Plant–Vitamin–Microorganism Interaction in Hydroponic Melon Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Study Design
2.2. Study Design and Plant Management
2.3. Gas Exchange Analysis
2.4. Plant and Fruit Analysis
2.5. Statistical Analysis
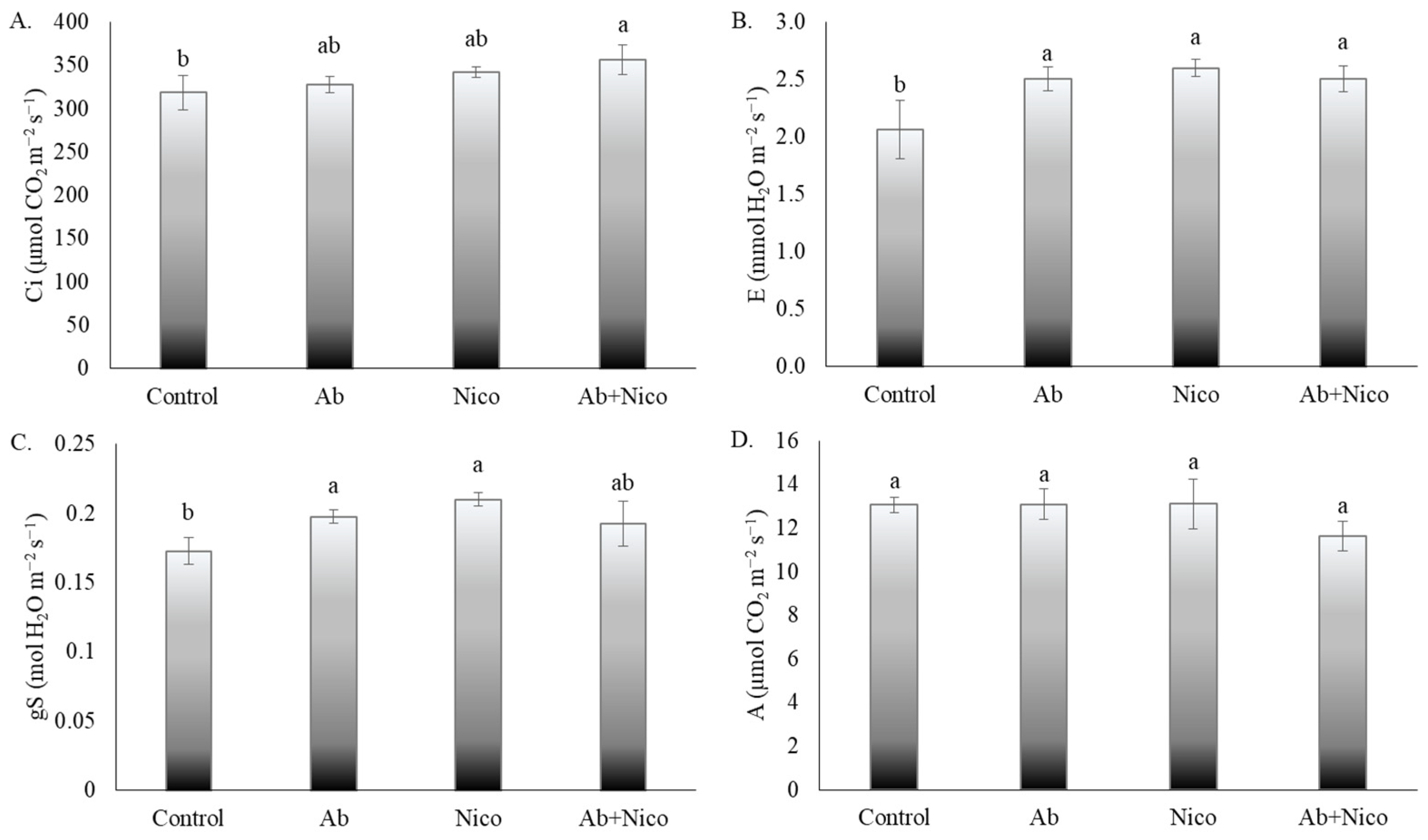
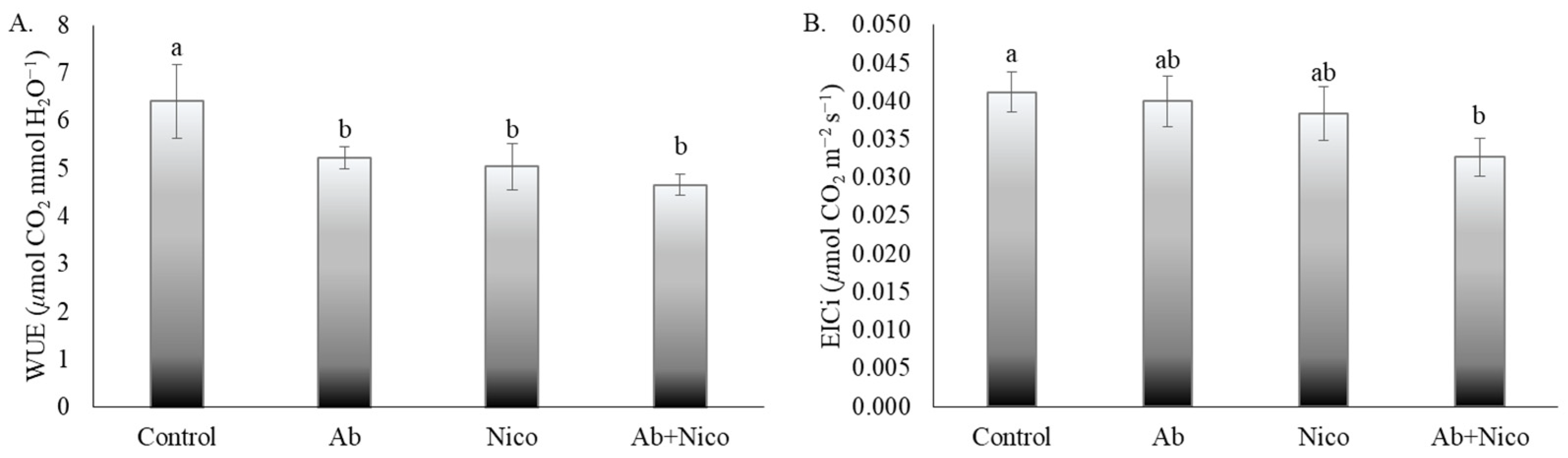
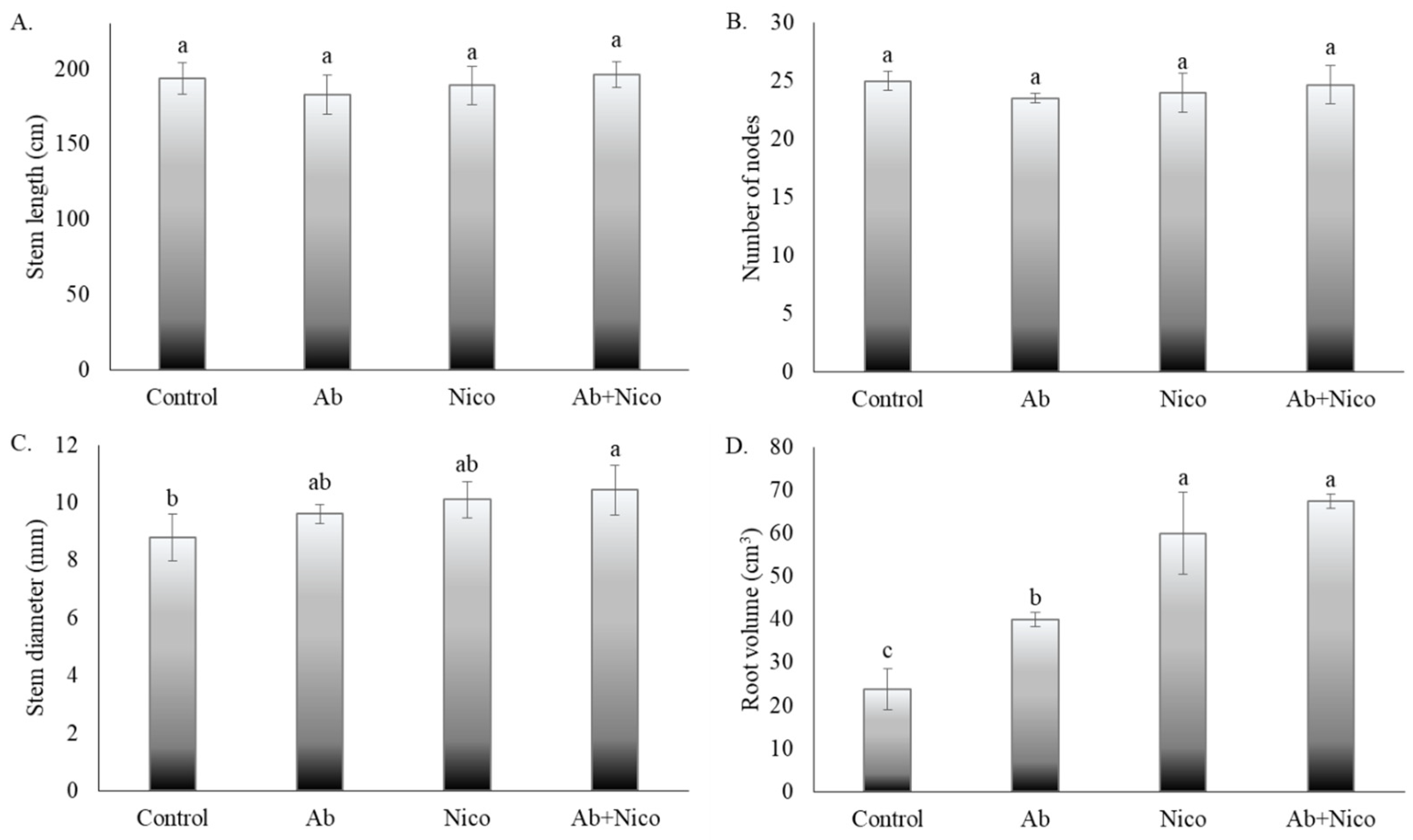
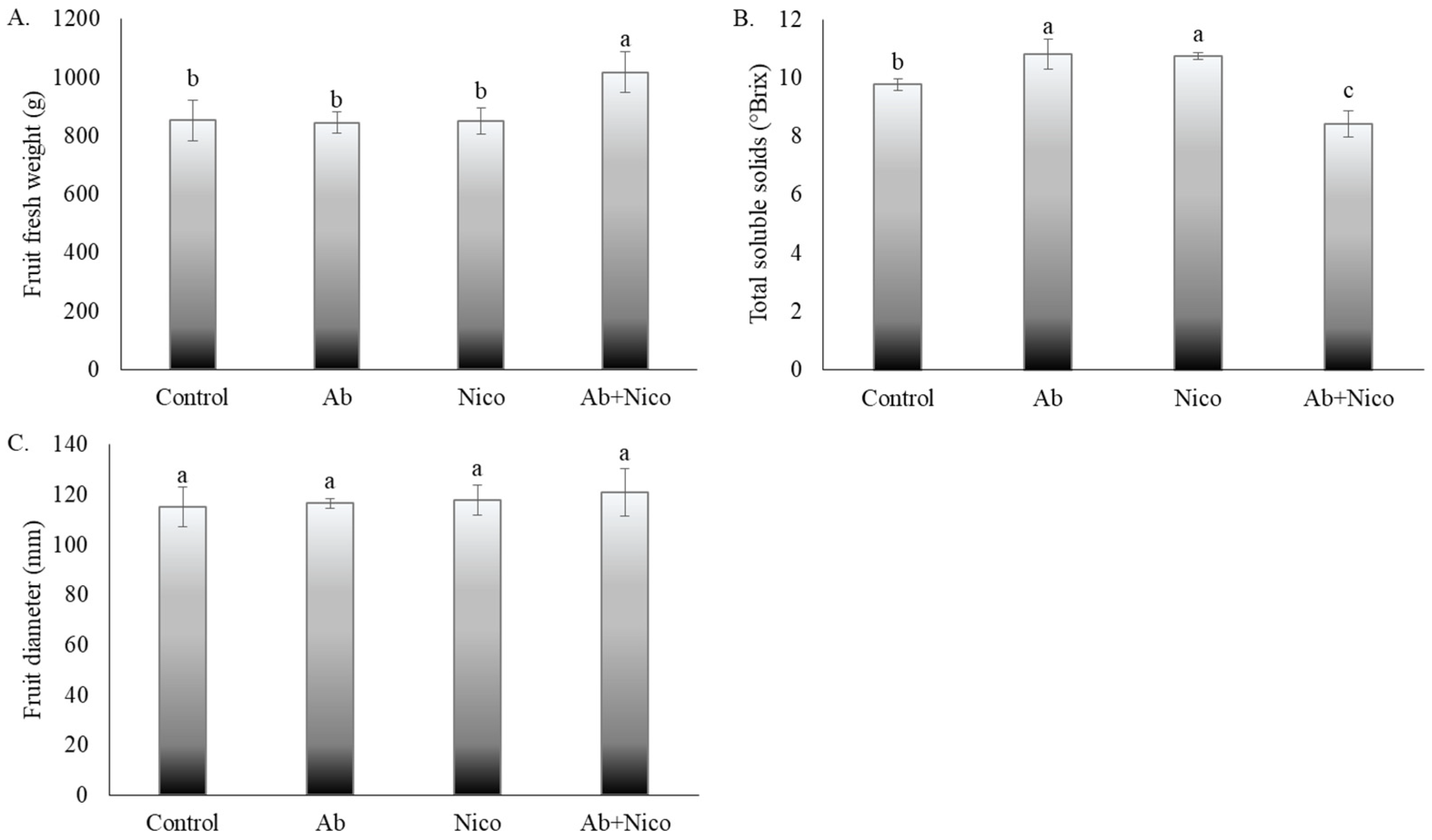
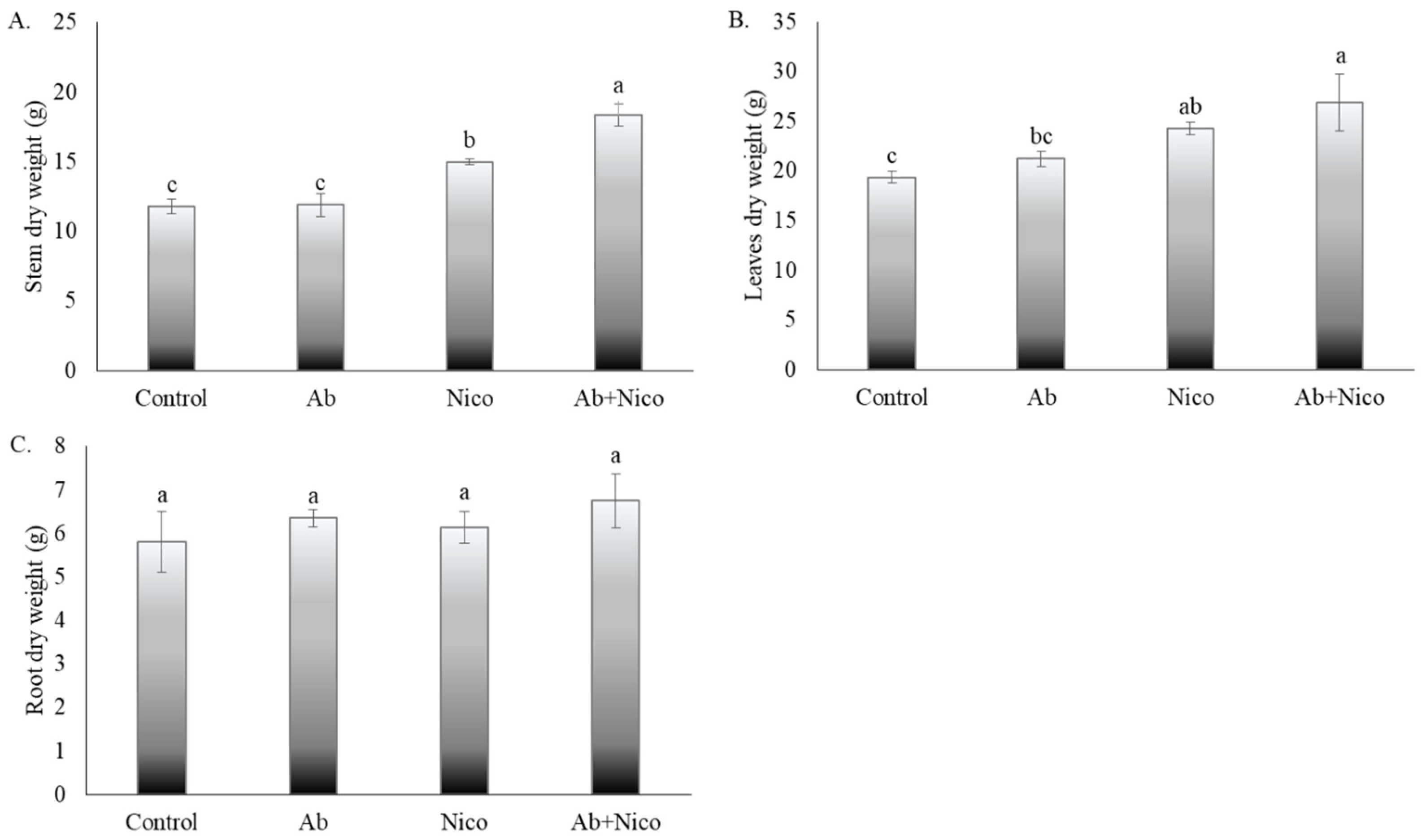
