Perioperative Fluoroquinolone Treatment Deteriorates Prognosis Following Coronary Artery Bypass Grafting
Abstract
:1. Introduction
2. Methods
2.1. Ethics Declaration
2.2. Data Extraction
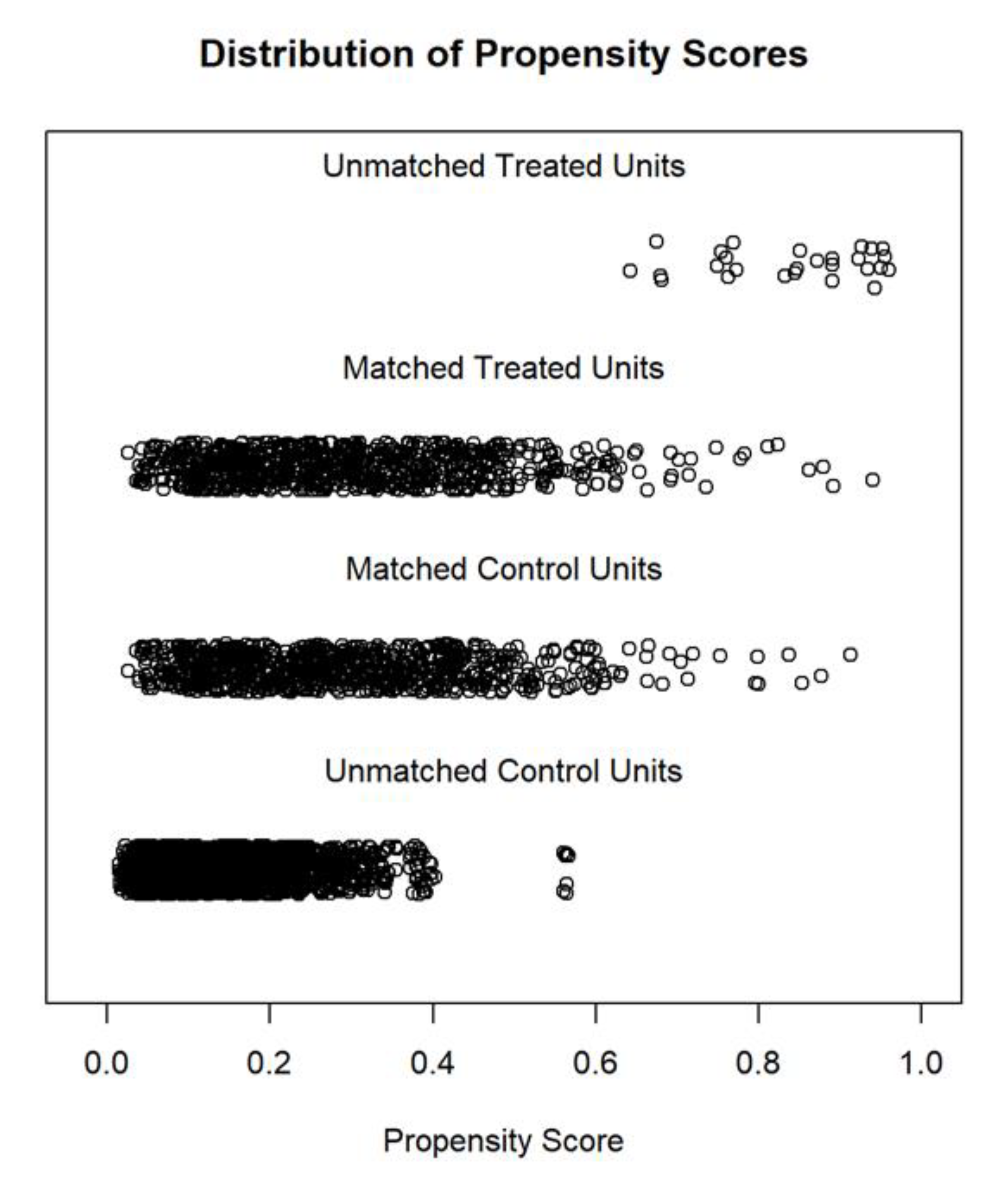
2.3. Statistical Analysis
3. Results
3.1. Baseline Data of Study Cohort
3.2. Primary Outcomes in the Matched Cohorts
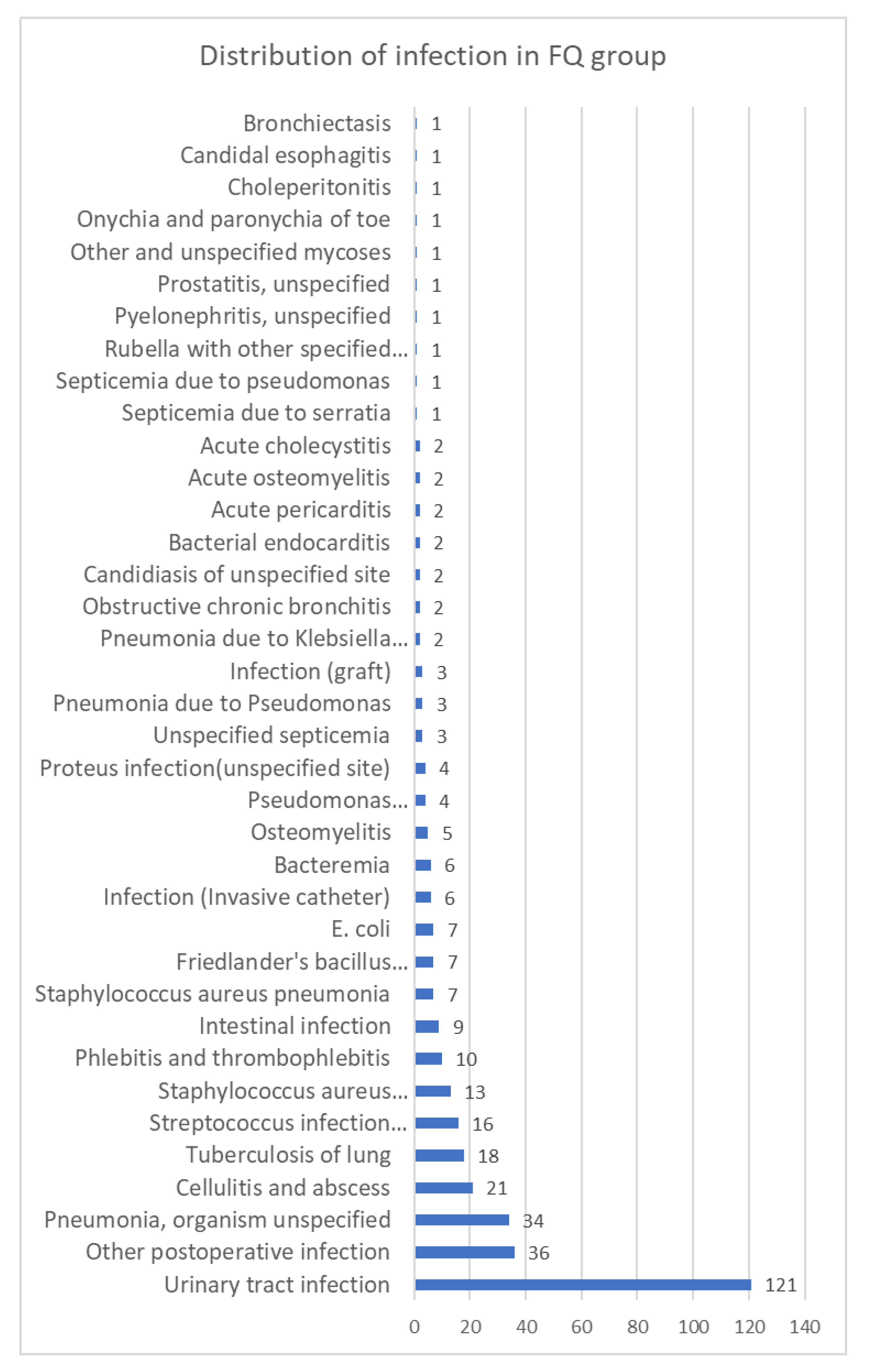
3.3. Secondary Outcomes in the Matched Cohorts
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reiner, Z. Hypertriglyceridaemia and risk of coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.H.; Smith, P.K. Coronary-Artery Bypass Grafting. N. Engl. J. Med. 2016, 374, 1954–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Jiang, D.-S.; Wang, J.; Wang, R.; Chen, T.; Wang, K.; Cao, S.; Wei, X. Fluoroquinolone Use and the Risk of Collagen-Associated Adverse Events: A Systematic Review and Meta-Analysis. Drug Saf. 2019, 42, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.C.; Bennett, C.L.; Witherspoon, B.J.; Knopf, K.B. An evaluation of reports of ciprofloxacin, levofloxacin, and moxifloxacin-association neuropsychiatric toxicities, long-term disability, and aortic aneurysms/dissections disseminated by the Food and Drug Administration and the European Medicines Agency. Expert Opin. Drug Saf. 2019, 18, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Bidell, M.R.; Lodise, T.P. Fluoroquinolone-Associated Tendinopathy: Does Levofloxacin Pose the Greatest Risk? Pharmacother. J. Hum. Pharmacol. Drug Ther. 2016, 36, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, B.; Inghammar, M.; Svanström, H. Fluoroquinolone use and risk of aortic aneurysm and dissection: Nationwide cohort study. BMJ 2018, 360, k678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Nautiyal, A. Aortic Dissection and Aortic Aneurysms Associated with Fluoroquinolones: A Systematic Review and Meta-Analysis. Am. J. Med. 2017, 130, 1449–1457.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankel, W.C.; Trautner, B.W.; Spiegelman, A.; Grigoryan, L.; LeMaire, S.A. Patients at Risk for Aortic Rupture Often Exposed to Fluoroquinolones during Hospitalization. Antimicrob. Agents Chemother. 