TNF-α Predicts Endothelial Function and Number of CD34+ Cells after Stimulation with G-CSF in Patients with Advanced Heart Failure
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Study Design
2.3. Blood Samples
2.4. Blood Analyses
2.5. Immunomagnetic Positive Selection of CD34+ Cells and Cell Counting
2.6. 6-min Walk Test
2.7. Brachial Artery Ultrasound Imaging
2.8. Transthoracic Echocardiography
2.9. Statistical Analysis
3. Results
3.1. Patients Clinical and Laboratory Characteristics
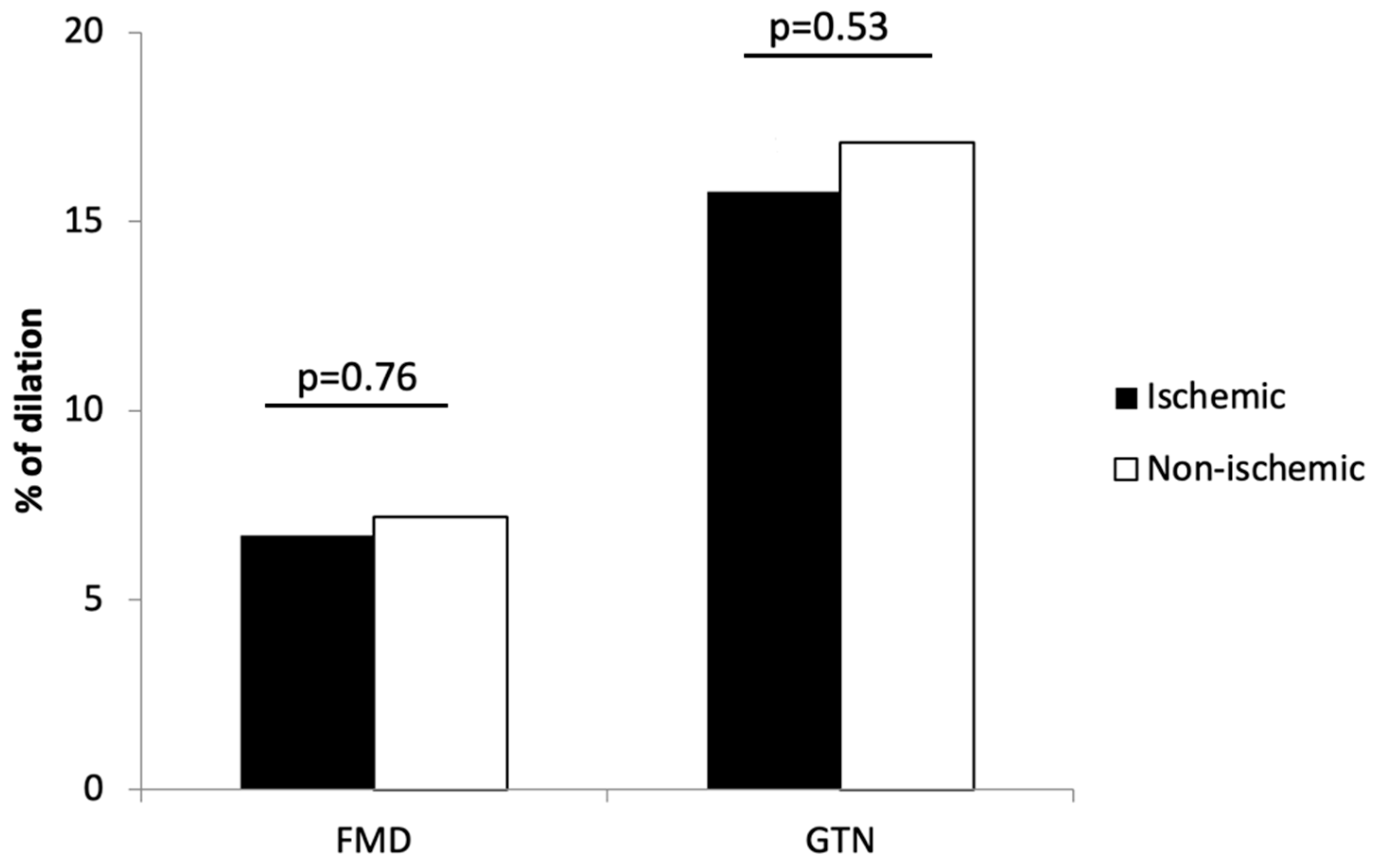
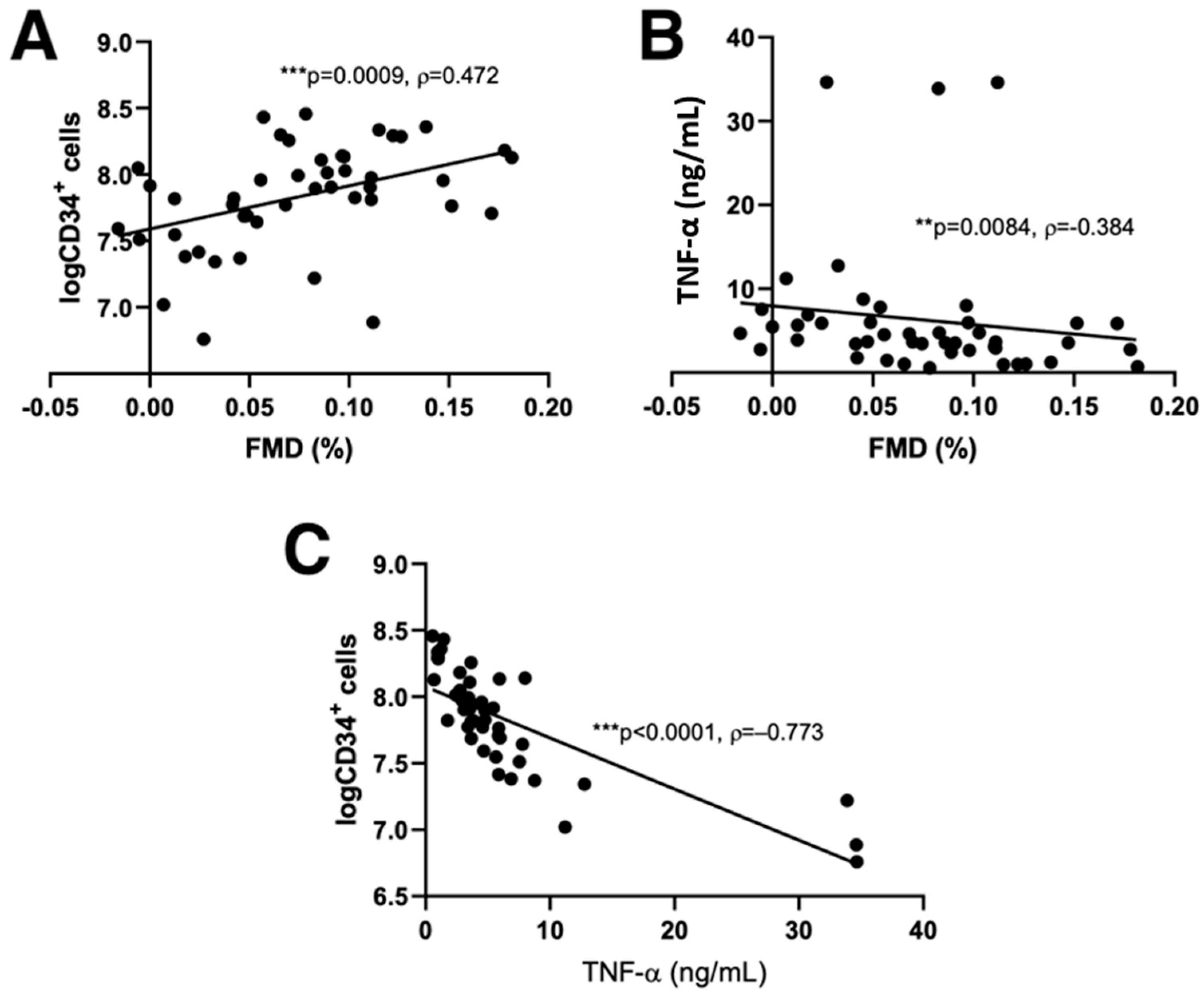
3.2. Flow-Mediated Dilation and CD34+ Cell Counts Predictors
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarkson, P.; Celermajer, D.S.; Powe, A.J.; Donald, A.E.; Henry, R.M.; Deanfield, J.E. Endothelium-dependent dilatation is impaired in young healthy subjects with a family history of premature coronary disease. Circulation 1997, 96, 3378–3383. [Google Scholar] [CrossRef] [PubMed]
- Mathier, M.A.; Rose, G.A.; Fifer, M.A.; Miyamoto, M.I.; Dinsmore, R.E.; Castaño, H.H.; Dec, G.W.; Palacios, I.F.; Semigran, M.J. Coronary endothelial dysfunction in patients with acute-onset idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 1998, 32, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Bank, A.J.; Lee, P.C.; Kubo, S.H. Endothelial dysfunction in patients with heart failure: Relationship to disease severity. J. Card. Fail. 2000, 6, 29–36. [Google Scholar] [CrossRef]
- Creager, M.A.; Cooke, J.P.; Mendelsohn, M.E.; Gallagher, S.J.; Coleman, S.M.; Loscalzo, J.; Dzau, V.J. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J. Clin. Investig. 1990, 86, 228–234. [Google Scholar] [CrossRef]
- Panza, J.A.; Quyyumi, A.A.; Brush, J.E., Jr.; Epstein, S.E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N. Engl. J. Med. 1990, 323, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Celermajer, D.S.; Sorensen, K.E.; Georgakopoulos, D.; Bull, C.; Thomas, O.; Robinson, J.; Deanfield, J.E. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 1993, 88, 2149–2155. [Google Scholar] [CrossRef] [Green Version]
- Celermajer, D.S. Endothelial dysfunction: Does it matter? Is it reversible? J. Am. Coll. Cardiol. 1997, 30, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Katz, S.D.; Hryniewicz, K.; Hriljac, I.; Balidemaj, K.; Dimayuga, C.; Hudaihed, A.; Yasskiy, A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation 2005, 111, 310–314. [Google Scholar] [CrossRef] [Green Version]
