Pregnancy Considerations in the Multidisciplinary Care of Patients with Pulmonary Arterial Hypertension
Abstract
1. Introduction
2. Comment on Dobbs V. Jackson Women’s Health Organization
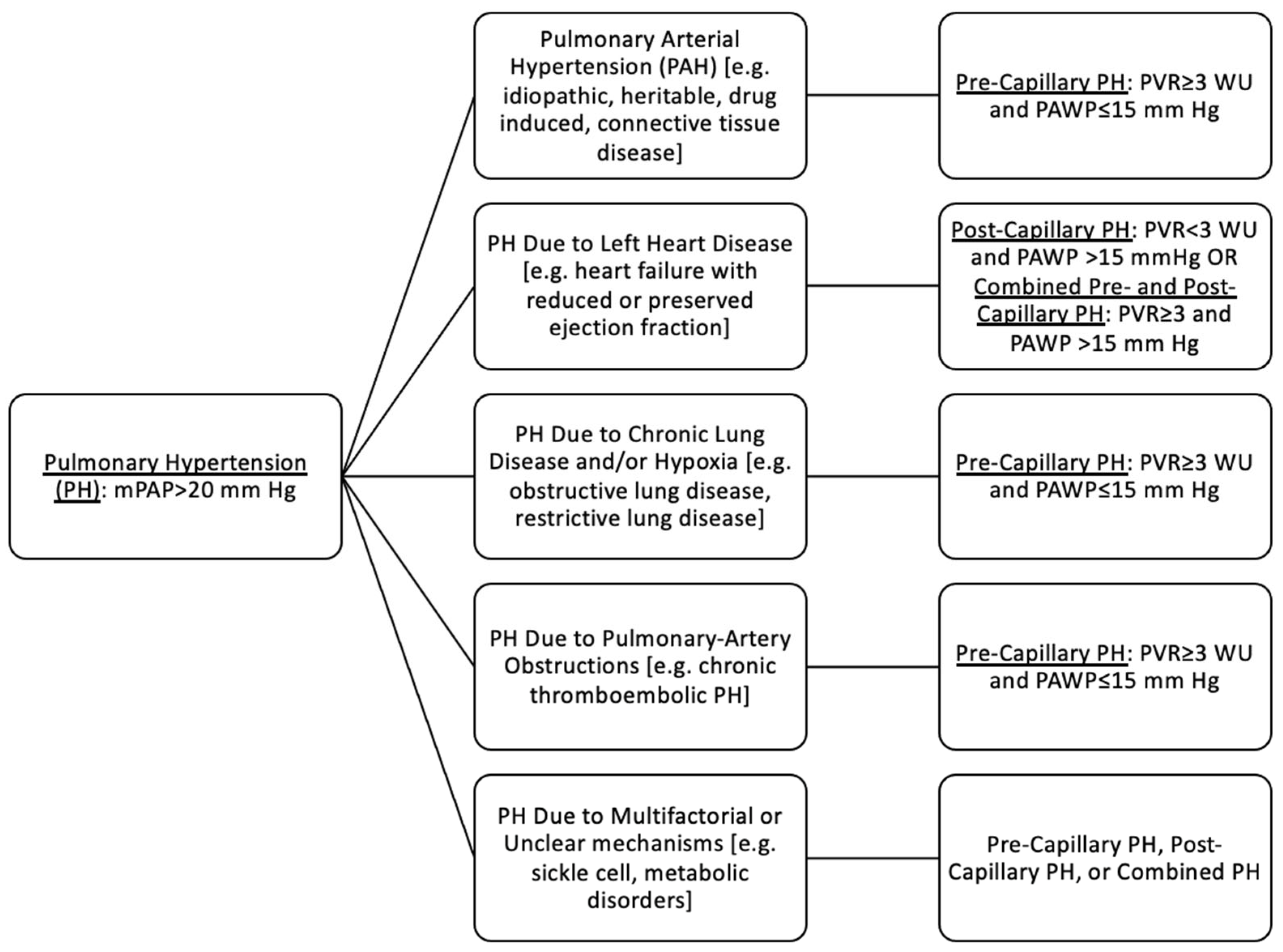
3. Background
3.1. Physiological Changes in Pregnancy and Pulmonary Arterial Hypertension
3.2. Reproductive Planning and Contraceptive Counseling Considerations in Patients with PAH
3.3. Pregnancy Counseling in Patients with PAH
3.4. Medication Counseling during Pregnancy in Patients with PAH
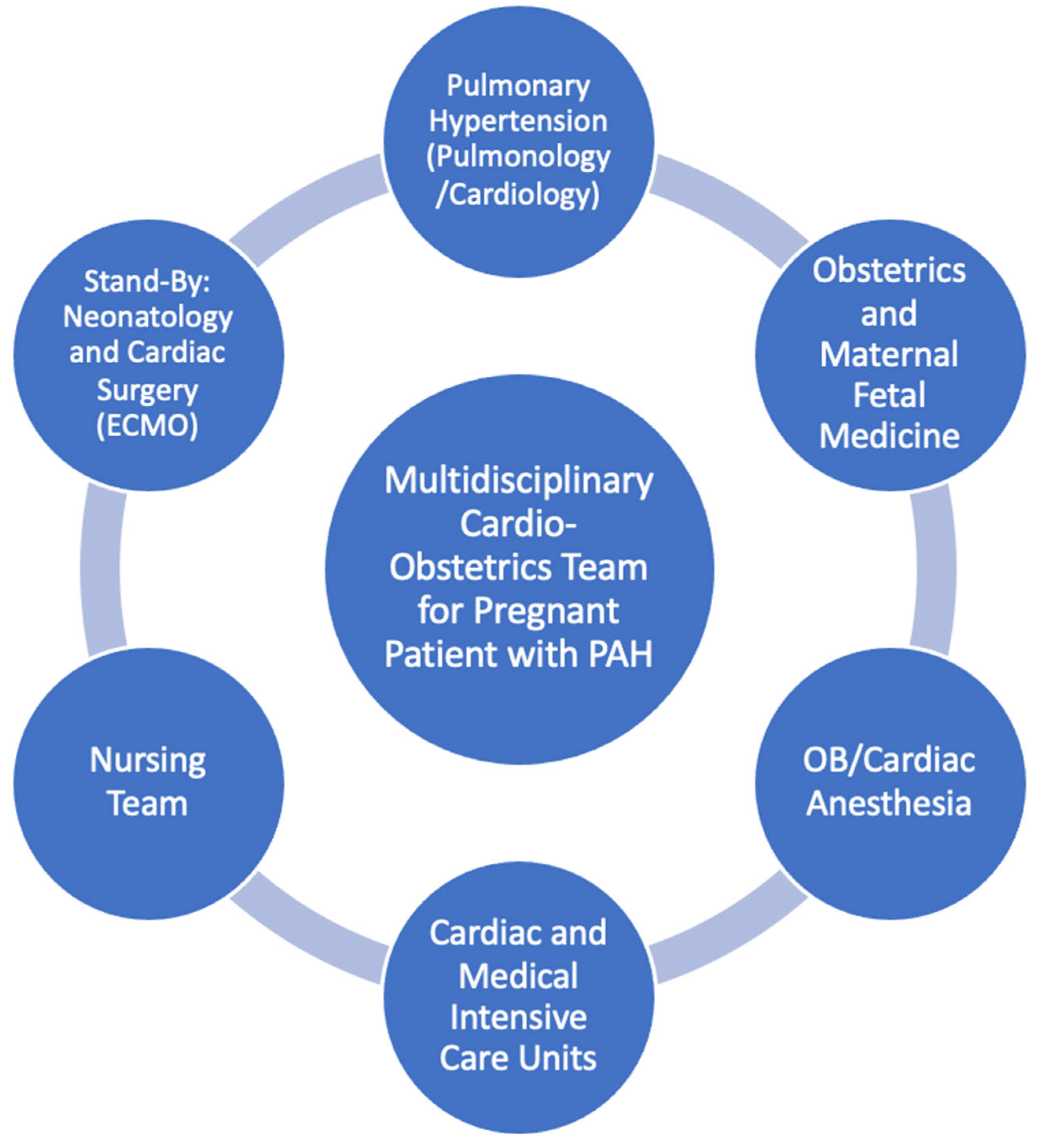
3.5. Delivery and Role of Multidisciplinary Care
3.6. Monitoring
3.7. Breastfeeding and Postpartum Management
3.8. Postpartum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vonk-Noordegraaf, A.; Haddad, F.; Chin, K.M.; Forfia, P.; Kawut, S.; Lumens, J.; Naeije, R.; Newman, J.; Oudiz, R.; Pro-vencher, S.; et al. Right heart adaptation to pulmonary arterial hypertension: Physiology and pathobiology. J. Am. Coll. Cardiol. 2013, 62, D22–D33. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, P.M. Pulmonary Arterial Hypertension. Taichman DB, ed. N. Engl. J. Med. 2021, 385, 2361–2376. [Google Scholar] [CrossRef] [PubMed]
- Vonk-Noordegraaf, A.; Chin, K.M.; Haddad, F.; Hassoun, P.; Hemnes, A.; Hopkins, S.; Kawut, S.; Langleben, D.; Lumens, J.; Naeije, R. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: An update. Eur. Respir. J. 2019, 53, 1801900. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simmonneau, G.; Peacock, A.; Vonk-Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Hemnes, A.R.; Kiely, D.G.; Cockrill, B.A.; Safdar, Z.; Wilson, V.; Hazmi, M.; Presto, I.; Maclean, M.; Lahm, T. Statement on pregnancy in pulmonary hypertension from the pulmonary vascular research institute. Pulm. Circ. 2015, 5, 435–465. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, V.V.; Archer, S.L.; Badesch, D.B.; Barst, R.; Farber, H.; Lindner, J.; Mathier, M.; McGoon, M.; Park, M.; Rosenson, R. ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension. Circulation 2009, 119, 2250–2294. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shi, H.; Xu, L.; Su, W.; Li, J. Pregnancy outcomes in patients with pulmonary arterial hypertension: A retrospective study. Medicine 2020, 99, e20285. [Google Scholar] [CrossRef]
- Duarte, A.G.; Thomas, S.; Safdar, Z.; Torres, F.; Pacheco, L.; Feldman, J.; DeBoisblanc, B. Management of pulmonary arterial hypertension during pregnancy: A retrospective, multicenter experience. Chest 2013, 143, 1330–1336. [Google Scholar] [CrossRef]
- Jaïs, X.; Olsson, K.M.; Barbera, J.A.; Blanco, I.; Torbicki, A.; Peacock, A.; Vizza, C.; Macdonald, P.; Humbert, M.; Hoeper, M. Pregnancy outcomes in pulmonary arterial hypertension in the modern management era. Eur. Respir. J. 2012, 40, 881–885. [Google Scholar] [CrossRef]