2019, 63, e01712-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-C.; Lee, M.-T.G.; Chen, Y.-S.; Lee, S.-H.; Chen, Y.-S.; Chen, S.-C.; Chang, S.-C. Risk of Aortic Dissection and Aortic Aneurysm in Patients Taking Oral Fluoroquinolone. JAMA Intern. Med. 2015, 175, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, A.E.W.; Pollard, T.J.; Shen, L.; Lehman, L.-W.H.; Feng, M.; Ghassemi, M.; Moody, B.; Szolovits, P.; Celi, L.A.; Mark, R.G. MIMIC-III, a freely accessible critical care database. Sci. Data 2016, 3, 160035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, C.; Mo, L.; Hong, Y. Effectiveness of sodium bicarbonate infusion on mortality in septic patients with metabolic acidosis. Intensiv. Care Med. 2018, 44, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.E.; Imai, K.; King, G.; Stuart, E.A. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Polit. Anal. 2007, 15, 199–236. [Google Scholar] [CrossRef] [Green Version]
- Jankowski, P.; Czarnecka, D.; Badacz, L.; Bogacki, P.; Dubiel, J.S.; Grodecki, J.; Grodzicki, T.; Maciejewicz, J.; Mirek-Bryniarska, E.; Nessler, J.; et al. Practice setting and secondary prevention of coronary artery disease. Arch. Med. Sci. 2018, 14, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, H.Y.; Li, J.H.; Schoepf, U.J.; Bayer, R.R.; Tinnefeld, F.C.; Di Jiang, M.; Yang, F.; Guo, B.J.; Zhou, C.S.; Ge, Y.Q.; et al. Prognostic implication of CT-FFR based functional SYNTAX score in patients with de novo three-vessel disease. Eur. Heart J.-Cardiovasc. Imaging 2020, 22, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Roques, F.; Nashef, S.A.M.; Michel, P.; Gauducheau, E.; De Vincentiis, C.; Baudet, E.; Cortina, J.; David, M.; Faichney, A.; Gavrielle, F.; et al. Risk factors and outcome in European cardiac surgery: Analysis of the EuroSCORE multinational database of 19030 patients. Eur. J. Cardio-Thorac. Surg. 1999, 15, 816–823. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Li, H.; Zhu, X.; Wang, L.; Yi, M.; Li, C.; Chen, L.; Shi, Y. Three non-invasive ventilation strategies for preterm infants with respiratory distress syndrome: A propensity score analysis. Arch. Med. Sci. 2020, 16, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, S.; Du, J.; Gu, D.; Wang, Y.; Hu, S.; Zheng, Z. An In-hospital Mortality Risk Model for Patients Undergoing Coronary Artery Bypass Grafting in China. Ann. Thorac. Surg. 2020, 109, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Zandecki, L.; Janion, M.; Sadowski, M.; Kurzawski, J.; Polonski, L.; Gierlotka, M.; Gasior, M. Associations of changes in patient characteristics and management with decrease in mortality rates of men and women with ST-elevation myocardial infarction—A propensity score-matched analysis. Arch. Med. Sci. 2020, 16, 772–780. [Google Scholar] [CrossRef] [PubMed]


| Covariates | Non-FQ | FQ | p | SMD |
|---|---|---|---|---|
| n | 4093 | 937 | ||
| Male (%) | 3143(76.8) | 592 (63.2) | <0.001 | 0.3 |
| Age (mean (SD)) | 67.25(10.74) | 70.99(10.78) | <0.001 | 0.348 |
| Admission type (%) | <0.001 | 0.438 | ||
| ELECTIVE | 1603 (39.2) | 214 (22.8) | ||
| EMERGENCY | 2268 (55.4) | 706 (75.3) | ||
| URGENT | 222 (5.4) | 17 (1.8) | ||
| Smoking (%) | 0.033 | 0.098 | ||
| Active smoking | 291 (7.1) | 72 (7.7) | ||
| No smoking | 3363 (82.2) | 791 (84.4) | ||
| Smoking history | 439 (10.7) | 74 (7.9) | ||
| Hypertension (%) | 2781 (67.9) | 555 (59.2) | <0.001 | 0.182 |
| COPD (%) | 563 (13.8) | 150 (16.0) | 0.083 | 0.063 |
| Hyperlipemia (%) | 2678 (65.4) | 459 (49.0) | <0.001 | 0.337 |
| Diabetes (%) | 1545 (37.7) | 379 (40.4) | 0.134 | 0.055 |
| Infectious endocarditis (%) | 6 (0.1) | 11 (1.2) | <0.001 | 0.127 |
| Neurological dysfunction (%) | 69 (1.7) | 36 (3.8) | <0.001 | 0.132 |
| Extracardiac arteriopathy (%) | 419 (10.2) | 145 (15.5) | <0.001 | 0.157 |
| Angina (%) | 1800 (44.0) | 263 (28.1) | <0.001 | 0.336 |
| Cardiac surgery history (%) | 30 (0.7) | 5 (0.5) | 0.664 | 0.025 |
| Coronary intervention history (%) | 426 (10.4) | 87 (9.3) | 0.335 | 0.038 |
| Pulmonary hypertension (%) | 177 (4.3) | 72 (7.7) | <0.001 | 0.142 |
| Plus other heart surgery (%) | 906 (22.1) | 355 (37.9) | <0.001 | 0.349 |
| Plus aorta surgery (%) | 62 (1.5) | 34 (3.6) | <0.001 | 0.134 |
| Myocardial infarction (%) | 900 (22.0) | 343 (36.6) | <0.001 | 0.325 |