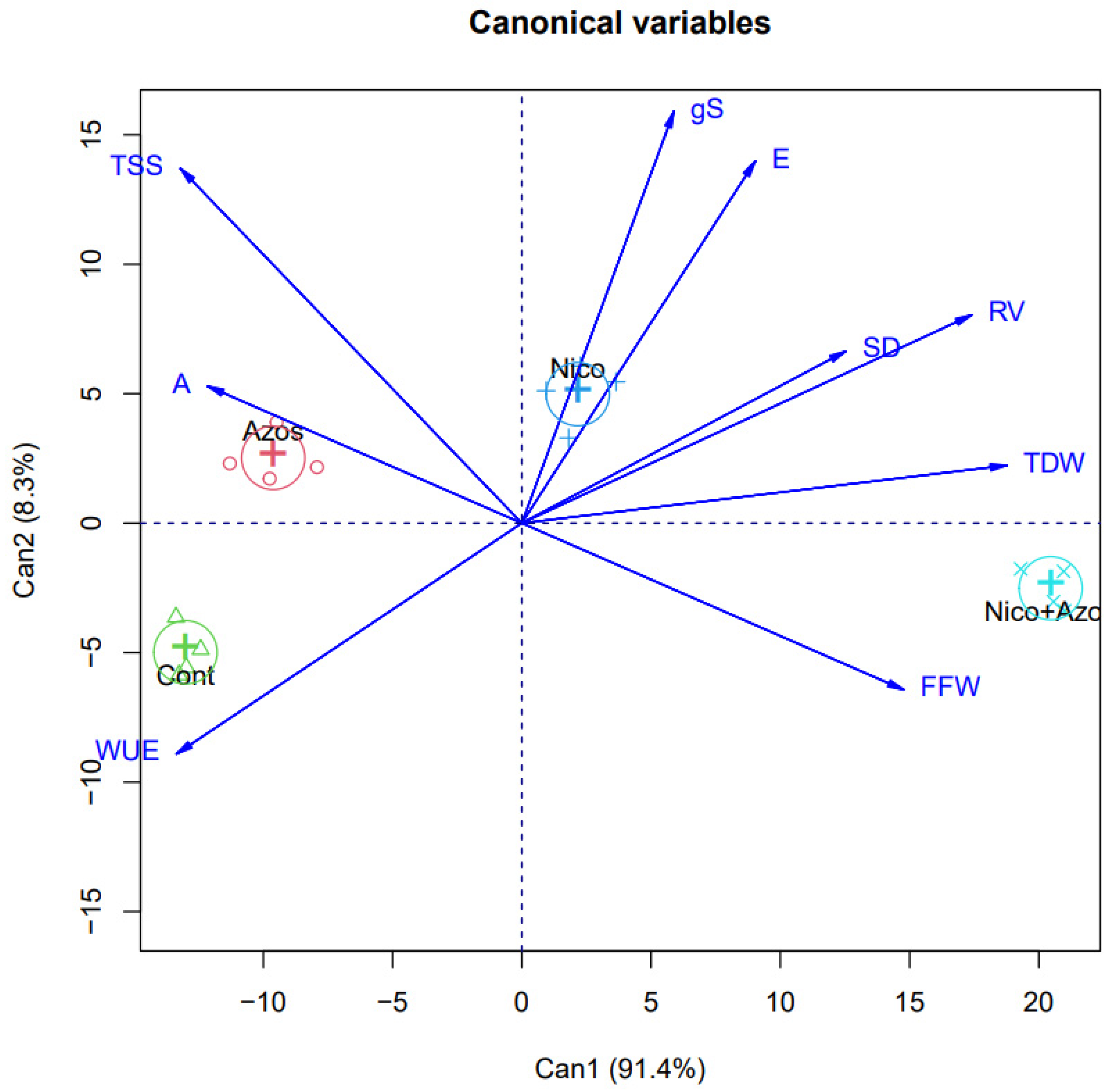
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, M.; Mao, J.; Wu, T.; Xiong, T.; Huang, Q.; Wu, H.; Hu, G. Transcriptomic analysis of salicylic acid promoting seed germination of melon under salt stress. Horticulturae 2023, 9, 375. [Google Scholar] [CrossRef]
- Medeiros, J.F.D.; Santos, S.C.L.; Câmara, M.J.T.; Negreiros, M.Z.D. Melon yield and fruit quality as influenced by soil coverages, agrotextile and irrigation depth in dry season. Hortic. Bras. 2007, 25, 538–543. [Google Scholar] [CrossRef]
- IBGE, Instituto Brasileiro de Geografia e Estatistica. Produção de Melão. 2022. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/melao/br (accessed on 13 March 2024).
