Lipoprotein(a), Immunity, and Inflammation in Polyvascular Atherosclerotic Disease
Abstract
1. Introduction
2. Material and Methods
3. Results
3.1. General Characteristics of Patients
3.2. The Association of Lp(a) and Other Markers with Atherosclerosis
3.3. Lp(a), IgM Autoantibodies, and Cardiovascular Diseases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steg, P.G.; Bhatt, D.L.; Wilson, P.W.F.; D’Agostino, R.; Ohman, E.M.; Röther, J.; Liau, C.-S.; Hirsch, A.T.; Mas, J.-L.; Ikeda, Y.; et al. One-Year Cardiovascular Event Rates in Outpatients With Atherothrombosis. JAMA 2007, 297, 1197–1206. [Google Scholar] [CrossRef]
- Alberts, M.J.; Bhatt, D.L.; Mas, J.-L.; Ohman, E.M.; Hirsch, A.T.; Röther, J.; Salette, G.; Goto, S.; Smith, S.C.; Liau, C.-S.; et al. Three-year follow-up and event rates in the international REduction of Atherothrombosis for Continued Health Registry. Eur. Heart J. 2009, 30, 2318–2326. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Eagle, K.A.; Ohman, E.M.; Hirsch, A.T.; Goto, S.; Mahoney, E.M.; Wilson, P.W.F.; Alberts, M.J.; D’Agostino, R.; Liau, C.-S.; et al. Comparative Determinants of 4-Year Cardiovascular Event Rates in Stable Outpatients at Risk of or With Atherothrombosis. JAMA 2010, 304, 1350–1357. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Flowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e726–e779. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration. Lipoprotein(a) Concentration and the Risk of Coronary Heart Disease, Stroke, and Nonvascular Mortality. JAMA 2009, 302, 412–423. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Extreme Lipoprotein(a) Levels and Improved Cardiovascular Risk Prediction. J. Am. Coll. Cardiol. 2013, 61, 1146–1156. [Google Scholar] [CrossRef]
- Dahlen, G.H. Incidence of Lp(a) among populations. In Lipoprotein; Scanu, A.M., Ed.; Academic Press: New York, NY, USA, 1990; pp. 151–173. [Google Scholar]
- Afanasieva, O.I.; Ezhov, M.V.; Razova, O.A.; Afanasieva, M.I.; Utkina, E.A.; Pokrovsky, S.N. Apolipoprotein(a) phenotype determines the correlations of lipoprotein(a) and proprotein convertase subtilisin/kexin type 9 levels in patients with potential familial hypercholesterolemia. Atherosclerosis 2018, 277, 477–482. [Google Scholar] [CrossRef]
- Afanasieva, O.I.; Klesareva, E.K.; Levashov, P.A.; Berestetskaya, Y.B.; Ezhov, M.V.; Artemieva, N.A.; Pokrovsky, S. Autoantibodies Against Lipoprotein(a) in Patients with Coronary Heart Disease. Kardiologiia 2014, 54, 4–8. [Google Scholar] [CrossRef]
- Cho, K.I.; Cho, S.H.; Her, A.-Y.; Singh, G.B.; Shin, E.-S. Prognostic Utility of Neutrophil-to-Lymphocyte Ratio on Adverse Clinical Outcomes in Patients with Severe Calcific Aortic Stenosis. PLoS ONE 2016, 11, e0161530. [Google Scholar] [CrossRef]