| Septal perforation (%) | 13 (0.3) | 1 (0.1) | 0.49 | 0.046 |
| Ejection fraction (%) | <0.001 | 0.252 | ||
| 30− | 290 (8.4) | 121 (14.3) | ||
| 30–50 | 1088 (31.4) | 312 (36.8) | ||
| 50+ | 2086 (60.2) | 415 (48.9) | ||
| Dialysis status (%) | 17 (0.4) | 7 (0.7) | 0.189 | 0.044 |
| Sepsis (%) | 25 (0.6) | 57 (6.1) | <0.001 | 0.308 |
| Grafts (%) | <0.001 | 0.191 | ||
| 1 graft | 730 (17.8) | 214 (22.8) | ||
| 2 grafts | 1584 (38.7) | 317 (33.8) | ||
| 3 grafts | 1238 (30.2) | 264 (28.2) | ||
| 4 or more grafts | 342 (8.4) | 83 (8.9) | ||
| Number of grafts unknown | 199 (4.9) | 59 (6.3) | ||
| on-pump CABG (%) | 3851 (94.1) | 876 (93.5) | 0.537 | 0.025 |
| Covariates | Non-FQ | FQ | p | SMD |
|---|---|---|---|---|
| n | 821 | 821 | ||
| Male (%) | 509 (62.0) | 515 (62.7) | 0.799 | 0.015 |
| Age (mean (SD)) | 71.12 (10.24) | 71.01 (10.80) | 0.837 | 0.01 |
| Admission type (%) | 0.567 | 0.053 | ||
| ELECTIVE | 171 (20.8) | 179 (21.8) | ||
| EMERGENCY | 628 (76.5) | 626 (76.2) | ||
| URGENT | 22 (2.7) | 16 (1.9) | ||
| Smoking (%) | 0.898 | 0.023 | ||
| Active smoking | 70 (8.5) | 67 (8.2) | ||
| No smoking | 678 (82.6) | 685 (83.4) | ||
| Smoking history | 73 (8.9) | 69 (8.4) | ||
| COPD (%) | 136 (16.6) | 130 (15.8) | 0.738 | 0.02 |
| Hyperlipemia (%) | 435 (53.0) | 421 (51.3) | 0.521 | 0.034 |
| Diabetes (%) | 345 (42.0) | 338 (41.2) | 0.764 | 0.017 |
| Hypertension (%) | 489 (59.6) | 489 (59.6) | 1 | <0.001 |
| Infectious endocarditis (%) | 4 (0.5) | 7 (0.9) | 0.547 | 0.045 |
| Neurological dysfunction (%) | 39 (4.8) | 33 (4.0) | 0.547 | 0.036 |
| Extracardiac arteriopathy (%) | 134 (16.3) | 126 (15.3) | 0.636 | 0.027 |
| Angina (%) | 225 (27.4) | 229 (27.9) | 0.869 | 0.011 |
| Cardiac surgery history (%) | 9 (1.1) | 4 (0.5) | 0.265 | 0.069 |
| Coronary intervention history (%) | 83 (10.1) | 81 (9.9) | 0.934 | 0.008 |
| Pulmonary hypertension (%) | 76 (9.3) | 67 (8.2) | 0.484 | 0.039 |
| Plus other heart surgery (%) | 311 (37.9) | 312 (38.0) | 1 | 0.003 |
| Plus aorta surgery (%) | 23 (2.8) | 27 (3.3) | 0.667 | 0.028 |
| Myocardial infarction (%) | 307 (37.4) | 302 (36.8) | 0.838 | 0.013 |
