Disagreement between Cardiac Troponin Tests Yielding a Higher Incidence of Myocardial Injury in the Emergency Setting
Abstract
1. Introduction
2. Materials and Methods
2.1. Study 1
2.2. Study 2
2.3. Statistical Analyses
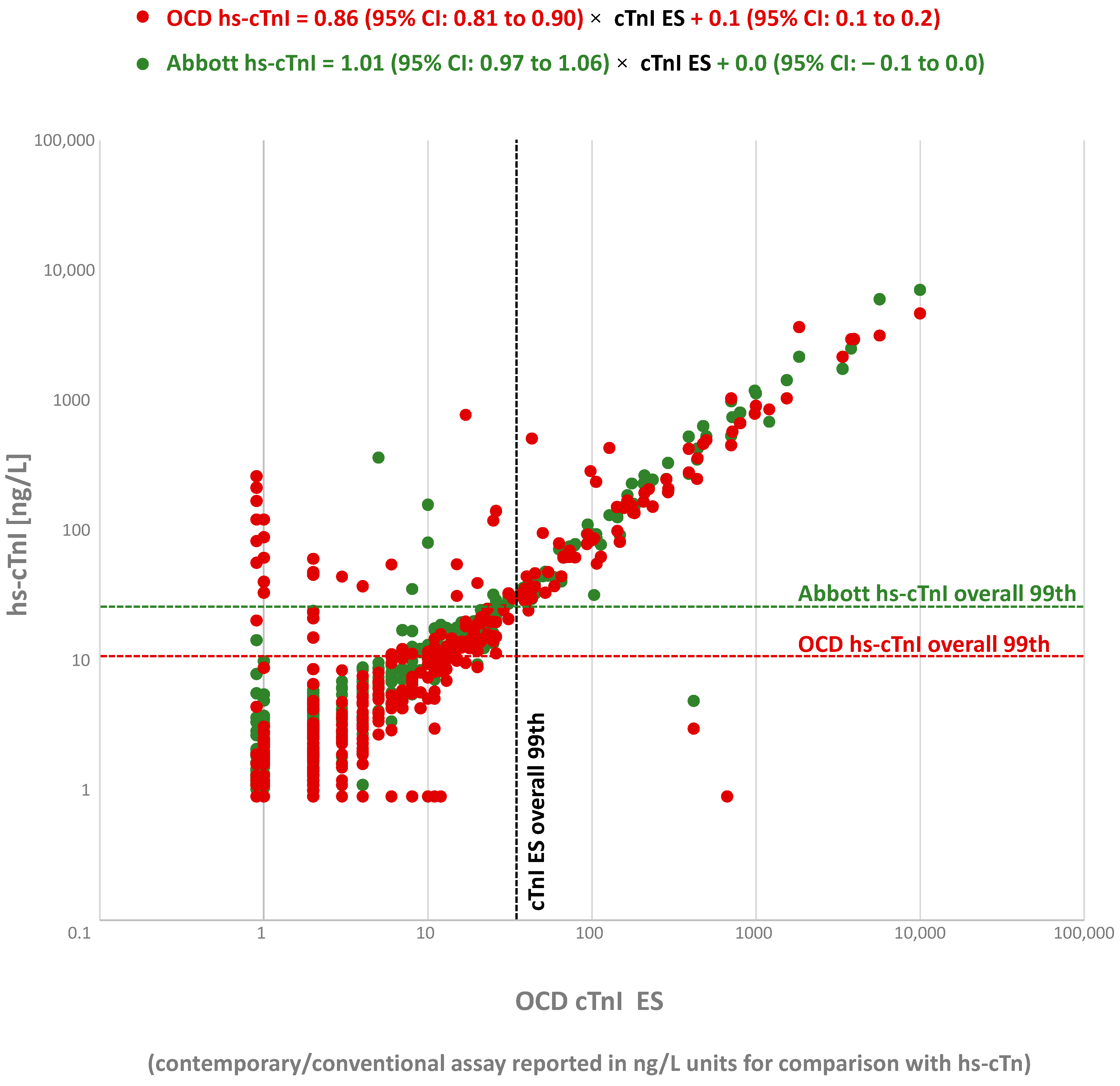
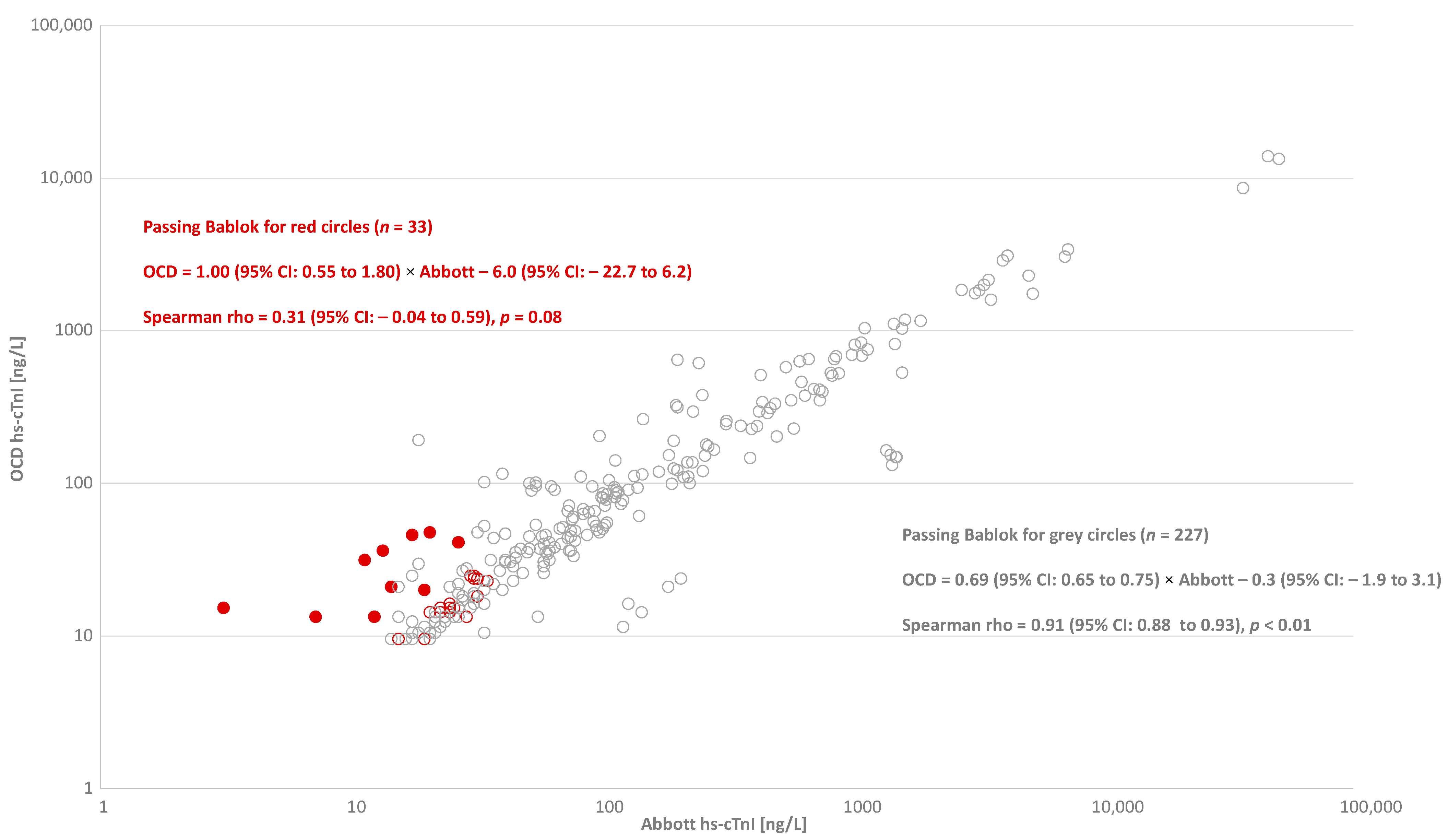
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boeddinghaus, J.; Twerenbold, R.; Nestelberger, T.; Koechlin, L.; Wussler, D.; Meier, M.; Troester, V.; Zimmermann, T.; Badertscher, P.; Wildi, K.; et al. Clinical Use of a New High-Sensitivity Cardiac Troponin I Assay in Patients with Suspected Myocardial Infarction. Clin. Chem. 2019, 65, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Kavsak, P.A.; Mondoux, S.E.; Sherbino, J.; Ma, J.; Clayton, N.; Hill, S.A.; McQueen, M.; Mehta, S.R.; Griffith, L.E.; Devereaux, P.J.; et al. Clinical evaluation of Ortho Clinical Diagnostics high-sensitivity cardiac Troponin I assay in patients with symptoms sug-gestive of acute coronary syndrome. Clin. Biochem. 2020, 80, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Kavsak, P.A.; Mondoux, S.E.; Ma, J.; Sherbino, J.; Hill, S.A.; Clayton, N.; Mehta, S.R.; Griffith, L.E.; McQueen, M.; Devereaux, P.J.; et al. Comparison of two biomarker only algorithms for early risk stratification in patients with suspected acute coronary syn-drome. Int. J. Cardiol. 2020, 319, 140–143. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Cerasuolo, J.O.; Ko, D.T.; Ma, J.; Sherbino, J.; Mondoux, S.E.; Clayton, N.; Hill, S.A.; McQueen, M.; Griffith, L.E.; et al. Using the clinical chemistry score in the emergency department to detect adverse cardiac events: A diagnostic accuracy study. CMAJ Open 2020, 8, E676–E684. [Google Scholar] [CrossRef] [PubMed]
- Kavsak, P.A.; Edge, T.; Roy, C.; Malinowski, P.; Bamford, K.; Clark, L.; Lamers, S.; Hill, S.; Worster, A. Analytical assessment of ortho clinical diagnostics high-sensitivity cardiac troponin I assay. Clin. Chem. Lab. Med. 2020. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint Euro-pean Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocar-dial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar]