- Bédard, E.; Dimopoulos, K.; Gatzoulis, M.A. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur. Heart J. 2009, 30, 256–265. [Google Scholar] [CrossRef]
- Meng, M.L.; Landau, R.; Viktorsdottir, O.; Banayan, J.; Grant, T.; Bateman, B.; Smiley, R.; Reitman, E. Pulmonary Hyper-tension in Pregnancy: A Report of 49 Cases at Four Tertiary North American Sites. Obstet. Gynecol. 2017, 129, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Roos-Hesselink, J.; Baris, L.; Johnson, M.; De Backer, J.; Otto, C.; Marelli, A.; Jondeau, G.; Budts, W.; Grewal, J.; Sliwa, K.; et al. Pregnancy outcomes in women with cardiovascular disease: Evolving trends over 10 years in the ESC Registry Of Pregnancy And Cardiac disease (ROPAC). Eur. Heart J. 2019, 40, 3848–3855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, J.; Zhou, X.; Xu, X.; Ye, Q.; Ou, Q.; Li, Y.; Huang, J. Perioperative Management of Pregnant Women With Idi-opathic Pulmonary Arterial Hypertension: An Observational Case Series Study From China. J. Cardiothorac. Vasc. Anesth. 2018, 32, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Peng, P.; Liu, X.; Liu, J.; Gao, J.; Chen, W. Evaluation of maternal and fetal outcomes in pregnancy complicated with pulmonary arterial hypertension. Ann. Palliat. Med. 2021, 10, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, M.; Kumar, S.; Roy, K.K.; Sharma, J.B.; Singh, N. Pregnancy outcome in women with pulmonary arterial hyper-tension: Single-center experience from India. Arch. Gynecol. Obstet. 2013, 288, 305–309. [Google Scholar] [CrossRef]
- Katsuragi, S.; Yamanaka, K.; Neki, R.; Kamiya, C.; Sasaki, Y.; Osato, K.; Miyoshi, T.; Kawasaki, K.; Horiuchi, C.; Kobayashi, Y.; et al. Maternal Outcome in Pregnancy Complicated With Pulmonary Arterial Hypertension. Circ. J. 2012, 76, 2249–2254. [Google Scholar] [CrossRef]
- Bonnin, M.; Mercier, F.J.; Sitbon, O.; Roger-Christoph, S.; Jais, X.; Humbert, M.; Audibert, F.; Frydman, R.; Simmonneau, G.; Benhamou, D. Severe Pulmonary Hypertension during Pregnancy: Mode of Delivery and Anesthetic Management of 15 Consecutive Cases. Anesthesiology 2005, 102, 1133–1137. [Google Scholar] [CrossRef]
- Monnery, L.; Nanson, J.; Charlton, G. Primary pulmonary hypertension in pregnancy; a role for novel vasodilators. Br. J. Anaesth. 2001, 87, 295–298. [Google Scholar] [CrossRef]
- Elliot, C.A.; Stewart, P.; Webster, V.J.; Mills, G.H.; Hutchinson, S.P.; Howarth, E.S.; Bu’lock, F.A.; Lawson, R.A.; Armstrong, I.J.; Kiely, D.G. The use of iloprost in early pregnancy in patients with pulmonary arterial hypertension. Eur. Respir. J. 2005, 26, 168–173. [Google Scholar] [CrossRef]
