf size expansion. Consequently, plants in the 0.20 m3·m−3 treatment did not demonstrate a significant increase in plant height (p = 0.705) or leaf size (p = 0.406), suggesting inhibited growth due to drought. However, the shoot fresh weight and leaf area of all the plants increased gradually with the increase in DAT. Nevertheless, under the 0.20 m3·m−3 treatment, significant reductions in plant height, leaf size, shoot fresh weight, and leaf area were observed at the final harvest at 21 DAT (Figure 2).
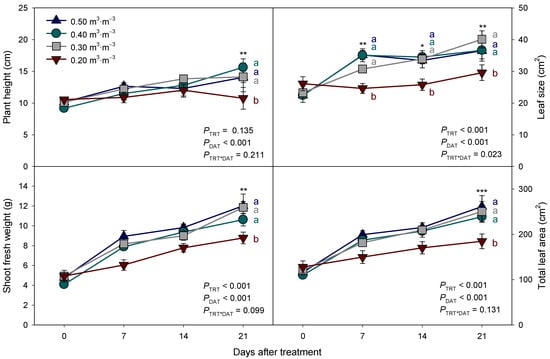
Figure 2.
Changes in plant height, leaf size, shoot fresh weight, and leaf area of Eruca sativa with maintaining different levels of substrate volumetric water content (VWC) (0.50, 0.40, 0.30, and 0.20 m3·m−3). Error bars indicate the standard error of the mean (n = 4). *, **, and *** indicate the significant VWC treatment effect within the days after treatment (DAT) at p < 0.05, 0.01, and 0.001, respectively. Means followed by the same letter are not significantly different by the least significant difference test at α = 0.05.
Although the stomatal conductance of arugula among the VWC treatments was similar at the final harvest (p = 0.349), the temporal analysis demonstrated that the lower VWC treatments decreased the stomatal conductance of arugula in the early period of treatment (PTRT*DAT = 0.007, Figure 3). The stomatal conductance of arugula under all the treatments was similar for the first two days until all the treatments reached the assigned VWC levels. However, at 3 DAT, when the VWC of the 0.20 m3·m−3 treatment reached the treatment threshold, the plants under the 0.20 m3·m−3 treatment demonstrated a dramatic decrease in the stomatal conductance that was significantly lower than those under the higher VWC treatments. These responses between VWC and stomatal conductance lasted until 14 DAT, excluding the days with a low light intensity (lower than 200 μmol·m−2·s−1). However, the stomatal conductance of arugula was observed to be similar after 15 DAT across the VWC treatments, even under high-light-intensity conditions. This indicates that the effect of VWC on stomatal conductance was attenuated, possibly because of the acclimation of the plants.

Figure 3.
Changes in midday stomatal conductance of Eruca sativa with maintaining different substrate levels of volumetric water content (VWC) (0.50, 0.40, 0.30, and 0.20 m3·m−3). The yellow bar graph shows the midday photosynthetic photon flux density. Error bars indicate the standard error of the mean (n = 4). *, **, and *** indicate the significant difference among the VWC treatment within a day at p < 0.05, 0.01, and 0.001, respectively.
3.3. Water Use and Water Use Efficiency
Although the cumulative irrigation amount for 21 days was the highest in the 0.50 m3·m−3 treatment, the 0.30 and 0.40 m3·m−3 treatments demonstrated a similar irrigation amount, while the 0.20 m3·m−3 treatment showed the least irrigation amount (Figure 4). Consequently, considering the shoot dry weight of arugula, the water use efficiency of the 0.20 and 0.30 m3·m−3 VWC treatments was higher than that of the 0.40 or 0.50 m3·m−3 VWC treatments (Figure 4).

Figure 4.
Cumulative irrigation amount and water use efficiency of Eruca sativa with maintaining different levels of substrate volumetric water content (VWC) (0.50, 0.40, 0.30, and 0.20 m3·m−3). Error bars indicate the standard error of the mean (n = 4). Means followed by the same letter are not significantly different by the least significant difference test at α = 0.05.
3.4. Changes in the Nitrate and Glucosinolate Content in Arugula by VWC Treatments
Although the nitrate and total glucosinolate contents of arugula changed over time (PDAT < 0.001), the VWC treatments did not affect the nitrate content, regardless of the DAT (Figure 5). The initial nitrate content of arugula was observed to be high (approximately 3800 ppm) before the application of the treatments. However, the nitrate content was only 10% (around 380 ppm) of the initial content a week after treatment. The low nitrate content was maintained under 700 ppm, regardless of the VWC treatments.