- Herman, D.S.; Kavsak, P.A.; Greene, D.N. Variability and Error in Cardiac Troponin Testing: An ACLPS Critical Review. Am. J. Clin. Pathol. 2017, 148, 281–295. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Roy, C.; Malinowski, P.; Mark, C.-T.; Scott, T.; Clark, L.; Lamers, S.; Ainsworth, C. Macrocomplexes and discordant high-sensitivity cardiac troponin concentrations. Ann. Clin. Biochem. Int. J. Lab. Med. 2017, 55, 500–504. [Google Scholar] [CrossRef]
- Kittanakom, S.; Ly, V.; Arnoldo, A.; Beattie, A.; Kavsak, P.A. Pre-analytical variables affecting discordant results on repeat sample testing for cardiac troponin I. Clin. Biochem. 2019, 63, 158–160. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Ainsworth, C.; Worster, A. An Approach to Investigating Discordant High-Sensitivity Cardiac Troponin I Results. Can. J. Cardiol. 2020. [Google Scholar] [CrossRef]
- Favresse, J.; Bayart, J.-L.; Gruson, D.; Bernardini, S.; Clerico, A.; Perrone, M. The underestimated issue of non-reproducible cardiac troponin I and T results: Case series and systematic review of the literature. Clin. Chem. Lab. Med. 2021. [Google Scholar] [CrossRef]
- Kavsak, P.A. Additional approaches for identifying non-reproducible cardiac troponin results. Clin. Chem. Lab. Med. 2021. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Caruso, N.; Mark, C.-T.; Millar, E.; Clark, L. Detection of repeated positive result biases for a high-sensitivity cardiac troponin I assay. Clin. Chim. Acta 2020, 510, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Kavsak, P.A.; Mondoux, S.; Worster, A.; Martin, J.; Tandon, V.; Ainsworth, C.; Devereaux, P. Misclassification of Myocardial Injury by a High-Sensitivity Cardiac Troponin I Assay. Can. J. Cardiol. 2021, 37, 523.e7–523.e8. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Um, K.; Mondoux, S.; Paré, G.; Ainsworth, C.; Worster, A. Important differences between manufacturers when transitioning from a contemporary cardiac troponin assay to a high-sensitivity cardiac troponin assay. CJC Open 2021. [Google Scholar] [CrossRef]