| Septal perforation (%) | 1 (0.1) | 1 (0.1) | 1 | <0.001 |
| Ejection fraction (%) | 0.492 | 0.059 | ||
| 30− | 132 (16.1) | 115 (14.0) | ||
| 30–50 | 289 (35.2) | 300 (36.5) | ||
| 50+ | 400 (48.7) | 406 (49.5) | ||
| Dialysis status (%) | 4 (0.5) | 6 (0.7) | 0.753 | 0.031 |
| Sepsis (%) | 20 (2.4) | 29 (3.5) | 0.246 | 0.064 |
| Grafts (%) | 0.78 | 0.065 | ||
| 1 graft | 198 (24.1) | 186 (22.7) | ||
| 2 grafts | 258 (31.4) | 274 (33.4) | ||
| 3 grafts | 224 (27.3) | 232 (28.3) | ||
| 4 or more grafts | 86 (10.5) | 75 (9.1) | ||
| Unknown number of grafts | 55 (6.7) | 54 (6.6) | ||
| on-pump CABG (%) | 755 (92.0) | 765 (93.2) | 0.397 | 0.046 |
| Univariable Cox Regression | Multivariable Cox Regression | |||||
|---|---|---|---|---|---|---|
| Covariates | HR | p | CI | HR | p | CI |
| Floxacin | 1.48 | 0.000 | 1.24–1.77 | 1.51 | 0.0000 | 1.26–1.81 |
| Male | 0.88 | 0.176 | 0.74–1.06 | |||
| Age | 1.03 | 0.000 | 1.02–1.04 | 1.03 | 0.0000 | 1.02–1.04 |
| Elective admission | 1.2 | 0.125 | 0.95–1.51 | |||
| Emergency admission | 1.12 | 0.636 | 0.69–1.82 | |||
| COPD | 1.37 | 0.007 | 1.09–1.71 | 1.39 | 0.0047 | 1.11–1.75 |
| Hypertension | 0.73 | 0.000 | 0.61–0.87 | 0.84 | 0.0729 | 0.7–1.02 |
| Neurological dysfunction | 1.32 | 0.143 | 0.91–1.92 | |||
| Extracardiac arteriopathy | 1.16 | 0.186 | 0.93–1.45 | |||
| Angina | 0.66 | 0.000 | 0.53–0.81 | 0.85 | 0.1865 | 0.66–1.08 |
| Cardiac surgery history | 1.11 | 0.851 | 0.36–3.47 | 1.09 | 0.3982 | 0.89–1.34 |
| Coronary intervention history | 0.61 | 0.007 | 0.43–0.88 | 0.68 | 0.0406 | 0.47–0.98 |
| Pulmonary hypertension | 1.27 | 0.115 | 0.94–1.72 | |||
| Plus other heart surgery | 1.28 | 0.007 | 1.07–1.53 | |||
| Myocardial infarction | 1.25 | 0.013 | 1.05–1.5 | 1.12 | 0.3117 | 0.9–1.4 |
| 30% ≤ EF < 50% | 1.02 | 0.902 | 0.8–1.29 | 0.97 | 0.7828 | 0.75–1.24 |
| EF ≥ 50% | 0.7 | 0.005 | 0.55–0.9 | 0.74 | 0.0228 | 0.58–0.96 |
| Septal perforation | 0.9 | 0.918 | 0.13–6.43 | |||
| Infectious endocarditis | 0.78 | 0.725 | 0.19–3.13 | |||
| Plus aorta surgery | 1 | 0.997 | 0.58–1.74 | |||
| Dialysis status | 0.77 | 0.718 | 0.19–3.11 | |||
| Hyperlipemia | 0.64 | 0.000 | 0.53–0.77 | 0.77 | 0.0068 | 0.63–0.93 |