- Gazzola, R.; Grundling, R.D.P.; Aragão, A.A. Melons: Growth Rates of World’s Production, Exports and Imports. Rev. Bras. Agrotecnol. 2020, 10, 75–80. [Google Scholar] [CrossRef]
- ASN. Agência Sebrae de Notícias. Available online: https://rn.agenciasebrae.com.br/economia-e-politica/meloes-frescos-elevam-exportacoes-potiguares-em-198-no-primeiro-trimestre/#:~:text=Economia%20%26%20Pol%C3%ADtica-,Mel%C3%B5es%20frescos%20elevam%20exporta%C3%A7%C3%B5es%20potiguares%20em%2019%2C8%25%20no%20primeiro,do%20total%20exportado%20no%20per%C3%ADodo (accessed on 22 October 2024).
- MAPA, Ministério da Agricultura e Pecuária. Secretaria de Política Agrícola. Projeções do Agronegócio: Brasil 2022/23 a 2032/33 Projeções de Longo Prazo. Brasília. 2023; pp. 57–63. Available online: https://www.gov.br/agricultura/pt-br/assuntos/noticias/producao-de-graos-brasileira-devera-chegar-a-390-milhoes-de-toneladas-nos-proximos-dez-anos/ProjeesdoAgronegcio20232033.pdf (accessed on 1 July 2024).
- Martínez-Gutiérrez, A.; Zamudio-González, B.; Tadeo-Robledo, M.; Espinosa-Calderón, A.; Cardoso-Galvão, J.C.; Vázquez-Carrillo, M.G. Yield of corn hybrids in response to foliar fertilization with biostimulants. Rev. Mex. Ciênc. Agrícolas 2022, 13, 289–301. [Google Scholar] [CrossRef]
- Araújo, G.P.; Batista, P.S.C.; de Souza Cangussú, L.V.; de Souza Cangussú, L.V.; de Oliveira, R.E.V.; Santiago, W.E. Growth of sorghum under different forms of application of biostimulants. Acta Iguazu 2020, 9, 83–93. [Google Scholar] [CrossRef]
- Ikiz, B.; Dasgan, H.Y.; Gruda, N.S. Utilizing the power of plant growth promoting rhizobacteria on reducing mineral fertilizer, improved yield, and nutritional quality of Batavia lettuce in a floating culture. Sci. Rep. 2024, 14, 1616. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Petropoulos, S.A.; Sun, W. Survey of the influences of microbial biostimulants on horticultural crops: Case studies and successful paradigms. Horticulturae 2023, 9, 193. [Google Scholar] [CrossRef]
- Friedrich, J.C.C.; Menegusso, F.J.; da Silva, L.S.; Lazaretti, N.S.; de Moraes Echer, M. Bio-stimulating: Use in production of changes and results in commercial production. Braz. J. Dev. 2020, 6, 27392–27409. [Google Scholar] [CrossRef]
- Oliveira, C.E.D.S.; Jalal, A.; Oliveira, J.R.; Tamburi, K.V.; Teixeira Filho, M.C.M. Leaf inoculation of Azospirillum brasilense and Trichoderma harzianum in hydroponic arugula improve productive components and plant nutrition and reduce leaf nitrate. Pesqui. Agropecuária Trop. 2022, 52, e72755. [Google Scholar] [CrossRef]
- Pérez-García, L.A.; Sáenz-Mata, J.; Fortis-Hernández, M.; Navarro-Muñoz, C.E.; Palacio-Rodríguez, R.; Preciado-Rangel, P. Plant-growth-promoting rhizobacteria improve germination and bioactive compounds in cucumber seedlings. Agronomy 2023, 13, 315. [Google Scholar] [CrossRef]
- Lima, S.F.; Pinto, P.H.; Soares, M.P.; Andrade, M.G.D.O.; Simon, C.A.; Vendruscolo, E.P.; Contardi, L.M.; Cordeiro, M.A.S.; Abreu, M.S. Nicotinamide and Azospirillum brasilense improves the quality of Coffea arabica seedlings. Rev. Bras. Eng. Agrícola Ambient. 2023, 27, 264–271. [Google Scholar] [CrossRef]
- Hu, Q.; Dong, Y.; Xiang, Y.; Luo, Z.; Lu, J.; Ban, Z.; Li, L. Exogenous nicotinamide accelerates pyridine nucleotides metabolism and redox homeostasis: A novel strategy for extension of potato dormancy. Postharvest Biol. Technol. 2025, 219, 113198. [Google Scholar] [CrossRef]