- Kavsak, P.; Clark, L.; Martin, J.; Mark, C.-T.; Paré, G.; Mondoux, S.; Chetty, V.; Ainsworth, C.; Worster, A. Acute Phase Response and Non-Reproducible Elevated Concentrations with a High-Sensitivity Cardiac Troponin I Assay. J. Clin. Med. 2021, 10, 1014. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Clark, L. Commercial Quality Control Imprecision Estimates for High-Sensitivity Cardiac Troponin Deltas Used to Rule-in Myocardial Infarction with the ESC 0/1-Hour Algorithm. J. Appl. Lab. Med. 2020, 5, 1122–1124. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Beattie, J.; Pickersgill, R.; Ford, L.; Caruso, N.; Clark, L. A practical approach for the validation and clinical imple-mentation of a high-sensitivity cardiac troponin I assay across a North American city. Pract. Lab. Med. 2015, 1, 28–34. [Google Scholar] [CrossRef]
- Wu, A.H.B.; Christenson, R.H.; Greene, D.N.; Jaffe, A.S.; Kavsak, P.A.; Ordonez-Llanos, J.; Apple, F.S. Clinical Laboratory Practice Rec-ommendations for the Use of Cardiac Troponin in Acute Coronary Syndrome: Expert Opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the In-ternational Federation of Clinical Chemistry and Laboratory Medicine. Clin. Chem. 2018, 64, 645–655. [Google Scholar]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020. [Google Scholar] [CrossRef]
- Apple, F.S.; Collinson, P.O.; Kavsak, P.A.; Body, R.; Ordóñez-Llanos, J.; Saenger, A.K.; Omland, T.; Hammarsten, O.; Jaffe, A.S.; IFCC Committee on Clinical Applications of Cardiac Bio-Markers Getting Cardiac Troponin Right. Appraisal of the 2020 European Society of Cardiology Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation by the International Federation of Clinical Chemistry and Laboratory Medicine Committee on Clinical Applications of Cardiac Bio-Markers. Clin. Chem. 2020. [Google Scholar] [CrossRef]
- Collinson, P.O.; Saenger, A.K.; Apple, F.S. High sensitivity, contemporary and point-of-care cardiac troponin assays: Educational aids developed by the IFCC Committee on Clinical Application of Cardiac Bio-Markers. Clin. Chem. Lab. Med. 2019, 57, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Wu, A.H.B.; Sandoval, Y.; Sexter, A.; Love, S.A.; Myers, G.; Schulz, K.; Duh, S.