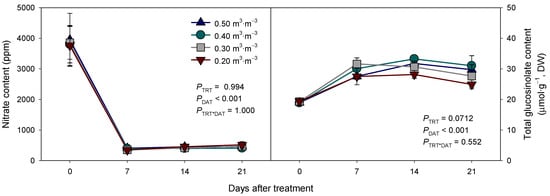
Figure 5.
Changes in leaf nitrate and total glucosinolate contents of Eruca sativa with maintaining different substrate volumetric water content levels (0.50, 0.40, 0.30, and 0.20 m3·m−3). Error bars indicate the standard error of the mean (n = 4).
The glucosinolate content analysis revealed six glucosinolates in arugula (Table S1). They were identified based on the retention time and mass fragment information. There were no significant differences in the total glucosinolate content (p = 0.089) at the final harvest at 21 DAT (Table 2). Although there was a tendency for increased glucosinolate content in arugula under the 0.40 m3·m−3 treatment, only glucoerucin demonstrated a significantly lower content in plants under the 0.20 m3·m−3 VWC treatment than that in plants under the higher VWC treatments. The temporal analysis demonstrated that the specific glucosinolate content changed with the DAT (PDAT < 0.05). The effect of VWC treatment was, therefore, not significant (PTRT > 0.05), regardless of the DAT (PTRT*DAT > 0.05).

Table 2.
Glucosinolate concentrations and profiles in extracts of Eruca sativa while maintaining different levels of substrate volumetric water content (VWC) (0.50, 0.40, 0.30, and 0.20 m3·m−3). Mean separation among the VWC treatments followed analysis of variance with the least significant difference at α = 0.05. Means followed by the same letter are not significantly different.
4. Discussion
4.1. Growth and Physiological Responses to VWC Treatment
Drought conditions can have diverse effects on plants but mostly limit their growth by reducing turgor and photosynthesis, decreasing the final yield [25,26,27]. However, the relatively low severity and slow rate of drought imposition may mitigate drought symptoms in plants, depending on the species. Arugula demonstrated similar drought responses in this study, with significantly reduced shoot growth (Table 1). These drought responses of arugula were similar to those previously reported [15,16]. It is, however, noteworthy that the expected mild drought condition (0.30 m3·m−3, −10 kPa) did not induce drought stress in arugula. Similar drought conditions as 0.30 m3·m−3 (−10 kPa) for soilless substrate have induced mild drought stress for Cymbidium [19], and Ocimum basilicum [20], with significantly reduced growth than that of plants under well-watered conditions. However, arugula reduced its growth only under much lower VWC conditions (0.20 m3·m−3, approximately –25 kPa), suggesting that this species has drought tolerance. A transcriptome analysis of arugula also showed that Eruca species could exert drought tolerance [28], implying that arugula endures lower VWC treatments than other species, even under automated irrigation systems, to maintain specific moisture conditions.
The temporal analysis of shoot growth indicated that arugula growth decreased only at a VWC level of 0.20 m3·m−3. Since arugula exhibits rosette-type growth, forming a cluster of leaves on the ground in a circular arrangement, the plant height of arugula largely depends on the size of its leaves. As the treatment started, the plants grown under 0.20 m3·m−3 exhibited minimal growth in leaf size. This appears to have contributed significantly to the consistent plant heights observed in arugula with rosette-shaped leaves during the experimental period. For plants sensing drought stress, the typical physiological responses to enhance water use efficiency involve diminishing leaf area growth and adjusting relative water content at the cellular level through osmotic regulation [29]. As stated above, arugula may be considered a drought-tolerant species. However, the 0.20 m3·m−3 treatment could be the VWC level simulating drought conditions for arugula, whereas those under higher VWC treatments demonstrated similar growth over time.