- Fischer, D.; Rossa, S.; Landmesser, U.; Spiekermann, S.; Engberding, N.; Hornig, B.; Drexler, H. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur. Heart J. 2005, 26, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Valgimigli, M.; Rigolin, G.M.; Fucili, A.; Porta, M.D.; Soukhomovskaia, O.; Malagutti, P.; Bugli, A.M.; Bragotti, L.Z.; Francolini, G.; Mauro, E.; et al. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation 2004, 110, 1209–1212. [Google Scholar] [CrossRef] [Green Version]
- Fritzenwanger, M.; Lorenz, F.; Jung, C.; Fabris, M.; Thude, H.; Barz, D.; Figulla, H.R. Differential number of CD34+, CD133+ and CD34+/CD133+ cells in peripheral blood of patients with congestive heart failure. Eur. J. Med. Res. 2009, 14, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusten, L.S.; Smeland, E.B.; Jacobsen, F.W.; Lien, E.; Lesslauer, W.; Loetscher, H.; Dubois, C.M.; Jacobsen, S.E. Tumor necrosis factor-alpha inhibits stem cell factor-induced proliferation of human bone marrow progenitor cells in vitro. Role of p55 and p75 tumor necrosis factor receptors. J. Clin. Investig. 1994, 94, 165–172. [Google Scholar] [CrossRef]
- Kissel, C.K.; Lehmann, R.; Assmus, B.; Aicher, A.; Honold, J.; Fischer-Rasokat, U.; Heeschen, C.; Spyridopoulos, I.; Dimmeler, S.; Zeiher, A.M. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J. Am. Coll. Cardiol. 2007, 49, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Theiss, H.D.; David, R.; Engelmann, M.G.; Barth, A.; Schotten, K.; Naebauer, M.; Reichart, B.; Steinbeck, G.; Franz, W.M. Circulation of CD34+ progenitor cell populations in patients with idiopathic dilated and ischaemic cardiomyopathy (DCM and ICM). Eur. Heart J. 2007, 28, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Geft, D.; Schwartzenberg, S.; Rogowsky, O.; Finkelstein, A.; Ablin, J.; Maysel-Auslender, S.; Wexler, D.; Keren, G.; George, J. Circulating apoptotic progenitor cells in patients with congestive heart failure. PLoS ONE 2008, 3, e3238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakzanov, Y.; Sevilya, Z.; Veturi, M.; Goldman, A.; Lev, E.I. Circulating endothelial progenitor cells in patients with heart failure with preserved versus reduced ejection fraction. Isr. Med. Assoc. J. 2021, 23, 364–368. [Google Scholar]