- Bendayan, D.; Hod, M.; Oron, G.; Sagie, A.; Eidelman, L.; Shitrit, D.; Kramer, M. Pregnancy outcome in patients with pul-monary arterial hypertension receiving prostacyclin therapy. Obstet. Gynecol. 2005, 106 Pt 2, 1206–1210. [Google Scholar] [CrossRef]
- Zwicke, D.; Paulus, S.; Thohan, V. Pulmonary Arterial Hypertension and Pregnancy. In Cardiac Problems in Pregnancy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 252–260. [Google Scholar] [CrossRef]
- Kamp, J.C.; von Kaisenberg, C.; Greve, S.; Winter, L.; Park, D.; Fuge, J.; Kuhn, C.; Hoeper, M.; Olsson, K. Pregnancy in pul-monary arterial hypertension: Midterm outcomes of mothers and offspring. J. Heart Lung Transplant. 2021, 40, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Kiely, D.G.; Condliffe, R.; Webster, V.; Mills, G.; Wrench, I.; Gandhi, S.; Selby, K.; Armstrong, I.; Martin, L.; Howarth, E.; et al. Improved survival in pregnancy and pulmonary hypertension using a multiprofessional approach. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 565–574. [Google Scholar] [CrossRef]
- Smith, J.S.; Mueller, J.; Daniels, C. Pulmonary Arterial Hypertension in the Setting of Pregnancy: A Case Series and Standard Treatment Approach. In C63. Management of Pulmonary Hypertension; American Thoracic Society International Conference Abstracts; American Thoracic Society: New York, NY, USA, 2012; p. A4779. [Google Scholar] [CrossRef]
- Shu, T.; Feng, P.; Liu, X.; Wen, L.; Chen, H.; Chen, Y.; Huang, W. Multidisciplinary Team Managements and Clinical Outcomes in Patients With Pulmonary Arterial Hypertension During the Perinatal Period. Front. Cardiovasc. Med. 2021, 8, 1964. [Google Scholar] [CrossRef] [PubMed]
- Daimon, A.; Iwanaga, N.; Ikeda, T.; Nakanishi, N.; Yoshimatsu, J.; Kamiya, C.A. Management of pulmonary vasodilator therapy in three pregnancies with pulmonary arterial hypertension. J. Obstet. Gynaecol. Res. 2017, 43, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Corbach, N.; Berlier, C.; Lichtblau, M.; Schwarz, E.; Gautschi, F.; Groth, A.; Schupbach, R.; Krahenmann, F.; Saxer, S.; Ulrich, S. Favorable Pregnancy Outcomes in Women With Well-Controlled Pulmonary Arterial Hypertension. Front. Med. 2021, 8, 689764. [Google Scholar] [CrossRef]
- Sliwa, K.; van Hagen, I.M.; Budts, W.; Swan, L.; Sinagra, G.; Caruana, M.; Blanco, M.; Wagenaar, L.; Johnson, M.; Webb, G.; et al. Pulmonary hypertension and pregnancy outcomes: Data from the Registry Of Pregnancy and Cardiac Disease (ROPAC) of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 1119–1128. [Google Scholar] [CrossRef]
- Supreme Court of the United States. Dobbs, State Health Officer of the Mississippi Department of Health; et al. v. Jackson Women’s Health Organization; et al. 2022. Available online: https://www.supremecourt.gov/opinions/21pdf/19-1392_6j37.pdf (accessed on 23 July 2022).