| Active smoking | 1.33 | 0.157 | 0.89–1.99 | |||
| No smoking | 1.06 | 0.834 | 0.63–1.79 | |||
| Diabetes | 1 | 0.983 | 0.84–1.2 | |||
| Sepsis | 1.89 | 0.001 | 1.31–2.73 | 1.68 | 0.0077 | 1.15–2.47 |
| 2 grafts | 1.05 | 0.696 | 0.83–1.32 | |||
| 3 grafts | 0.84 | 0.186 | 0.66–1.09 | |||
| 4 or more grafts | 1.02 | 0.916 | 0.71–1.47 | |||
| Unknown number of grafts | 1.09 | 0.638 | 0.76–1.56 | |||
| Hospital Stay | ICU Stay | Ventilation | Vasopressors Duration | ||
|---|---|---|---|---|---|
| FQ vs. non-FQ (paired t test, p) | <0.001 | <0.001 | <0.001 | <0.001 | |
| Covariates (Covariance Test, p) | Gender (Male) | 0.792 | 0.630 | 0.409 | 0.792 |
| COPD | 0.755 | 0.500 | 0.106 | 0.755 | |
| Neurological dysfunction | 0.130 | 0.016 | <0.001 | 0.130 | |
| Extracardiac arteriopathy | 0.366 | 0.263 | 0.671 | 0.366 | |
| Angina | 0.901 | 0.472 | 0.701 | 0.901 | |
| Cardiac surgery history | 0.394 | 0.339 | 0.044 | 0.394 | |
| Coronary intervention history | 0.718 | 0.796 | 0.468 | 0.718 | |
| Pulmonary hypertension | 0.810 | 0.152 | 0.305 | 0.810 | |
| Plus other heart surgery | 0.135 | 0.050 | 0.046 | 0.135 | |
| Myocardial infarction | 0.727 | 0.667 | 0.881 | 0.727 | |
| Ejection fraction | 0.612 | 0.958 | 0.590 | 0.612 | |
| Admission type | 0.560 | 0.953 | 0.823 | 0.560 | |
| Septal perforation | 0.854 | 0.634 | 0.580 | 0.854 | |
| Infectious endocarditis | 0.796 | 0.009 | 0.712 | 0.796 | |
| Plus aorta surgery | 0.033 | 0.000 | <0.001 | 0.033 | |
| Dialysis status | 0.726 | 0.971 | 0.521 | 0.726 | |
| Age | 0.490 | 0.818 | 0.423 | 0.490 | |
| Hyperlipemia | 0.005 | 0.001 | 0.135 | 0.005 | |
| Smoking | 0.193 | 0.089 | 0.182 | 0.193 | |
| Diabetes | 0.623 | 0.416 | 0.626 | 0.623 | |
| Sepsis | 0.257 | 0.208 | 0.416 | 0.257 | |
| In Hospital Death | Post-Operative Stroke | IABP | |
|---|---|---|---|
| FQ | 0.0055 | 0.0124 | 0.0011 |
| Male | 0.1747 | 0.0066 | 0.0683 |
| Age | 0.0072 | 0.0591 | 0.0022 |
| Elective admission | 0.9407 | 0.9909 | 0.0065 |
| Emergency admission | 0.3858 | 0.7185 | 0.0574 |
| No smoking | 0.9844 | 0.9369 | 0.9913 |
| Smoking history | 0.9855 | 0.8746 | 0.4540 |
| Dialysis status | 0.9954 | 0.3650 | 0.2139 |