-H.; Christenson, R.H. Sex-Specific 99th Percentile Upper Reference Limits for High Sensitivity Cardiac Troponin Assays Derived Using a Universal Sample Bank. Clin. Chem. 2020, 66, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.V.; Marshall, G.A. High incidence of macrotroponin I with a high-sensitivity troponin I assay. Clin. Chem. Lab. Med. 2016, 54, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.; Aspin, L.; Heron, R.C.; Ha, L.; Kyle, C. Discrepancy between Cardiac Troponin Assays Due to Endogenous Antibodies. Clin. Chem. 2020, 66, 445–454. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Tandon, V.; Ainsworth, C. A Three-Site Immunoassay for High-Sensitivity Cardiac Troponin I with Low Immu-noreactivity for Macrocomplexes. Clin. Chem. 2020, 66, 854–855. [Google Scholar] [CrossRef]
- Clerico, A.; Zaninotto, M.; Ripoli, A.; Masotti, S.; Prontera, C.; Passino, C.; Plebani, M.; Study Group on Cardio-vascular Risk Biomarkers of the Italian Society of Clinical Biochemistry (SIBioC). The 99th percentile of reference population for cTnI and cTnT assay: Methodology, pathophysiology and clinical implications. Clin. Chem. Lab. Med. 2017, 55, 1634–1651. [Google Scholar] [CrossRef]

| OCD Poor Reproducibility Results | ||||||
|---|---|---|---|---|---|---|
| OCD hs-cTnI 1st | OCD hs-TnI 2nd | Average | Difference between OCD Results | OCD TnI-ES | Abbott hs-cTnI 1st | Abbott hs-cTnI 2nd |
| 8.1 | 11.7 | 9.9 | 3.6 | 5 | 6.1 | 5.5 |
| 8.6 | 14.6 | 11.6 | 6 | 2 | 1.7 | 2.8 |
| 4.4 | 10.7 | 7.6 | 6.3 | 0.9 | 0.9 | 0.9 |
| 8.8 | 2.1 | 5.5 | 6.7 | 1 | 1.8 | 1.3 |
| 47.0 | 58.3 | 52.7 | 21% | 45 | 32.6 | 31.6 |
| 168.1 | 210.9 | 189.5 | 23% | 0.9 | 0.9 | 0.9 |
| 83.3 | 62.6 | 73.0 | 28% | 0.9 | 0.9 | 0.9 |
| 33.2 | 24.4 | 28.8 | 31% | 1 | 3.2 | 3.3 |
| 39.4 | 55.2 | 47.3 | 33% | 20 | 18.3 | 17.8 |
| 55.1 | 79.1 | 67.1 | 36% | 15 | 15.1 | 16.3 |
| 286.9 | 195.8 | 241.4 | 38% | 98 | 83.6 | 84.4 |
| 121.3 | 81.7 | 101.5 | 39% | 0.9 | 2.0 | 2.3 |
| 141.8 | 213.2 | 177.5 | 40% | 26 | 29.2 | 28.6 |
| 20.3 | 11.1 | 15.7 | 59% | 0.9 | 0.9 | 0.9 |
| 44.2 | 24.0 | 34.1 | 59% | 3 | 3.5 | 3.9 |
| 89.0 | 45.7 | 67.4 | 64% | 1 | 1.7 | 2.1 |
| 54.9 | 26.7 | 40.8 | 69% | 6 | 7.0 | 8.2 |
| 48.2 | 22.2 | 35.2 | 74% | 2 | 6.5 | 6.1 |
| 37.3 | 85.7 | 61.5 | 79% | 4 | 6.0 | 6.4 |