- Gutierrez, J.A.; Mulder, H.; Jones, W.S.; Rockhold, F.W.; Baumgartner, I.; Berger, J.S.; Blomster, J.I.; Fowkes, F.G.R.; Held, P.; Katona, B.G.; et al. Polyvascular Disease and Risk of Major Adverse Cardiovascular Events in Peripheral Artery Disease. JAMA Netw. Open 2018, 1, e185239. [Google Scholar] [CrossRef]
- Varvel, S.; McConnell, J.P.; Tsimikas, S. Prevalence of Elevated Lp(a) Mass Levels and Patient Thresholds in 532 359 Patients in the United States. Arter. Thromb. Vasc. Biol. 2016, 36, 2239–2245. [Google Scholar] [CrossRef]
- Laschkolnig, A.; Kollerits, B.; Lamina, C.; Meisinger, C.; Rantner, B.; Stadler, M.; Peters, A.; Koenig, W.; Stöckl, A.; Dähnhardt, D.; et al. Lipoprotein (a) concentrations, apolipoprotein (a) phenotypes, and peripheral arterial disease in three independent cohorts. Cardiovasc. Res. 2014, 103, 28–36. [Google Scholar] [CrossRef]
- Bérard, A.M.; Bedel, A.; Le Trequesser, R.; Freyburger, G.; Nurden, A.; Colomer, S.; Guérin, V.; Vergnes, M.-C.; Becker, F.; Camelot, G.; et al. Novel Risk Factors for Premature Peripheral Arterial Occlusive Disease in Non-Diabetic Patients: A Case-Control Study. PLoS ONE 2013, 8, e37882. [Google Scholar] [CrossRef]
- Gurdasani, D.; Sjouke, B.; Tsimikas, S.; Hovingh, G.K.; Luben, R.N.; Wainwright, N.W.J.; Pomilla, C.; Wareham, N.J.; Khaw, K.-T.; Boekholdt, S.M.; et al. Lipoprotein(a) and Risk of Coronary, Cerebrovascular, and Peripheral Artery Disease. Arter. Thromb. Vasc. Biol. 2012, 32, 3058–3065. [Google Scholar] [CrossRef]
- Dieplinger, B.; Lingenhel, A.; Baumgartner, N.; Poelz, W.; Dieplinger, H.; Haltmayer, M.; Kronenberg, F.; Mueller, T. Increased Serum Lipoprotein(a) Concentrations and Low Molecular Weight Phenotypes of Apolipoprotein(a) Are Associated with Symptomatic Peripheral Arterial Disease. Clin. Chem. 2007, 53, 1298–1305. [Google Scholar] [CrossRef]
- Klarin, D.; Program, V.M.V.; Lynch, J.A.; Aragam, K.; Chaffin, M.; Assimes, T.L.; Huang, J.; Lee, K.M.; Shao, Q.; Huffman, J.E.; et al. Genome-wide association study of peripheral artery disease in the Million Veteran Program. Nat. Med. 2019, 25, 1274–1279. [Google Scholar] [CrossRef]
- Ooi, E.M.; Ellis, K.L.; Barrett, P.H.R.; Watts, G.F.; Hung, J.; Beilby, J.P.; Thompson, P.L.; Stobie, P.; McQuillan, B. Lipoprotein(a) and apolipoprotein(a) isoform size: Associations with angiographic extent and severity of coronary artery disease, and carotid artery plaque. Atherosclerosis 2018, 275, 232–238. [Google Scholar] [CrossRef]
- Bos, S.; Duvekot, M.H.; Touw-Blommesteijn, A.C.; Verhoeven, A.J.M.; Mulder, M.T.; Watts, G.F.; Sijbrands, E.J.G.; Van Lennep, J.E.R. Lipoprotein (a) levels are not associated with carotid plaques and carotid intima media thickness in statin-treated patients with familial hypercholesterolemia. Atherosclerosis 2015, 242, 226–229. [Google Scholar] [CrossRef]