- Meluzín, J.; Mayer, J.; Groch, L.; Janousek, S.; Hornácek, I.; Hlinomaz, O.; Kala, P.; Panovský, R.; Prásek, J.; Kamínek, M.; et al. Autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction: The effect of the dose of transplanted cells on myocardial function. Am. Heart J. 2006, 152, e9–e15. [Google Scholar] [CrossRef]
- Martin-Rendon, E.; Brunskill, S.J.; Hyde, C.J.; Stanworth, S.J.; Mathur, A.; Watt, S.M. Autologous bone marrow stem cells to treat acute myocardial infarction: A systematic review. Eur. Heart J. 2008, 29, 1807–1818. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Xie, Y.; Gu, J.; Sun, L.; He, S.; Xu, R.; Duan, J.; Zhao, J.; Hang, F.; Xu, H.; et al. Repeated intracoronary infusion of peripheral blood stem cells with G-CSF in patients with refractory ischemic heart failure--a pilot study. Circ. J. 2011, 75, 955–963. [Google Scholar] [CrossRef] [Green Version]
- Vrtovec, B.; Poglajen, G.; Sever, M.; Lezaic, L.; Domanovic, D.; Cernelc, P.; Haddad, F.; Torre-Amione, G. Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. J. Card. Fail. 2011, 17, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, M.; Perrella, M.A.; Burnett, J.C., Jr.; Lee, M.E. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ. Res. 1993, 73, 205–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitges, M.; Roig, E.; Morales, M.; Azqueta, M.; Pérez Villa, F.; Paré, C.; Orús, J.; Heras, M.; Sanz, G. La disfunción endotelial periférica en la miocardiopatía dilatada idiopática se asocia con mayor disfunción ventricular y concentraciones plasmáticas elevadas de factor de necrosis tumoral [Impaired endothelium-dependent forearm vasodilation in idiopathic dilated cardiomyopathy is related to severe left ventricular dysfunction and elevated serum tumor necrosis factor levels]. Rev. Esp. Cardiol. 2005, 58, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Torre-Amione, G.; Kapadia, S.; Benedict, C.; Oral, H.; Young, J.B.; Mann, D.L. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the Studies of Left Ventricular Dysfunction (SOLVD). J. Am. Coll. Cardiol. 1996, 27, 1201–1206. [Google Scholar] [CrossRef] [Green Version]
- Zemljic, G.; Poglajen, G.; Sever, M.; Cukjati, M.; Frljak, S.; Androcec, V.; Cernelc, P.; Haddad, F.; Vrtovec, B. Electroanatomic properties of the myocardium predict response to CD34+ cell therapy in patients with ischemic and nonischemic heart failure. J. Card. Fail. 2017, 23, 153–160. [Google Scholar] [CrossRef]