- The American College of Obstetricians and Gynecologists. ACOG Statement on Reports of a Draft Opinion in Dobbs v. Jackson. Available online: https://www.acog.org/news/news-releases/2022/05/acog-statement-on-reports-of-a-draft-opinion-in-dobbs-v-jackson (accessed on 10 June 2022).
- American Academy of Family Physicians, American Academy of Pediatrics, American College of Obstetricians and Gyne-cologists, American College of Physicians, American Psychiatric Association. Physicians: SCOTUS Decision Jeopardizes Pa-tient-Physician Relationship, Penalizes Evidence-Based Care. Available online: https://www.aafp.org/news/media-center/statements/physicians-scotus-decision-jeopardizes-patient-physician-relationship-penalizes-evidence-based-care.html (accessed on 23 July 2022).
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.; Gatzoulis, M.; Krowka, M.; Williams, P.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Blomstrom Lundqvist, C.; Borghi, C.; Cifkova, R.; Ferreira, R.; Foidart, J.; Gibbs, J.; Gohlke-Baerwolf, C.; Gorenek, B.; Iung, B.; et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2011, 32, 3147–3197. [Google Scholar] [CrossRef]
- Silversides, C.K.; Grewal, J.; Mason, J.; Sermer, M.; Kiess, M.; Rychel, V.; Wald, R.; Colman, J.; Siu, S. Pregnancy Outcomes in Women With Heart Disease: The CARPREG II Study. J. Am. Coll. Cardiol. 2018, 71, 2419–2430. [Google Scholar] [CrossRef]
- Sharma, G.; Ying, W.; Silversides, C.K. The Importance of Cardiovascular Risk Assessment and Pregnancy Heart Team in the Management of Cardiovascular Disease in Pregnancy. Cardiol. Clin. 2021, 39, 7–19. [Google Scholar] [CrossRef]
- Galiè, N.; Channick, R.N.; Frantz, R.P.; Grünig, E.; Jing, Z.-C.; Moiseeva, O.; Preston, I.R.; Pulido, T.; Safdar, Z.; Tamura, Y.; et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801889. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Hoeper, M.M.; Humbert, M.; Torbicki, A.; Vachiery, J.; Barbera, J.; Beghetti, M.; Corris, P.; Gaine, S.; Gibbs, J.; et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2009, 30, 2493–2537. [Google Scholar] [CrossRef]
- Vachiéry, J.L.; Simonneau, G. Management of severe pulmonary arterial hypertension. Eur. Respir. Rev. 2010, 19, 279–287. [Google Scholar] [CrossRef] [PubMed]
- D’Alonzo, G.E.; Barst, R.J.; Ayres, S.M.; Bergofsky, E.; Brundage, B.; Detre, K.; Fishman, A.; Goldring, R.; Groves, B.; Kernis, J.; et al. Survival in Patients with Primary Pulmonary Hypertension. Ann. Intern. Med. 1991, 115, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kylhammar, D.; Kjellström, B.; Hjalmarsson, C.; Jansson, K.; Nisell, M.; Soderberg, S.; Wikstrom, G.; Radegran, G. A com-prehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur. Heart J. 2018, 39, 4175–4181. [Google Scholar] [CrossRef]
- Boucly, A.; Weatherald, J.; Savale, L.; Jais, X.; Cottin, V.; Prevot, G.; Picard, F.; De Groote, P.; Jevnikar, M.; Bergot, E.; et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur. Respir. J. 2017, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barst, R.J.; McGoon, M.; Torbicki, A.; Sitbon, O.; Krowka, M.J.; Olschewski, H.; Gaine, S. Diagnosis and differential as-sessment of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004, 43, 40S–47S. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Kramer, T.; Pan, Z.; Eichstaedt, C.; Spiesshoefer, J.; Benjamin, N.; Olsson, K.; Meyer, K.; Vizza, C.; Vonk-Noordegraaf, A.; et al. Mortality in pulmonary arterial hypertension: Prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur. Respir. J. 2017, 50, 1700740. [Google Scholar] [CrossRef]
- Benza, R.L.; Farber, H.W.; Frost, A.; Ghofrani, H.; Gomez-Sanchez, M.; Langleben, D.; Rosenkranz, S.; Busse, D.; Meier, C.; Nikkho, S.; et al. REVEAL risk scores applied to riociguat-treated patients in PATENT-2: Impact of changes in risk score on survival. J. Heart Lung Transplant. 2018, 37, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Gomberg-Maitland, M.; Elliott, C.G.; Farber, H.W.; Foreman, A.J.; Frost, A.E.; McGoon, M.D.; Pasta, D.J.; Selej, M.; Burger, C.D.; et al. Predicting Survival in Patients with Pulmonary Arterial Hypertension: The REVEAL Risk Score Calculator 2.0 and Comparison with ESC/ERS-Based Risk Assessment Strategies. Chest 2019, 156, 323–337. [Google Scholar] [CrossRef]
- Benza, R.L.; Farber, H.W.; Frost, A.E.; Ghofrani, H.A.; Corris, P.A.; Lambelet, M.; Nikkho, S.; Meier, C.; Hoeper, M.M. Application of the REVEAL risk score calculator 2.0 in the PATENT study. Int. J. Cardiol. 2021, 332, 189–192. [Google Scholar] [CrossRef]