The changes in the stomatal conductance of arugula indicated a considerable decrease under the lower VWC treatment. However, this significant decrease only occurred when the VWC decreased close to 0.20 m3·m−3. A decrease in stomatal conductance is a quick response to drought conditions in petunia [18], even at 0.3 m3·m−3. However, arugula demonstrated a significant reduction in stomatal conductance later at lower VWC levels. Two weeks after the VWC treatment, all plants demonstrated similar stomatal conductance levels, regardless of treatment, indicating their acclimation to a certain VWC level. This stomatal acclimation of plants under drought has been reported; however, the limitation of stomatal conductance in the early period of drought led to a decrease in photosynthetic activity, resulting in the smallest fresh weight and leaf area in the lowest VWC treatment from 7 DAT. These findings suggest that arugula may possess drought tolerance and adaptation abilities under drought stress.
Although a VWC of 0.30 m3·m−3 may be regarded as a mild drought for other species, arugula exhibited sustained growth even under these conditions, demonstrating a lower water use and, consequently, a higher water use efficiency (Figure 4). Generally, when plants cope with drought conditions, the water use efficiency can significantly increase with a very low water use; however, in most cases, the highest water use efficiency may not be applicable for practical cultivation considering the reduced yield. Even though the 0.20 m3·m−3 treatment had the highest water use efficiency, it led to a decrease in the yield of arugula. Consequently, the 0.30 m3·m−3 treatment, which also demonstrated a high water use efficiency, proved to be the most effective in supporting the growth of arugula compared to the other treatments. Maintaining the VWC at 0.30 m3·m−3 would, therefore, be optimal for arugula to secure its yield with a high water use efficiency.
4.2. VWC Effects on Arugula Quality
Although drought may be considered an abiotic stress that can enhance the quality of crops by the accumulation of more sugar or secondary metabolites [30,31], the current study did not demonstrate the significant effect of VWC levels on the quality of arugula in terms of nitrate or the total glucosinolate content. Considering excessive nitrate content in arugula is considered a potential market problem [11], a proper VWC level was considered in this study to control the nitrate content in arugula. However, this study did not demonstrate any effect on the nitrate content in arugula in terms of the VWC levels; instead, the nitrate content decreased much less than that regulated by the EU (6000 ppm). Previous studies have indicated that arugula leaves contain approximately 500 ppm nitrate, particularly under nitrogen-deficient environmental conditions [32,33,34]. Although the plants were provided with a sufficient level of slow-release fertilizer with nitrogen, the nitrate content of all the treatments decreased to below 500 ppm at 7 DAT, regardless of the VWC levels. This may be because the soil-moisture-sensor-based automated irrigation system provided irrigation only when the substrate reached specific VWC levels, thereby restricting excessive fertilizer release from slow-release fertilizers. Previously, maintaining a specific VWC with a soil-moisture-sensor-based automated irrigation system was demonstrated to enhance the secondary metabolites in basil [20]. The effect of efficient nutrient management on the quality of crop production through automated irrigation systems should, however, be carefully quantified with separate research.
Unlike the decrease in nitrate content, the total glucosinolate content in arugula increased with the increase in DAT. However, there were no effects of the VWC treatment. Previous studies have suggested an inverse relationship between glucosinolates and nitrates under nitrogen-deficient and nitrogen-abundant conditions [35]. A competitive relationship may exist between nitrate and glucosinolate metabolism and storage in plant leaves for nitrogen utilization. A previous study on arugula glucosinolate content using temporal analysis demonstrated that the levels of glucosinolates can vary based on DAT and environmental factors, with different patterns observed for each glucosinolate species [35]. However, the glucosinolate content in arugula in this study changed as the DAT increased. VWC treatment did not affect the glucosinolate contents except for the increase in glucoerucin contents under the 0.30 and 0.40 m3·m−3 treatments compared to that under the 0.20 m3·m−3 treatment. Similarly, decreased levels of carotenoids (neoxanthin and antheraxanthin) have been reported with increasing drought stress in kale [36]. However, in the same study, moderate drought stress (0.25 m3·m−3 VWC) was found to elevate the concentrations of linoleic acid, certain glucosinolates (glucoiberin, progoitrin, and sinigrin), and total phenolic content, suggesting that specific metabolic pathways are stimulated under moderate drought stress. This study underscores the sensitivity of glucoerucin synthesis to water availability and suggests that managing irrigation levels could be a strategic approach for enhancing the nutritional and health-promoting properties of cruciferous vegetables. Arugula lacks the activity of the epithiospecifier protein, resulting in the complete conversion of glucoerucin to erucin, a chemopreventive compound [5]. The pungent, bitter, and radish-like flavor of arugula is derived from glucosinolates such as glucosativin, glucoibervirin, and glucoerucin [37]. The reduction in health-beneficial compounds could negatively affect the crop value of arugula, and the decrease in arugula flavor-related glucosinolates could influence consumer preferences [38,39,40]. There was a general decreasing tendency in glucosinolate content observed in this study with the decrease in VWC threshold levels; only the glucoerucin contents in arugula grown under 0.20 m3·m−3 demonstrated significantly lower contents than those under higher VWC treatments. In other words, the drought response of arugula grown under the 0.20 m3·m−3 treatment not only reduced its growth but also decreased its comprehensive quality, including health-promoting activity and flavor. However, arugula grown under VWC levels of 0.30 m3·m−3 or higher demonstrated similar nitrate and glucosinolate contents, suggesting it is desirable to maintain a sufficient VWC level (at least 0.30 m3·m−3) to ensure high-quality arugula production.