- Olsson, L.G.; Swedberg, K.; Clark, A.L.; Witte, K.K.; Cleland, J.G.F. Six minute corridor walk test as an outcome measure for the assessment of treatment in randomized, blinded intervention trials of chronic heart failure: A systematic review. Eur. Heart J. 2005, 26, 778–793. [Google Scholar] [CrossRef] [Green Version]
- Ärnlöv, J.; Sang, Y.; Ballew, S.H.; Vaidya, D.; Michos, E.D.; Jacobs, D.R., Jr.; Lima, J.; Shlipak, M.G.; Bertoni, A.G.; Coresh, J.; et al. Endothelial dysfunction and the risk of heart failure in a community-based study: The Multi-Ethnic Study of Atherosclerosis. ESC Heart Fail. 2020, 7, 4231–4240. [Google Scholar] [CrossRef]
- Shah, A.; Gkaliagkousi, E.; Ritter, J.M.; Ferro, A. Endothelial function and arterial compliance are not impaired in subjects with heart failure of non-ischemic origin. J. Card. Fail. 2010, 16, 114–120. [Google Scholar] [CrossRef]
- Teragawa, H.; Ueda, K.; Matsuda, K.; Kimura, M.; Higashi, Y.; Oshima, T.; Yoshizumi, M.; Chayama, K. Relationship between endothelial function in the coronary and brachial arteries. Clin. Cardiol. 2005, 28, 460–466. [Google Scholar] [CrossRef]
- Soman, P.; Dave, D.M.; Udelson, J.E.; Han, H.; Ouda, H.Z.; Patel, A.R.; Karas, R.H.; Kuvin, J.T. Vascular endothelial dysfunction is associated with reversible myocardial perfusion defects in the absence of obstructive coronary artery disease. J. Nucl. Cardiol. 2006, 13, 756–760. [Google Scholar] [CrossRef]
- Stolen, K.Q.; Kemppainen, J.; Kalliokoski, K.K.; Karanko, H.; Toikka, J.; Janatuinen, T.; Raitakari, O.T.; Airaksinen, K.E.; Nuutila, P.; Knuuti, J. Myocardial perfusion reserve and peripheral endothelial function in patients with idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2004, 93, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.H.; Rector, T.S.; Bank, A.J.; Williams, R.E.; Heifetz, S.M. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation 1991, 84, 1589–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drexler, H.; Hayoz, D.; Münzel, T.; Hornig, B.; Just, H.; Brunner, H.R.; Zelis, R. Endothelial function in chronic congestive heart failure. Am. J. Cardiol. 1992, 69, 1596–1601. [Google Scholar] [CrossRef]