| 21.2 | 8.5 | 14.9 | 86% | 2 | 1.9 | 2.1 |
| Abbott Poor Reproducible Results | ||||||
| Abbott hs-cTnI 1st | Abbott hs-cTnI 2nd | Average | Difference between Abbott Results | OCD TnI-ES | OCD hs-cTnI 1st | OCD hs-TnI 2nd |
| 2514.3 | 3115.7 | 2815.0 | 21% | 3790 | 2958.0 | 2864.0 |
| 2938.5 | 3704.9 | 3321.7 | 23% | 3950 | 2975.0 | 2924.0 |
| 31.9 | 41.5 | 36.7 | 26% | 103 | 87.2 | 94.6 |
| 687.6 | 897.6 | 792.6 | 26% | 1200 | 854.1 | 830.0 |
| 273.4 | 398.6 | 336.0 | 37% | 390 | 279.5 | 271.8 |
| 206.3 | 302.6 | 254.4 | 38% | 292 | 209.3 | 225.7 |
| 40.6 | 62.8 | 51.7 | 43% | 65 | 44.5 | 44.5 |
| 12.1 | 18.8 | 15.5 | 43% | 20 | 8.9 | 8.0 |
| 15.4 | 25.1 | 20.2 | 48% | 19 | 16.5 | 16.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavsak, P.A.; Mondoux, S.E.; Martin, J.; Hewitt, M.K.; Clark, L.; Caruso, N.; Mark, C.-T.; Chetty, V.T.; Ainsworth, C.; Worster, A. Disagreement between Cardiac Troponin Tests Yielding a Higher Incidence of Myocardial Injury in the Emergency Setting. J. Cardiovasc. Dev. Dis. 2021, 8, 31. https://doi.org/10.3390/jcdd8030031
Kavsak PA, Mondoux SE, Martin J, Hewitt MK, Clark L, Caruso N, Mark C-T, Chetty VT, Ainsworth C, Worster A. Disagreement between Cardiac Troponin Tests Yielding a Higher Incidence of Myocardial Injury in the Emergency Setting. Journal of Cardiovascular Development and Disease. 2021; 8(3):31. https://doi.org/10.3390/jcdd8030031
Chicago/Turabian StyleKavsak, Peter A., Shawn E. Mondoux, Janet Martin, Mark K. Hewitt, Lorna Clark, Nadia Caruso, Ching-Tong Mark, V. Tony Chetty, Craig Ainsworth, and Andrew Worster. 2021. "Disagreement between Cardiac Troponin Tests Yielding a Higher Incidence of Myocardial Injury in the Emergency Setting" Journal of Cardiovascular Development and Disease 8, no. 3: 31. https://doi.org/10.3390/jcdd8030031
APA StyleKavsak, P. A., Mondoux, S. E., Martin, J., Hewitt, M. K., Clark, L., Caruso, N., Mark, C.-T., Chetty, V. T., Ainsworth, C., & Worster, A. (2021). Disagreement between Cardiac Troponin Tests Yielding a Higher Incidence of Myocardial Injury in the Emergency Setting. Journal of Cardiovascular Development and Disease, 8(3), 31. https://doi.org/10.3390/jcdd8030031