In conclusion, this study explored the influence of VWC on the growth, physiological characteristics, and biochemical composition of arugula, highlighting the plant’s response to varying substrate water contents. Our findings indicated that arugula could tolerate mild drought conditions but display reduced growth and physiological activity under severe water stress (0.20 m3·m−3, −24.6 kPa). Notably, the plants maintained at a 0.30 m3·m−3 (−9.8 kPa) exhibited optimal growth without compromising yield or water use efficiency, suggesting that this irrigation threshold may be optimal for quality arugula production. Further research should explore the mechanisms underlying the observed responses of glucoerucin to different irrigation levels. Understanding these mechanisms could lead to more precise recommendations for water management in the cultivation of cruciferous vegetables, ultimately contributing to the production of crops with optimal nutritional value and health-promoting properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10050483/s1, Table S1. Desulfo-glucosinolates isolated from extracts of Eruca sativa while maintaining different levels of substrate volumetric water content (0.50, 0.40, 0.30, and 0.20 m3·m−3) using a soil-moisture-sensor-based automated irrigation system.
Author Contributions
Investigation, formal analysis, software, and writing—original draft preparation, K.L.; conceptualization, methodology, data curation, writing—original draft preparation, visualization, and formal analysis, S.K.A.; methodology, validation, data curation, and writing—review and editing, K.-M.K.; conceptualization, methodology, validation, writing—review and editing, supervision, and funding acquisition, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Rural Development Administration of Korea (grant number: PJ016184), the National Research Foundation of Korea (grant number: 2021R1C1C1007733), and a Korea University Grant.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barazani, O.; Ziffer-Berger, J. Eruca sativa, a Tasty Salad Herb with Health-Promoting Properties. In Medicinal and Aromatic Plants of the Middle-East. Medicinal and Aromatic Plants of the World; Yaniv, Z., Dudai, N., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 269–279. [Google Scholar] [CrossRef]
- Matev, G.; Dimitrova, P.; Petkova, N.; Ivanov, I.; Mihaylova, D. Antioxidant activity and mineral content of rocket (Eruca sativa) plant from Italian and Bulgarian. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 759. [Google Scholar] [CrossRef]
- Garg, G.; Sharma, V. Eruca sativa (L.): Botanical description, crop improvement, and medicinal properties. J. Herbs Spices Med. Plants 2014, 20, 171–182. [Google Scholar] [CrossRef]
- Vo, Q.V.; Trenerry, C.; Rochfort, S.; Wadeson, J.; Leyton, C.; Hughes, A.B. Synthesis and anti-inflammatory activity of indole glucosinolates. Bioorg. Med. Chem. 2014, 22, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Kim, M.J.; Jeffery, E.H.; Kang, Y.H.; Juvik, J.A. Profiles of glucosinolates, their hydrolysis products, and quinone reductase inducing activity from 39 arugula (Eruca sativa Mill.) accessions. J. Agric. Food Chem. 2016, 64, 6524–6532. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Talalay, P. Antioxidant functions of sulforaphane: A potent inducer of phase II detoxication enzymes. Food Chem. Toxicol. 1999, 37, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Agerbirk, N.; Olsen, C.E.; Nielsen, J.K. Seasonal variation in leaf glucosinolates and insect resistance in two types of Barbarea vulgaris ssp. arcuata. Phytochemistry 2001, 58, 91–100. [Google Scholar] [CrossRef]