- Erbs, S.; Beck, E.B.; Linke, A.; Adams, V.; Gielen, S.; Kränkel, N.; Möbius-Winkler, S.; Höllriegel, R.; Thiele, H.; Hambrecht, R.; et al. High-dose rosuvastatin in chronic heart failure promotes vasculogenesis, corrects endothelial function, and improves cardiac remodeling--results from a randomized, double-blind, and placebo-controlled study. Int. J. Cardiol. 2011, 146, 56–63. [Google Scholar] [CrossRef]
- Oikonomou, E.; Siasos, G.; Zaromitidou, M.; Hatzis, G.; Mourouzis, K.; Chrysohoou, C.; Zisimos, K.; Mazaris, S.; Tourikis, P.; Athanasiou, D.; et al. Atorvastatin treatment improves endothelial function through endothelial progenitor cells mobilization in ischemic heart failure patients. Atherosclerosis 2015, 238, 159–164. [Google Scholar] [CrossRef]
- Laufs, U.; La Fata, V.; Plutzky, J.; Liao, J.K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998, 97, 1129–1135. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Mikhailidis, D.P.; Rizzo, M.; von Haehling, S.; Rysz, J.; Banach, M. The influence of atorvastatin on parameters of inflammation left ventricular function, hospitalizations and mortality in patients with dilated cardiomyopathy--5-year follow-up. Lipids Health Dis. 2013, 12, 47. [Google Scholar] [CrossRef] [Green Version]
- Vita, J.A.; Yeung, A.C.; Winniford, M.; Hodgson, J.M.; Treasure, C.B.; Klein, J.L.; Werns, S.; Kern, M.; Plotkin, D.; Shih, W.J.; et al. Effect of cholesterol-lowering therapy on coronary endothelial vasomotor function in patients with coronary artery disease. Circulation 2000, 102, 846–851. [Google Scholar] [CrossRef] [Green Version]
- Gounari, P.; Tousoulis, D.; Antoniades, C.; Kampoli, A.M.; Stougiannos, P.; Papageorgiou, N.; Roulia, G.; Stefanadi, E.; Siasos, G.; Tsioufis, C.; et al. Rosuvastatin but not ezetimibe improves endothelial function in patients with heart failure, by mechanisms independent of lipid lowering. Int. J. Cardiol. 2010, 142, 87–91. [Google Scholar] [CrossRef]
- Castro, P.F.; Miranda, R.; Verdejo, H.E.; Greig, D.; Gabrielli, L.A.; Alcaino, H.; Chiong, M.; Bustos, C.; Garcia, L.; Mellado, R.; et al. Pleiotropic effects of atorvastatin in heart failure: Role in oxidative stress, inflammation, endothelial function, and exercise capacity. J. Heart Lung Transpl. 2008, 27, 435–441. [Google Scholar] [CrossRef]