- Kim, B.; Jung, H.; Bang, O.; Chung, C.-S.; Lee, K.; Kim, G. Elevated serum lipoprotein(a) as a potential predictor for combined intracranial and extracranial artery stenosis in patients with ischemic stroke. Atherosclerosis 2010, 212, 682–688. [Google Scholar] [CrossRef]
- Kim, S.J.; Song, P.; Park, J.H.; Lee, Y.T.; Kim, W.S.; Park, Y.G.; Bang, O.Y.; Chung, C.-S.; Lee, K.H.; Kim, G.-M. Biomarkers of Asymptomatic Carotid Stenosis in Patients Undergoing Coronary Artery Bypass Grafting. Stroke 2011, 42, 734–739. [Google Scholar] [CrossRef]
- Hippe, D.S.; Phan, B.A.P.; Sun, J.; Isquith, D.A.; O’Brien, K.D.; Crouse, J.R.; Anderson, T.; Huston, J.; Marcovina, S.M.; Hatsukami, T.S.; et al. Lp(a) (Lipoprotein(a)) Levels Predict Progression of Carotid Atherosclerosis in Subjects with Atherosclerotic Cardiovascular Disease on Intensive Lipid Therapy. Arter. Thromb. Vasc. Biol. 2018, 38, 673–678. [Google Scholar] [CrossRef]
- Nasr, N.; Ruidavets, J.B.; Farghali, A.; Guidolin, B.; Perret, B.; Larrue, V. Lipoprotein (a) and Carotid Atherosclerosis in Young Patients with Stroke. Stroke 2011, 42, 3616–3618. [Google Scholar] [CrossRef][Green Version]
- Libby, P.; Loscalzo, J.; Ridker, P.M.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, Immunity, and Infection in Atherothrombosis. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [Google Scholar] [CrossRef]
- Iseme, R.A.; McEvoy, M.; Kelly, B.; Agnew, L.; Walker, F.R.; Handley, T.; Oldmeadow, C.; Attia, J.; Boyle, M. A role for autoantibodies in atherogenesis. Cardiovasc. Res. 2017, 113, 1102–1112. [Google Scholar] [CrossRef]
- Tsiantoulas, D.; Perkmann, T.; Afonyushkin, T.; Mangold, A.; Prohaska, T.A.; Papac-Milicevic, N.; Millischer, V.; Bartel, C.; Hörkkö, S.; Boulanger, C.M.; et al. Circulating microparticles carry oxidation-specific epitopes and are recognized by natural IgM antibodies. J. Lipid Res. 2015, 56, 440–448. [Google Scholar] [CrossRef]
- Rahman, M.; Sing, S.; Golabkesh, Z.; Fiskesund, R.; Gustafsson, T.; Jogestrand, T.; Frostegård, A.G.; Ingiäld Ingiald Hafstrom for the BARFOT Study Group; Liu, A.; Frostegård, J. IgM antibodies against malondialdehyde and phosphorylcholine are together strong protection markers for atherosclerosis in systemic lupus erythematosus: Regulation and underlying mechanisms. Clin. Immunol. 2016, 166, 27–37. [Google Scholar] [CrossRef]
- Afanasieva, O.I.; Pylaeva, E.A.; Klesareva, E.A.; Potekhina, A.V.; Provatorov, S.I.; Krasnikova, T.L.; Masenko, V.P.; Arefieva, T.I.; Pokrovsky, S. Lipoprotein(a), its autoantibodies, and circulating T lymphocyte subpopulations as independent risk factors for coronary artery atherosclerosis. Ther. Arch. 2016, 88, 31–38. [Google Scholar] [CrossRef]