- Vendruscolo, E.P.; Seron, C.D.C.; Leonel, E.A.; Lima, S.F.D.; Araujo, S.L.; Martins, M.B.; Sant’ana, G.R.; Oliveira, J.J.D. Do vitamins affect the morphophysiology of lettuce in a hydroponic system? Rev. Bras. Eng. Agrícola Ambient. 2023, 27, 698–703. [Google Scholar] [CrossRef]
- Martins, F.A.D.; Andrade, A.T.; Condé, A.B.T.; Godinho, D.B.; Caixeta, C.G.; Costa, R.L.; Pomel, A.W.V.; Soares, C.M.S. Avaliação de híbridos de milho inoculados com Azospirillum brasilense. Pesqui. Agropecuária Gaúcha 2012, 18, 102–109. [Google Scholar]
- Yadav, D.; Dasgupta, M.D.; Dey, A. Mechanisms and applications of nitrogen fixing Azotobacter and Azospirillum in horticultural crops. In Bio-Inoculants in Horticultural Crops; Woodhead Publishing: Cambridge, UK, 2024; pp. 139–154. [Google Scholar] [CrossRef]
- Vendruscolo, E.P.; Campos, L.F.C.; Seleguini, A.; De Lima, S.F.; Bortolheiro, F.P.D.A.P.; Martins, M.B.; Seron, C.C.; De Souza, M.I. Azospirillum brasilense and nitrogen fertilizer affect the development and quality of Cantaloupe melons. J. Plant Growth Regul. 2023, 42, 5452–5460. [Google Scholar] [CrossRef]
- Khurshid, N.; Bukhari, M.A.; Ahmad, T.; Ahmad, Z.; Jatoi, W.N.; Abbas, S.M.; Latif, A.; Raza, A.; Aurangzaib, M.; Hashem, A.; et al. Exogenously applied nicotinic acid alleviates drought stress by enhancing morpho-physiological traits and antioxidant defense mechanisms in wheat. Ecotoxicol. Environ. Saf. 2023, 263, 115350. [Google Scholar] [CrossRef]
- Elsayed, A.; Abdelsattar, A.M.; Heikal, Y.M.; El-Esawi, M.A. Synergistic effects of Azospirillum brasilense and Bacillus cereus on plant growth, biochemical attributes and molecular genetic regulation of steviol glycosides biosynthetic genes in Stevia rebaudiana. Plant Physiol. Biochem. 2022, 189, 24–34. [Google Scholar] [CrossRef]
- Vendruscolo, E.P.; Sant’Ana, G.R.; Lima, S.F.D.; Gaete, F.I.; Bortolheiro, F.P.D.A.; Serafim, G.M. Biostimulant potential of Azospirillum brasilense and nicotinamide for hydroponic pumpkin cultivation. Rev. Bras. Eng. Agrícola Ambient. 2024, 28, e278962. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Badr, E.A.; Sadak, M.S.; Khedr, H.H. Effect of garlic extract, ascorbic acid and nicotinamide on growth, some biochemical aspects, yield and its components of three faba bean (Vicia faba L.) cultivars under sandy soil conditions. Bull. Natl. Res. Cent. 2020, 44, 100. [Google Scholar] [CrossRef]
- Ocwa, A.; Mohammed, S.; Mousavi, S.M.N.; Illés, Á.; Bojtor, C.; Ragán, P.; Harsányi, E. Maize Grain Yield and Quality Improvement Through Biostimulant Application: A Systematic Review. J. Soil Sci. Plant Nutr. 2024, 24, 1609–1649. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A Guide for its Bootstrap procedures in multiple comparisons. Cienc. Agrotecnol. 2014, 38, 109–112. [Google Scholar] [CrossRef]
- Ferreira, E.S.; da Silva Binotti, F.F.; Costa, E.; Vendruscolo, E.P.; Binotti, E.D.C.; Salles, J.S.; Salles, J.S. Vitamin B3 with action on biological oxide/reduction reactions and growth biostimulant in Chlorella vulgaris cultivation. Algal Res. 2023, 76, 103306. [Google Scholar] [CrossRef]