- Tousoulis, D.; Andreou, I.; Tsiatas, M.; Miliou, A.; Tentolouris, C.; Siasos, G.; Papageorgiou, N.; Papadimitriou, C.A.; Dimopoulos, M.A.; Stefanadis, C. Effects of rosuvastatin and allopurinol on circulating endothelial progenitor cells in patients with congestive heart failure: The impact of inflammatory process and oxidative stress. Atherosclerosis 2011, 214, 151–157. [Google Scholar] [CrossRef] [PubMed]
| Variable (Unit) | Ischemic (n = 18) | Non-Ischemic (n = 38) | p |
|---|---|---|---|
| Age (years) | 58.00 ± 5.89 | 52.39 ± 10.40 | 0.01 |
| LVEF (%) | 27.28 ± 4.45 | 29.67 ± 4.13 | 0.08 |
| Na+ (mmol/L) | 141 ± 3 | 141 ± 3 | 0.34 |
| K+ (mmol/L) | 4.5 ± 0.4 | 4.6 ± 0.4 | 0.91 |
| BUN (mmol/L) | 8.15 ± 2.9 | 6.85± 4.1 | 0.40 |
| Creatinine (μmol/L) | 92.94 ± 24.41 | 91.58 ± 23.02 | 0.84 |
| Total cholesterol (mmol/L) | 3.56 ± 0.66 | 4.70 ± 1.21 | <0.001 |
| LDL cholesterol (mmol/L) | 1.96 ± 0.50 | 2.85 ± 0.73 | <0.001 |
| HDL cholesterol (mmol/L) | 0.97 ± 0.20 | 1.20 ± 0.40 | 0.01 |
| Triglycerides (mmol/L) | 1.50 ± 1.0 | 1.42 ± 1.2 | 0.99 |
| NT-proBNP (pg/mL) | 1575 (425–2439) | 1273 (225–2239) | 0.40 |
| 6-min walk test (m) | 417.61 ± 105.46 | 460.80 ± 109.69 | 0.17 |
| WBC (×109/L) | 7.25 ± 1.73 | 7.22 ± 1.57 | 0.95 |
| RBC (×1012/L) | 4.77 ± 0.38 | 4.79 ± 0.77 | 0.72 |
| Hb (g/L) | 146.39 ± 12.44 | 145.79 ± 10.42 | 0.85 |
| Platelets (×109/L) | 195.00 ± 48.47 | 218.55 ± 47.30 | 0.09 |
| RDW (%) | 14.68 ± 1.48 | 14.23 ± 1.08 | 0.21 |
| Blood glucose (mmol/L) | 5.65 ±2.8 | 5.4± 1.6 | 0.48 |
| CD34+ cells (×106) | 67.54 ± 102.32 | 89.76 ± 71.21 | 0.32 |
| TNF-α (ng/mL) | 8.72 ± 10.30 | 4.96 ± 6.16 | 0.13 |
| ASA | 14/18 (77.8%) | 6/38 (15.8%) | <0.001 |
| β-blocker | 18/18 (100.0%) | 37/38 (97.4%) | 0.99 |
| ACE inhibitor/ARBs | 18/18 (100.0%) | 36/38 (94.7%) | 0.99 |
| Statins | 17/18 (94.4%) | 14/38 (36.8%) | <0.001 |
| Aldosterone inhibitors | 15/18 (83.3%) | 23/38 (84.2%) | 0.99 |
| Furosemide | 11/18 (61.1%) | 23/38 (60.5%) | 0.97 |
| Ivabradine | 1/18 (5.6%) | 4/38 (10.5%) | 0.99 |
| Digoxin | 1/18 (5.6%) | 6/38 (15.8%) | 0.41 |
| Ischemic HF (n = 18) | Non-Ischemic HF (n = 38) | |||
|---|---|---|---|---|
| Variable | ρ | p | ρ | p |
| Age | 0.01 | 0.98 | −0.08 | 0.64 |
| LVEF | 0.36 | 0.14 | 0.24 | 0.14 |
| Na+ | 0.21 | 0.40 | 0.17 | 0.32 |
| K+ | 0.01 | 0.96 | −0.22 | 0.19 |
| BUN | −0.16 | 0.53 | −0.20 | 0.22 |
| Creatinine | −0.28 | 0.25 | −0.08 | 0.64 |
| Total cholesterol | 0.66 | 0.003 | 0.01 | 0.96 |