- Engelen-Eigles, G.; Holden, G.; Cohen, J.D.; Gardner, G. The effect of temperature, photoperiod, and light quality on gluconasturtiin concentration in watercress (Nasturtium officinale R. Br.). J. Agric. Food Chem. 2006, 54, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Shawon, R.A.; Kang, B.S.; Lee, S.G.; Kim, S.K.; Ju Lee, H.J.; Katrich, E.; Gorinstein, S.; Ku, Y.G. Influence of drought stress on bioactive compounds, antioxidant enzymes and glucosinolate contents of Chinese cabbage (Brassica rapa). Food Chem. 2020, 308, 125657. [Google Scholar] [CrossRef] [PubMed]
- Sarabi, B.; Ghaderi, N.; Ghashghaie, J. Light-emitting diode combined with humic acid improve the nutritional quality and enzyme activities of nitrate assimilation in rocket (Eruca sativa (Mill.) Thell.). Plant Physiol. Biochem. 2022, 187, 11–24. [Google Scholar] [CrossRef]
- European Union (EU) Commission Regulation. 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC). Off. J. Eur. Union 2023, L119, 103–157. [Google Scholar]
- Jin, J.; Koroleva, O.A.; Gibson, T.; Swanston, J.; Magan, J.; Zhang, Y.; Rowland, I.R.; Wagstaff, C. Analysis of phytochemical composition and chemoprotective capacity of rocket (Eruca sativa and Diplotaxis tenuifolia) leafy salad following cultivation in different environments. J. Agric. Food Chem. 2009, 57, 5227–5234. [Google Scholar] [CrossRef]
- Jasper, J.; Wagstaff, C.; Bell, L. Growth temperature influences postharvest glucosinolate concentrations and hydrolysis product formation in first and second cuts of rocket salad. Postharvest Biol. Technol. 2020, 163, 111157. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; Bell, L.; Santamaria, P.; Wagstaff, C.; Van Labeke, M.C. Red light is effective in reducing nitrate concentration in rocket by increasing nitrate reductase activity, and contributes to increased total glucosinolates content. Front. Plant Sci. 2020, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, E.M.; Giovanelli, L.B.; Delazari, F.T.; dos Santos, M.L.; Pereira, S.B.; da Silva, D.J. Arugula production as a function of irrigation depths and potassium fertilization. Rev. Bras. Eng. Agric. Ambient. 2017, 21, 197–202. [Google Scholar] [CrossRef]
- dos Santos, S.K.; da Silva Gomes, D.; dos Santos, L.W.O.; de Azevedo Soares, V.; Dantas, E.F.O.; Henschel, J.M.; Batista, D.S. Exogenous carnitine mitigates the deleterious effects of mild-water stress on arugula by modulating morphophysiological responses. J. Plant Growth Regul. 2023, 42, 4073–4082. [Google Scholar] [CrossRef]
- Jones, H.G. Monitoring plant and soil water status: Established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 2007, 58, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Malladi, A.; van Iersel, M.W. Physiological and molecular responses to drought in petunia: The importance of stress severity. J. Exp. Bot. 2012, 63, 6335–6345. [Google Scholar] [CrossRef] [PubMed]
- An, S.K.; Lee, H.B.; Kim, J.; Kim, K.S. Efficient water management for cymbidium grown in coir dust using a soil moisture sensor-based automated irrigation system. Agronomy 2020, 11, 41. [Google Scholar] [CrossRef]
- Nam, S.; Kang, S.; Kim, J. Maintaining a constant soil moisture level can enhance the growth and phenolic content of sweet basil better than fluctuating irrigation. Agric. Water Manag. 2020, 238, 106203. [Google Scholar] [CrossRef]
- Nemali, K.S.; Montesano, F.; Dove, S.K.; van Iersel, M.W. Calibration and performance of moisture sensors in soilless substrates: ECH2O and Theta probes. Sci. Hortic. 2007, 112, 227–234. [Google Scholar] [CrossRef]
- de Boodt, M.; Verdonck, O. The physical properties of the substrates in horticulture. Acta Hortic. 1972, 26, 37–44. [Google Scholar] [CrossRef]
- Raviv, M.; Leith, J.H.; Bar-Tal, A. Soilless Culture: Theory and Practice; Elsevier: Boston, MA, USA, 2019; pp. 79–84. [Google Scholar]