- Benza, R.L.; Miller, D.P.; Gomberg-Maitland, M.; Frantz, R.P.; Foreman, A.J.; Coffey, C.S.; Frost, A.; Barst, R.J.; Badesch, D.B.; Elliott, C.G.; et al. Predicting survival in pulmonary arterial hypertension: Insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL). Circulation 2010, 122, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.; Holman, T.; Novak, T. Idiopathic Pulmonary Arterial Hypertension Unmasked by Pregnancy. Acute Med. 2020, 19, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Madden, B.P. Pulmonary hypertension and pregnancy. Int. J. Obstet. Anesth. 2009, 18, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Limoges, M.; Langleben, D.; Fox, B.D.; Shear, R.; Wieczorek, P.; Rudski, L.; Hirsch, A.; Schlesinger, R.; Lesenko, L. Pregnancy as a possible trigger for heritable pulmonary arterial hypertension. Pulm. Circ. 2016, 6, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, M.; Rutherford, J.D. Cardiovascular physiology of pregnancy. Circulation 2014, 130, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- LoMauro, A.; Aliverti, A. Respiratory physiology of pregnancy. Breathe 2015, 11, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Ouzounian, J.G.; Elkayam, U. Physiologic Changes During Normal Pregnancy and Delivery. Cardiol. Clin. 2012, 30, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Paulin, R.; Michelakis, E.D. The estrogen puzzle in pulmonary arterial hypertension. Circulation 2012, 126, 1016–1019. [Google Scholar] [CrossRef]
- Ventetuolo, C.; Ouyang, P.; Bluemke, D.; Tandri, H.; Barr, R.G.; Bagiella, E.; Cappola, A.R.; Bristow, M.R.; Johnson, C.; Kronmal, R.A.; et al. Sex Hormones Are Associated with Right Ventricular Structure and Function: The MESA-right ventricle study. Am. J. Respir. Crit. Care Med. 2011, 183, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, W.; van de Veerdonk, M.C.; Trip, P.; De Man, F.; Heymans, M.; Marcus, J.; Kawut, S.; Bogaard, H.; Boonstra, A.; Vonk-Noordegraaf, A. The Right Ventricle Explains Sex Differences in Survival in Idiopathic Pulmonary Arterial Hyperten-sion. Chest 2014, 145, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Rabinovitch, M.; Eghbali, M. Estrogen paradox in pulmonary hypertension: Current controversies and future perspectives. Am. J. Respir. Crit. Care Med. 2012, 186, 125–131. [Google Scholar] [CrossRef] [PubMed]
- James, A.H. Venous thromboembolism in pregnancy. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 326–331. [Google Scholar] [CrossRef]
- Weiss, B.M.; Hess, O.M. Pulmonary Vascular Disease and Pregnancy: Current, Management Strategies, and Perspectives. Eur. Heart J. 2000, 21, 104–115. [Google Scholar] [CrossRef]
- Thorne, S.; Nelson-Piercy, C.; Rosenthal, E.; MacGregor, A.; Gibbs, S.; Crowhurst, J.; Panay, N.; Walker, F.; Williams, D.; de Swiet, M.; et al. Pregnancy and contraception in heart disease and pulmonary arterial hypertension. J. Fam. Plan. Reprod. Health Care 2006, 32, 75. [Google Scholar] [CrossRef]
- Lindley, K.J.; Merz, C.N.B.; Davis, M.B.; Madden, T.; Park, K.; Bello, N.A. Contraception and Reproductive Planning for Women With Cardiovascular Disease: JACC Focus Seminar 5/5. J. Am. Coll. Cardiol. 2021, 77, 1823–1834. [Google Scholar] [CrossRef]
- Hill, W.; Holy, R.; Traiger, G. Intimacy, contraception, and pregnancy prevention in patients with pulmonary arterial hy-pertension: Are we counseling our patients? Pulm. Circ. 2020, 10, 2045894018785259. [Google Scholar] [CrossRef]
- Badesch, D.B.; Raskob, G.E.; Elliott, C.G.; Krichman, A.M.; Farber, H.W.; Frost, A.E.; Barst, R.J.; Benza, R.L.; Liou, T.G.; Turner, M.; et al. Pulmonary Arterial Hypertension: Baseline Characteristics From the REVEAL Registry. Chest 2010, 137, 376–387. [Google Scholar] [CrossRef]
- Humbert, M.; Sitbon, O.; Chaouat, A.; Bertocchi, M.; Habib, G.; Gressin, V.; Yaici, A.; Weitzenblum, E.; Cordier, J.; Chabot, F. Pulmonary arterial hypertension in France: Results from a national registry. Am. J. Respir. Crit. Care Med. 2006, 173, 1023–1030. [Google Scholar] [CrossRef]
- Humbert, M.; Sitbon, O.; Chaouat, A.; Bertocchi, M.; Habib, G.; Gressin, V.; Yaici, A.; Weitzenblum, E.; Cordier, J.; Chabot, F.; et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010, 122, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Sarayani, A.; Albogami, Y.; Thai, T.N.; Smolinski, N.; Patel, P.; Wang, Y.; Nduaguba, S.; Rasmussen, S.; Winterstein, A. Prenatal exposure to teratogenic medications in the era of Risk Evaluation and Mitigation Strategies. Am. J. Obstet. Gynecol. 2022, 227, 263.e1–263.e38. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.M.; Channick, R. Pregnancy in pulmonary arterial hypertension. Eur. Respir. Rev. 2016, 25, 431–437. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, V.V.; McGoon, M.D. Pulmonary arterial hypertension. Circulation 2006, 114, 1417–1431. [Google Scholar] [CrossRef] [PubMed]
- Kiely, D.G.; Condliffe, R.; Wilson, V.J.; Gandhi, S.V.; Elliot, C.A. Pregnancy and pulmonary hypertension: A practical approach to management. Obstet. Med. 2013, 6, 144–154. [Google Scholar] [CrossRef]