| Hyperlipemia | 0.4447 | 0.0288 | 0.3194 |
| Diabetes | 0.8175 | 0.3973 | 0.0381 |
| COPD | 0.8013 | 0.2617 | 0.4999 |
| Neurological dysfunction | 0.1964 | 0.0000 | 0.1151 |
| Extracardiac arteriopathy | 0.0000 | 0.3843 | 0.0077 |
| Angina | 0.7161 | 0.8917 | 0.0797 |
| Myocardium infarction | 0.0066 | 0.2593 | 0.0000 |
| Cardiac surgery history | 0.9955 | 0.9900 | 0.2738 |
| Coronary intervention history | 0.2402 | 0.6902 | 0.9584 |
| Pulmonary hypertension | 0.3829 | 0.4057 | 0.0197 |
| Septal perforation | 0.9984 | 0.9963 | 0.5800 |
| Plus other heart surgery | 0.4736 | 0.1292 | 0.8322 |
| EF 30–50 | 0.2449 | 0.7837 | 0.0000 |
| EF 50+ | 0.0740 | 0.9964 | 0.0000 |
| Sepsis | 0.0000 | 0.0259 | 0.1294 |
| Hypertension | 0.0070 | 0.2375 | 0.0085 |
| on-pump CABG | 0.0426 | 0.3812 | 0.7707 |
| 2 grafts | 0.1016 | 0.4998 | 0.6797 |
| 3 grafts | 0.2520 | 0.1353 | 0.3002 |
| 4 or more grafts | 0.7899 | 0.3423 | 0.9350 |
| Unknown number of grafts | 0.7755 | 0.3209 | 0.2536 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Jian, L.; Min, X.; Li, B.; Cai, X.; Wang, Z.; Hu, Z. Perioperative Fluoroquinolone Treatment Deteriorates Prognosis Following Coronary Artery Bypass Grafting. J. Cardiovasc. Dev. Dis. 2022, 9, 173. https://doi.org/10.3390/jcdd9060173
Zhang M, Jian L, Min X, Li B, Cai X, Wang Z, Hu Z. Perioperative Fluoroquinolone Treatment Deteriorates Prognosis Following Coronary Artery Bypass Grafting. Journal of Cardiovascular Development and Disease. 2022; 9(6):173. https://doi.org/10.3390/jcdd9060173
Chicago/Turabian StyleZhang, Min, Lijuan Jian, Xinping Min, Bowen Li, Xin Cai, Zhiwei Wang, and Zhipeng Hu. 2022. "Perioperative Fluoroquinolone Treatment Deteriorates Prognosis Following Coronary Artery Bypass Grafting" Journal of Cardiovascular Development and Disease 9, no. 6: 173. https://doi.org/10.3390/jcdd9060173
APA StyleZhang, M., Jian, L., Min, X., Li, B., Cai, X., Wang, Z., & Hu, Z. (2022). Perioperative Fluoroquinolone Treatment Deteriorates Prognosis Following Coronary Artery Bypass Grafting. Journal of Cardiovascular Development and Disease, 9(6), 173. https://doi.org/10.3390/jcdd9060173