- Chae, S.H.; Lee, O.N.; Park, H.Y.; Ku, K.M. Seasonal effects of glucosinolate and sugar content determine the pungency of small-type (Altari) radishes (Raphanus sativus L.). Plants 2022, 11, 312. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S.; Siddique, K.H.M.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Cornic, G. Drought stress inhibits photosynthesis by decreasing stomatal aperture –not by affecting ATP synthesis. Trends Plant Sci. 2000, 5, 187–188. [Google Scholar] [CrossRef]
- Liang, G.; Liu, J.; Zhang, J.; Guo, J. Effects of drought stress on photosynthetic and physiological parameters of tomato. J. Am. Soc. Hortic. Sci. 2020, 145, 12–17. [Google Scholar] [CrossRef]
- Huang, B.L.; Li, X.; Liu, P.; Ma, L.; Wu, W.; Zhang, X.; Li, Z.; Huang, B. Transcriptomic analysis of Eruca vesicaria subs. sativa lines with contrasting tolerance to polyethylene glycol-simulated drought stress. BMC Plant Biol. 2019, 19, 419. [Google Scholar] [CrossRef]
- Taiz, L.; Møller, I.M.; Murphy, A.; Peer, W.A. Fundamentals of Plant Physiology; Sinauer Associates: Sunderland, MA, USA, 2018; pp. 537–560. [Google Scholar]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Drought Stress Tolerance in Plants; Hossain, M.A., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 1, pp. 1–33. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Cavarianni, R.L.; Cecílio Filho, A.B.C.; Cazetta, J.O.; May, A.; Corradi, M.M. Nutrient contents and production of rocket as affected by nitrogen concentrations in the nutritive solution. Sci. Agric. 2008, 65, 652–658. [Google Scholar] [CrossRef]
- Fontana, E.; Nicola, S. Traditional and soilless culture systems to produce corn salad (Valerianella olitoria L.) and rocket (Eruca sativa Mill.) with low nitrate content. J. Food Agric. Environ. 2009, 7, 405–410. [Google Scholar]
- Kim, S.J.; Chiami, K.; Ishii, G. Effect of ammonium: Nitrate nutrient ratio on nitrate and glucosinolate contents of hydroponically-grown rocket salad (Eruca sativa Mill.). Soil Sci. Plant Nutr. 2006, 52, 387–393. [Google Scholar] [CrossRef]
- Omirou, M.; Papastefanou, C.; Katsarou, D.; Papastylianou, I.; Passam, H.C.; Ehaliotis, C.; Papadopoulou, K.K. Relationships between nitrogen, dry matter accumulation and glucosinolates in Eruca sativa Mills. The applicability of the critical NO3-N levels approach. Plant Soil 2012, 354, 347–358. [Google Scholar] [CrossRef]
- Barickman, T.C.; Ku, K.-M.; Sams, C.E. Differing precision irrigation thresholds for kale (Brassica oleracea L. var. acephala) induces changes in physiological performance, metabolites, and yield (Brassica oleracea L. var. acephala). Environ. Exp. Bot. 2020, 180, 104253. [Google Scholar] [CrossRef]
- Bennett, R.N.; Mellon, F.A.; Botting, N.P.; Eagles, J.; Rosa, E.A.; Williamson, G. Identification of the major glucosinolate (4-mercaptobutyl glucosinolate) in leaves of Eruca sativa L. (salad rocket). Phytochemistry 2002, 61, 25–30. [Google Scholar] [CrossRef]
- Bell, L.; Lignou, S.; Wagstaff, C. High glucosinolate content in rocket leaves (Diplotaxis tenuifolia and Eruca sativa) after multiple harvests is associated with increased bitterness, pungency, and reduced consumer liking. Foods 2020, 9, 1799. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, L.F.; Elementi, S.; Neri, R. Exploring new potential health-promoting vegetables: Glucosinolates and sensory attributes of rocket salads and related Diplotaxis and Eruca species. J. Sci. Food Agric. 2009, 89, 713–722. [Google Scholar] [CrossRef]
- Bell, L.; Chadwick, M.; Puranik, M.; Jasper, J.; Tudor, R.; Methven, L.; Wagstaff, C. Genotypes of Eruca vesicaria subsp. sativa grown in contrasting field environments differ on transcriptomic and metabolomic levels, significantly impacting nutritional quality. Front. Plant Sci. 2023, 14, 1218984. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).