- Lan, N.; Massam, B.; Kulkarni, S.; Lang, C. Pulmonary Arterial Hypertension: Pathophysiology and Treatment. Diseases 2018, 6, 38. [Google Scholar] [CrossRef]
- Guiahi, M.; Davis, A. First-trimester abortion in women with medical conditions. Contraception 2012, 86, 622–630. [Google Scholar] [CrossRef]
- Bartlett, L.A.; Berg, C.J.; Shulman, H.B.; Zane, S.; Green, C.; Whitehead, S.; Atrash, H. Risk factors for legal induced abor-tion-related mortality in the United States. Obstet. Gynecol. 2004, 103, 729–737. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomstrom-Lundqvist, C.; Cifkova, R.; De Bonis, M.; Iung, B.; Johnson, M.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef]
- Lima, F.V.; Yang, J.; Xu, J.; Stergiopoulos, K. National Trends and In-Hospital Outcomes in Pregnant Women with Heart Disease in the United States. Am. J. Cardiol. 2017, 119, 1694–1700. [Google Scholar] [CrossRef]
- Davis, M.B.; Walsh, M.N. Cardio-obstetrics: Team-based care to improve maternal outcomes. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005417. [Google Scholar] [CrossRef] [PubMed]
- Briller, J.; Koch, A.R.; Geller, S.E. Maternal Cardiovascular Mortality in Illinois, 2002–2011. Obstet. Gynecol. 2017, 129, 819–826. Available online: https://journals.lww.com/greenjournal/Fulltext/2017/05000/Maternal_Cardiovascular_Mortality_in_Illinois,.7.aspx (accessed on 10 June 2022). [CrossRef] [PubMed]
- Vachiéry, J.L.; Yerly, P.; Huez, S. How to detect disease progression in pulmonary arterial hypertension. Eur. Respir. Rev. 2012, 21, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Hamm, C. Role of B-type natriuretic peptide (BNP) and NT-PROBNP in clinical routine. Heart 2006, 92, 843–849. [Google Scholar] [CrossRef]
- Magee, L.A.; Schick, B.; Donnenfeld, A.E.; Sage, S.R.; Conover, B.; Cook, L.; McElhatton, P.R.; Schmidt, M.A.; Koren, G. The safety of calcium channel blockers in human pregnancy: A prospective, multicenter cohort study. Am. J. Obstet. Gynecol. 1996, 174, 823–828. [Google Scholar] [CrossRef]
- Rich, S.; Kaufmann, E.; Levy, P.S. The Effect of High Doses of Calcium-Channel Blockers on Survival in Primary Pulmonary Hypertension. N. Engl. J. Med. 1992, 327, 76–81. [Google Scholar] [CrossRef]
- Food and Drug Administration. Norvasc (Amlodipine Besylate) Tablets Label. 2011. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019787s047lbl.pdf (accessed on 23 July 2020).
- Galiè, N.; Barberà, J.A.; Frost, A.E.; Ghofrani, H.-A.; Hoeper, M.M.; McLaughlin, V.V.; Peacock, A.J.; Simonneau, G.; Vachiery, J.-L.; Grünig, E.; et al. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N. Engl. J. Med. 2015, 373, 834–844. [Google Scholar] [CrossRef]
- Goland, S.; Tsai, F.; Habib, M.; Janmohamed, M.; Goodwin, T.M.; Elkayam, U. Favorable outcome of pregnancy with an elective use of epoprostenol and sildenafil in women with severe pulmonary hypertension. Cardiology 2010, 115, 205–208. [Google Scholar] [CrossRef]
- Food and Drug Administration. Label: VIAGRA (Sildenafil Citrate) Tablets. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/20895s039s042lbl.pdf (accessed on 23 July 2022).
- Wang, T.; Lu, J.; Li, Q.; Chen, Y.; Ye, Q.; Gao, J.; Yang, D.; Zhao, L.; Huang, J.; Zhang, J. Rapid Titration of Intravenous Treprostinil to Treat Severe Pulmonary Arterial Hypertension Postpartum: A Retrospective Observational Case Series Study. Anesth. Analg. 2019, 129, 1607–1612. [Google Scholar] [CrossRef]
- Streit, M.; Speich, R.; Fischler, M.; Ulrich, S. Successful pregnancy in pulmonary arterial hypertension associated with systemic lupus erythematosus: A case report. J. Med. Case Rep. 2009, 3, 7255. [Google Scholar] [CrossRef]
- Badalian, S.S.; Silverman, R.K.; Aubry, R.H.; Longo, J. Twin pregnancy in a woman on long-term epoprostenol therapy for primary pulmonary hypertension. A case report. J. Reprod. Med. 2000, 45, 149–152. [Google Scholar] [PubMed]
- Stewart, R.; Tuazon, D.; Duarte, A.G.; Olson, G. Pregnancy and Primary Pulmonary Hypertension: Successful Outcome With Epoprostenol Therapy. Chest 2001, 119, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.M.; Maggiorini, M.; Jenni, R.; Lauper, U.; Popov, V.; Bombeli, T.; Spahn, D.R. Pregnant patient with primary pulmonary hypertension: Inhaled pulmonary vasodilators and epidural anesthesia for cesarean delivery. J. Am. Soc. Anesthesiol. 2000, 92, 1191. [Google Scholar] [CrossRef]
- Hsu, C.H.; Gomberg-Maitland, M.; Glassner, C.; Chen, J.H. The management of pregnancy and pregnancy-related medical conditions in pulmonary arterial hypertension patients. Int. J. Clin. Pract. 2011, 65 (Suppl. S172), 6–14. [Google Scholar] [CrossRef]
- Food and Drug Administration. Highlights of Prescribing Information (Remodulin). 2018. Available online: www.fda.gov/medwatch (accessed on 10 June 2022).