| LDL | 0.56 | 0.01 | 0.04 | 0.79 |
| HDL | 0.57 | 0.01 | −0.02 | 0.92 |
| Triglycerides | 0.30 | 0.22 | −0.19 | 0.24 |
| NT-proBNP | −0.17 | 0.51 | 0.06 | 0.73 |
| 6-min walk test | 0.34 | 0.16 | 0.02 | 0.92 |
| WBC | −0.18 | 0.47 | 0.03 | 0.87 |
| RBC | −0.12 | 0.62 | 0.10 | 0.56 |
| Hb | −0.10 | 0.70 | −0.04 | 0.82 |
| Platelets | 0.36 | 0.14 | 0.38 | 0.02 |
| RDW | 0.12 | 0.64 | 0.24 | 0.15 |
| Blood glucose | −0.26 | 0.29 | −0.30 | 0.06 |
| CD34+ cells | 0.62 | 0.01 | 0.45 | 0.004 |
| TNF-α | 0.54 | 0.02 | 0.57 | 0.001 |
| Variable | B | SEB | β | p |
|---|---|---|---|---|
| Intercept | 254.343 | 92.312 | 0.002 | |
| Age | −1.321 | 1.226 | −0.153 | 0.39 |
| EF | 0.783 | 1.1215 | 0.054 | 0.52 |
| NT-proBNP | −7.234 | 2.453 | −0.223 | 0.14 |
| Platelets | −0.234 | 0.263 | −0.176 | 0.52 |
| Ischemic heart failure | 0.543 | 1.235 | 0.456 | 0.62 |
| FMD | 597.145 | 230.654 | 0.543 | 0.001 |
| TNF-α | −392.432 | 187.480 | −0.654 | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugovšek, S.; Rehberger Likozar, A.; Finderle, S.; Poglajen, G.; Okrajšek, R.; Vrtovec, B.; Šebeštjen, M. TNF-α Predicts Endothelial Function and Number of CD34+ Cells after Stimulation with G-CSF in Patients with Advanced Heart Failure. J. Cardiovasc. Dev. Dis. 2022, 9, 281. https://doi.org/10.3390/jcdd9080281
Ugovšek S, Rehberger Likozar A, Finderle S, Poglajen G, Okrajšek R, Vrtovec B, Šebeštjen M. TNF-α Predicts Endothelial Function and Number of CD34+ Cells after Stimulation with G-CSF in Patients with Advanced Heart Failure. Journal of Cardiovascular Development and Disease. 2022; 9(8):281. https://doi.org/10.3390/jcdd9080281
Chicago/Turabian StyleUgovšek, Sabina, Andreja Rehberger Likozar, Sanjo Finderle, Gregor Poglajen, Renata Okrajšek, Bojan Vrtovec, and Miran Šebeštjen. 2022. "TNF-α Predicts Endothelial Function and Number of CD34+ Cells after Stimulation with G-CSF in Patients with Advanced Heart Failure" Journal of Cardiovascular Development and Disease 9, no. 8: 281. https://doi.org/10.3390/jcdd9080281
APA StyleUgovšek, S., Rehberger Likozar, A., Finderle, S., Poglajen, G., Okrajšek, R., Vrtovec, B., & Šebeštjen, M. (2022). TNF-α Predicts Endothelial Function and Number of CD34+ Cells after Stimulation with G-CSF in Patients with Advanced Heart Failure. Journal of Cardiovascular Development and Disease, 9(8), 281. https://doi.org/10.3390/jcdd9080281