- Waskell, L.; Kim, J.J.P. Electron transfer partners of cytochrome P450. In Cytochrome P450: Structure, Mechanism, and Biochemistry; Springer: Cham, Switzerland, 2015; pp. 33–68. [Google Scholar]
- Ahmad, Z.; Bashir, K.; Matsui, A.; Tanaka, M.; Sasaki, R.; Oikawa, A.; Hirai, M.Y.; Chaomurilege; Zu, Y.; Kawai-Yamada, M.; et al. Overexpression of nicotinamidase 3 (NIC3) gene and the exogenous application of nicotinic acid (NA) enhance drought tolerance and increase biomass in Arabidopsis. Plant Mol. Biol. 2021, 107, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Laurell, C.; Berglund, T.; Ohlsson, A.B. Transcriptome analysis shows nicotinamide seed treatment alters expression of genes involved in defense and epigenetic processes in roots of seedlings of Picea abies. J. Forest. Res. 2022, 33, 1365–1375. [Google Scholar] [CrossRef]
- Lima, S.F.; Vendruscolo, E.P.; Alves, V.C.D.; Arguelho, J.C.; Pião, J.D.A.; Seron, C.D.C.; Martins, M.B.; Witt, T.W.; Serafim, G.M.; Contardi, L.M. Nicotinamide as a biostimulant improves soybean growth and yield. Open Agric. 2024, 9, 20220259. [Google Scholar] [CrossRef]
- Ferreira, E.S.; da Silva Binotti, F.F.; Binotti, E.D.C.; Costa, E.; Vendruscolo, E.P.; de Lima, S.F.; Neto, F.A.C. Potentiating Chlorella vulgaris bioinput as a growth biostimulant in the production of basil seedlings with the addition of vitamin B3. Algal Res. 2024, 83, 103706. [Google Scholar] [CrossRef]
- Lade, S.B.; Román, C.; Cueto-Ginzo, A.I.; Serrano, L.; Sin, E.; Achón, M.A.; Medina, V. Host-specific proteomic and growth analysis of maize and tomato seedlings inoculated with Azospirillum brasilense Sp7. Plant Physiol. Biochem. 2018, 129, 381–393. [Google Scholar] [CrossRef]
- Kolega, S.; Miras-Moreno, B.; Buffagni, V.; Lucini, L.; Valentinuzzi, F.; Maver, M.; Cesco, S. Nutraceutical profiles of two hydroponically grown sweet basil cultivars as affected by the composition of the nutrient solution and the inoculation with Azospirillum brasilense. Front. Plant Sci. 2020, 11, 596000. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, V.; Vendruscolo, E.P.; Conceição, J.S.; Lima, S.F.d.; Binotti, F.F.d.S.; Bortolheiro, F.P.d.A.P.; Oliveira, C.E.d.S.; Costa, E.; Lafleur, L. Plant–Vitamin–Microorganism Interaction in Hydroponic Melon Cultivation. Horticulturae 2024, 10, 1329. https://doi.org/10.3390/horticulturae10121329
Ribeiro V, Vendruscolo EP, Conceição JS, Lima SFd, Binotti FFdS, Bortolheiro FPdAP, Oliveira CEdS, Costa E, Lafleur L. Plant–Vitamin–Microorganism Interaction in Hydroponic Melon Cultivation. Horticulturae. 2024; 10(12):1329. https://doi.org/10.3390/horticulturae10121329
Chicago/Turabian StyleRibeiro, Vanessa, Eduardo Pradi Vendruscolo, Jessé Santarém Conceição, Sebastião Ferreira de Lima, Flávio Ferreira da Silva Binotti, Fernanda Pacheco de Almeida Prado Bortolheiro, Carlos Eduardo da Silva Oliveira, Edilson Costa, and Luc Lafleur. 2024. "Plant–Vitamin–Microorganism Interaction in Hydroponic Melon Cultivation" Horticulturae 10, no. 12: 1329. https://doi.org/10.3390/horticulturae10121329
APA StyleRibeiro, V., Vendruscolo, E. P., Conceição, J. S., Lima, S. F. d., Binotti, F. F. d. S., Bortolheiro, F. P. d. A. P., Oliveira, C. E. d. S., Costa, E., & Lafleur, L. (2024). Plant–Vitamin–Microorganism Interaction in Hydroponic Melon Cultivation. Horticulturae, 10(12), 1329. https://doi.org/10.3390/horticulturae10121329