- Mathier, M.A.; McDevitt, S.; Saggar, R. Subcutaneous treprostinil in pulmonary arterial hypertension: Practical considerations. J. Heart Lung Transplant. 2010, 29, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.D. UPTRAVI ® (Selexipag) UPTRAVI ® (Selexipag) Use during Pregnancy and Effect on Fertility. 2022. Available online: https://www.janssenmd.com/uptravi/special-populations/pregnancy/uptravi-selexipag-use-during-pregnancy-and-effect-on-fertility (accessed on 23 July 2022).
- Sahni, S.; Palkar, A.V.; Rochelson, B.L.; Kȩpa, W.; Talwar, A. Pregnancy and pulmonary arterial hypertension: A clinical conundrum. Pregnancy Hypertens. 2015, 5, 157–164. [Google Scholar] [CrossRef]
- Kenyon, K.W.; Nappi, J.M. Bosentan for the Treatment of Pulmonary Arterial Hypertension. Ann. Pharmacother. 2003, 37, 1055–1062. [Google Scholar] [CrossRef]
- Food and Drug Administration. Highlights of Prescribing Information (Adempas). 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/204819s006lbl.pdf (accessed on 10 June 2022).
- Food and Drug Administration. Highlights of Prescribing Information (Tracleer). 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021290s012lbl.pdf (accessed on 10 June 2022).
- Food and Drug Administration. Highlights of Prescribing Information (Letairis). 2015. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022081s033lbl.pdf (accessed on 10 June 2022).
- Food and Drug Administration. Highlights of Prescribing Information (Opsumit). 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204410s017lbl.pdf (accessed on 10 June 2022).
- Olsson, K.M.; Delcroix, M.; Ghofrani, H.A.; Tiede, H.; Huscher, D.; Speich, R.; Grunig, E.; Staehler, G.; Rosenkranz, S.; Halank, M.; et al. Anticoagulation and survival in pulmonary arterial hypertension: Results from the comparative, prospective registry of newly initiated therapies for pulmonary hypertension (COMPERA). Circulation 2014, 129, 57–65. [Google Scholar] [CrossRef]
- Alshawabkeh, L.; Economy, K.E.; Valente, A.M. Anticoagulation During Pregnancy Evolving Strategies With a Focus on Mechanical Valves. J. Am. Coll. Cardiol. 2016, 68, 1804–1813. [Google Scholar] [CrossRef]
- Berresheim, M.; Wilkie, J.; Nerenberg, K.A.; Ibrahim, Q.; Bungard, T.J. A case series of LMWH use in pregnancy: Should trough anti-Xa levels guide dosing? Thromb. Res. 2014, 134, 1234–1240. [Google Scholar] [CrossRef]
- McNeil, A.; Chen, J.; Meng, M.L. Pulmonary hypertension in pregnancy-the Anesthesiologist’s perspective. Int. J. Cardiol. Congenit. Heart Dis. 2021, 5, 100234. [Google Scholar] [CrossRef]
- Nowroozpoor, A.; Malekmohammad, M.; Seyyedi, S.R.; Hashemian, S.M. Pulmonary Hypertension in Intensive Care Units: An Updated Review. Tanaffos 2019, 18, 180–207. [Google Scholar] [PubMed]
- Breen, T.W.; Janzen, J.A. Clinical Reports Pulmonary Hypertension and Cardiomyopathy: Anaesthetic Management for Caesarean Section. Can. J. Anaesth. 1991, 38, 895–899. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wolla, C.D.; Matos, J.R.; Wineland, R. Anesthetic Management of Severe Pulmonary Hypertension in Pregnancy. J. Cardiothorac. Vasc. Anesth. 2022, 36, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Smedstad, M.B.; Cramb, R.M.; Morison, D.H. Pulmonary Hyper-Tension and Pregnancy: A Series of Eight Cases. Can. J. Anaesth. 1994, 41, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Höhn, L.; Schweizer, A.; Morel, D.R.; Spiliopoulos, A.; Licker, M. Circulatory Failure after Anesthesia Induction in a Patient with Severe Primary Pulmonary Hypertension. Anesthesiology 1999, 91, 1943. [Google Scholar] [CrossRef]
- Decoene, C.; Bourzoufi, K.; Moreau, D.; Narducci, F.; Crepin, F.; Krivosic-Horber, R. Use of Inhaled Nitric Oxide for Emer-gency Cesarean section in a woman with unexpected primary pulmonary hypertension. Can. J. Anaesth. 2001, 48, 584–587. [Google Scholar] [CrossRef]
- Pieper, P.G.; Lameijer, H.; Hoendermis, E.S. Pregnancy and pulmonary hypertension. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 579–591. [Google Scholar] [CrossRef]
- Prasad, C.S.; Kumar, S.; Sumathy, S.; Kunjukutty, R.; Puthenveettil, N.; Sen, A.; Sivabalakrishnan, J.; Kumar, R. Pregnancy and pulmonary arterial hypertension—Improving surveillance and outcomes with multidisciplinary care and N terminal pro-brain natriuretic peptide trends. J. Matern.-Fetal Neonatal Med. 2020, 35, 3533–3539. [Google Scholar] [CrossRef]
- Fijalkowska, A.; Kurzyna, M.; Torbicki, A.; Szewczyk, G.; Florczyk, M.; Pruszczyk, P.; Szturmowicz, M. Serum N-Terminal Brain Natriuretic Peptide as a Prognostic Parameter in Patients With Pulmonary Hypertension. Chest 2006, 129, 1313–1321. [Google Scholar] [CrossRef]
- Ghanem, F.A.; Movahed, A. Use of antihypertensive drugs during pregnancy and lactation. Cardiovasc. Ther. 2008, 26, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Drug Levels and Effects Summary of Use during Lactation. Available online: https://www.ncbi.nlm.nih.gov/books/ (accessed on 23 July 2022).
- Rosengarten, D.; Blieden, L.C.; Kramer, M.R. Pregnancy outcomes in pulmonary arterial hypertension in the modern management era. Eur. Respir. J. 2012, 40, 1304–1305. [Google Scholar] [CrossRef] [PubMed]
- Garabedian, M.J.; Hansen, W.F.; Gianferrari, E.A.; Lain, K.Y.; Fragneto, R.Y.; Campbell, C.L.; Booth, D.C. Epoprostenol treatment for idiopathic pulmonary arterial hyper-tension in pregnancy. J. Perinatol. 2010, 30, 628–631. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nahapetian, A.; Oudiz, R.J. Serial Hemodynamics and Complications of Pregnancy in Severe Pulmonary Arterial Hypertension. Cardiology 2008, 109, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Price, L.C.; Dimopoulos, K.; Marino, P.; Alonso-Gonzalez, R.; McCabe, C.; Kemnpy, A.; Swan, L.; Boutsikou, M.; Al Zahrani, A.; Coghlan, G.; et al. The CRASH report: Emergency management dilemmas facing acute physicians in patients with pulmonary arterial hypertension. Thorax 2017, 72, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Low, T.T.; Guron, N.; Ducas, R.; Yamamura, K.; Charla, P.; Granton, J.; Silversides, C. Pulmonary arterial hypertension in pregnancy—A systematic review of outcomes in the modern era. Pulm. Circ. 2021, 11, 20458940211013671. [Google Scholar] [CrossRef]
- Penning, S.; Thomas, N.; Atwal, D.; Nageotte, M.; McConnell, D. Cardiopulmonary bypass support for emergency cesarean delivery in a patient with severe pulmonary hypertension. Am. J. Obstet. Gynecol. 2001, 184, 225–226. [Google Scholar] [CrossRef][Green Version]
- Abid Memon, H.; Safdar, Z.; Goodarzi, A. Use of Extracorporeal Membrane Oxygenation in Postpartum Management of a Patient with Pulmonary Arterial Hypertension. Case Rep. Pulmonol. 2018, 2018, 7031731. [Google Scholar] [CrossRef]
- Yang, J.Z.; Fernandes, T.M.; Kim, N.H.; Poch, D.S.; Kerr, K.M.; Lombardi, S.; Melber, D.; Kelly, T.; Papamatheakis, D.G. Pregnancy and pulmonary arterial hypertension: A case series and literature review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100358. [Google Scholar] [CrossRef]
- Phoophiboon, V.; Jaimchariyatam, N.; Srimahachota, S.; Sirinawin, C. Successful multimodality management of severe pulmonary arterial hypertension during pregnancy with VA-ECMO and atrial septostomy using stent. BMJ Case Rep. 2019, 12, e231916. [Google Scholar] [CrossRef]
- Bostock, S.; Sheares, K.; Cannon, J.; Taboada, D.; Pepke-Zaba, J.; Toshner, M. The potential effects of pregnancy in a patient with idiopathic pulmonary arterial hypertension responding to calcium channel blockade. Eur. Respir. J. 2017, 50, 1701141. [Google Scholar] [CrossRef] [PubMed]


|
|
|
|
| Delivery Method | Strengths | Weaknesses |
|---|---|---|
| Vaginal Delivery |
|
|
| Cesarean Delivery |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coursen, J.; Simpson, C.E.; Mukherjee, M.; Vaught, A.J.; Kutty, S.; Al-Talib, T.K.; Wood, M.J.; Scott, N.S.; Mathai, S.C.; Sharma, G. Pregnancy Considerations in the Multidisciplinary Care of Patients with Pulmonary Arterial Hypertension. J. Cardiovasc. Dev. Dis. 2022, 9, 260. https://doi.org/10.3390/jcdd9080260
Coursen J, Simpson CE, Mukherjee M, Vaught AJ, Kutty S, Al-Talib TK, Wood MJ, Scott NS, Mathai SC, Sharma G. Pregnancy Considerations in the Multidisciplinary Care of Patients with Pulmonary Arterial Hypertension. Journal of Cardiovascular Development and Disease. 2022; 9(8):260. https://doi.org/10.3390/jcdd9080260
Chicago/Turabian StyleCoursen, Julie, Catherine E. Simpson, Monica Mukherjee, Arthur J. Vaught, Shelby Kutty, Tala K. Al-Talib, Malissa J. Wood, Nandita S. Scott, Stephen C. Mathai, and Garima Sharma. 2022. "Pregnancy Considerations in the Multidisciplinary Care of Patients with Pulmonary Arterial Hypertension" Journal of Cardiovascular Development and Disease 9, no. 8: 260. https://doi.org/10.3390/jcdd9080260
APA StyleCoursen, J., Simpson, C. E., Mukherjee, M., Vaught, A. J., Kutty, S., Al-Talib, T. K., Wood, M. J., Scott, N. S., Mathai, S. C., & Sharma, G. (2022). Pregnancy Considerations in the Multidisciplinary Care of Patients with Pulmonary Arterial Hypertension. Journal of Cardiovascular Development and Disease, 9(8), 260. https://doi.org/10.3390/jcdd9080260