- Kyaw, T.; Tipping, P.; Bobik, A.; Toh, B.-H. Protective Role of Natural IgM-Producing B1a Cells in Atherosclerosis. Trends Cardiovasc. Med. 2012, 22, 48–53. [Google Scholar] [CrossRef]
- Tsimikas, S.; Willeit, P.; Willeit, J.; Santer, P.; Mayr, M.; Xu, Q.; Mayr, A.; Witztum, J.L.; Kiechl, S. Oxidation-Specific Biomarkers, Prospective 15-Year Cardiovascular and Stroke Outcomes, and Net Reclassification of Cardiovascular Events. J. Am. Coll. Cardiol. 2012, 60, 2218–2229. [Google Scholar] [CrossRef]
- Prasad, A.; Clopton, P.; Ayers, C.; Khera, A.; De Lemos, J.A.; Witztum, J.L.; Tsimikas, S. Relationship of Autoantibodies to MDA-LDL and ApoB-Immune Complexes to Sex, Ethnicity, Subclinical Atherosclerosis, and Cardiovascular Events. Arter. Thromb. Vasc. Biol. 2017, 37, 1213–1221. [Google Scholar] [CrossRef]
- Ravandi, A.; Boekholdt, S.M.; Mallat, Z.; Talmud, P.J.; Kastelein, J.J.P.; Wareham, N.J.; Miller, E.R.; Benessiano, J.; Tedgui, A.; Witztum, J.L.; et al. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: Results from the EPIC-Norfolk Study. J. Lipid Res. 2011, 52, 1829–1836. [Google Scholar] [CrossRef]
- Tsimikas, S. A Test in Context: Lipoprotein(a). J. Am. Coll. Cardiol. 2017, 69, 692–711. [Google Scholar] [CrossRef]
- Ali, L.; Schnitzler, J.G.; Kroon, J. Metabolism. Curr. Opin. Lipidol. 2018, 29, 474–480. [Google Scholar] [CrossRef]
- Kose, N.; Akin, F.; Yildirim, T.; Ergun, G.; Altun, I. The association between the lymphocyte-to-monocyte ratio and coronary artery disease severity in patients with stable coronary artery disease. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2570–2575. [Google Scholar]
- Bressi, E.; Mangiacapra, F.; Ricottini, E.; Cavallari, I.; Colaiori, I.; Di Gioia, G.; Creta, A.; Capuano, M.; Viscusi, M.M.; Di Sciascio, G. Impact of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio on 5-Year Clinical Outcomes of Patients with Stable Coronary Artery Disease Undergoing Elective Percutaneous Coronary Intervention. J. Cardiovasc. Transl. Res. 2018, 11, 517–523. [Google Scholar] [CrossRef]
- Nam, K.-W.; Kwon, H.-M.; Jeong, H.-Y.; Park, J.-H.; Kim, S.H.; Jeong, S.-M. High neutrophil to lymphocyte ratios predict intracranial atherosclerosis in a healthy population. Atherosclerosis 2018, 269, 117–121. [Google Scholar] [CrossRef]
- Ridker, P.M. A Test in Context. J. Am. Coll. Cardiol. 2016, 67, 712–723. [Google Scholar] [CrossRef]
- Moriya, J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 2019, 73, 22–27. [Google Scholar] [CrossRef]
- Suciu, C.F.; Prete, M.; Caporali, R.; Favoino, E.; Giacomelli, R.; Perosa, F. Oxidized low density lipoproteins: The bridge between atherosclerosis and autoimmunity. Possible implications in accelerated atherosclerosis and for immune intervention in autoimmune rheumatic disorders. Autoimmun. Rev. 2018, 17, 366–375. [Google Scholar] [CrossRef]
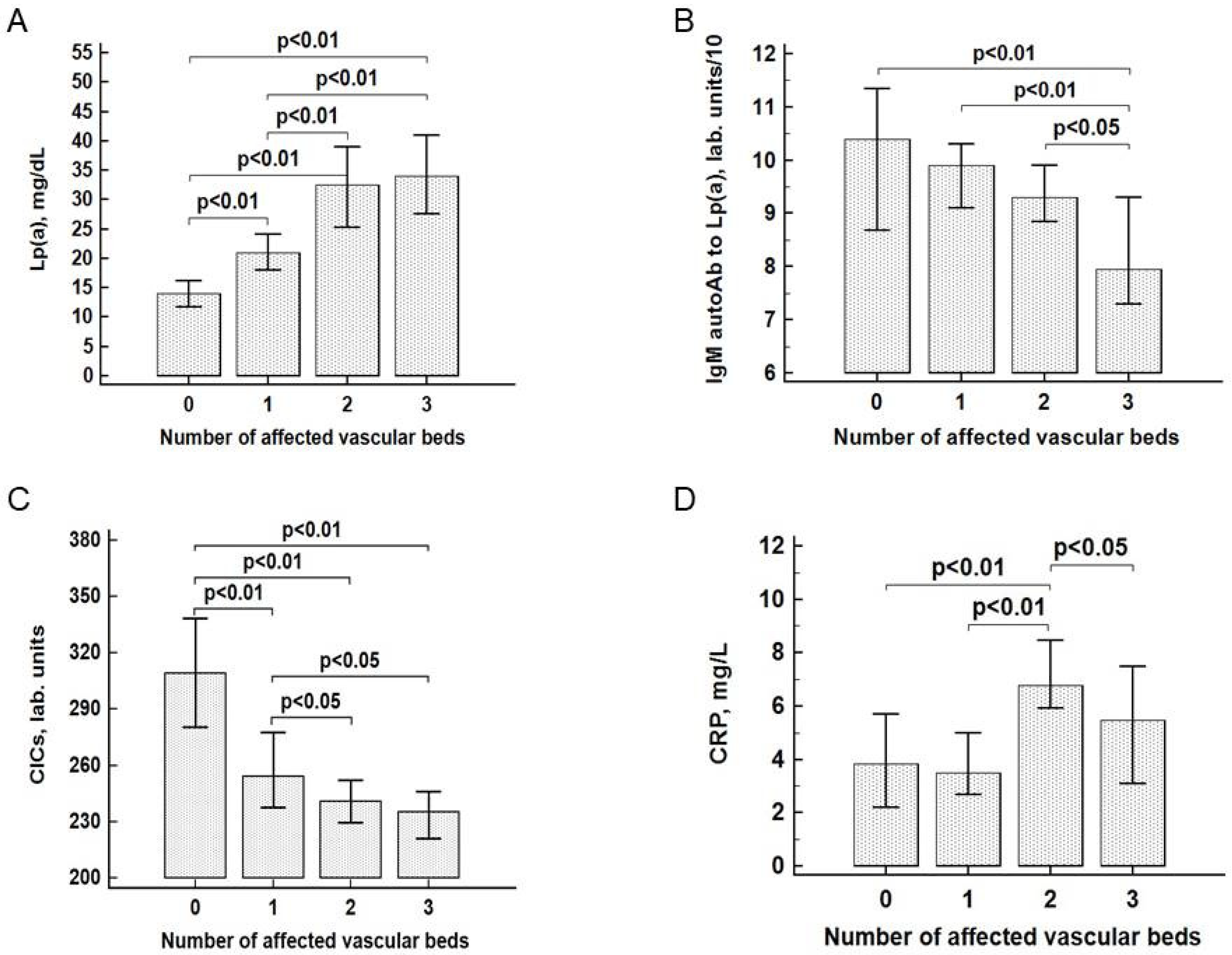
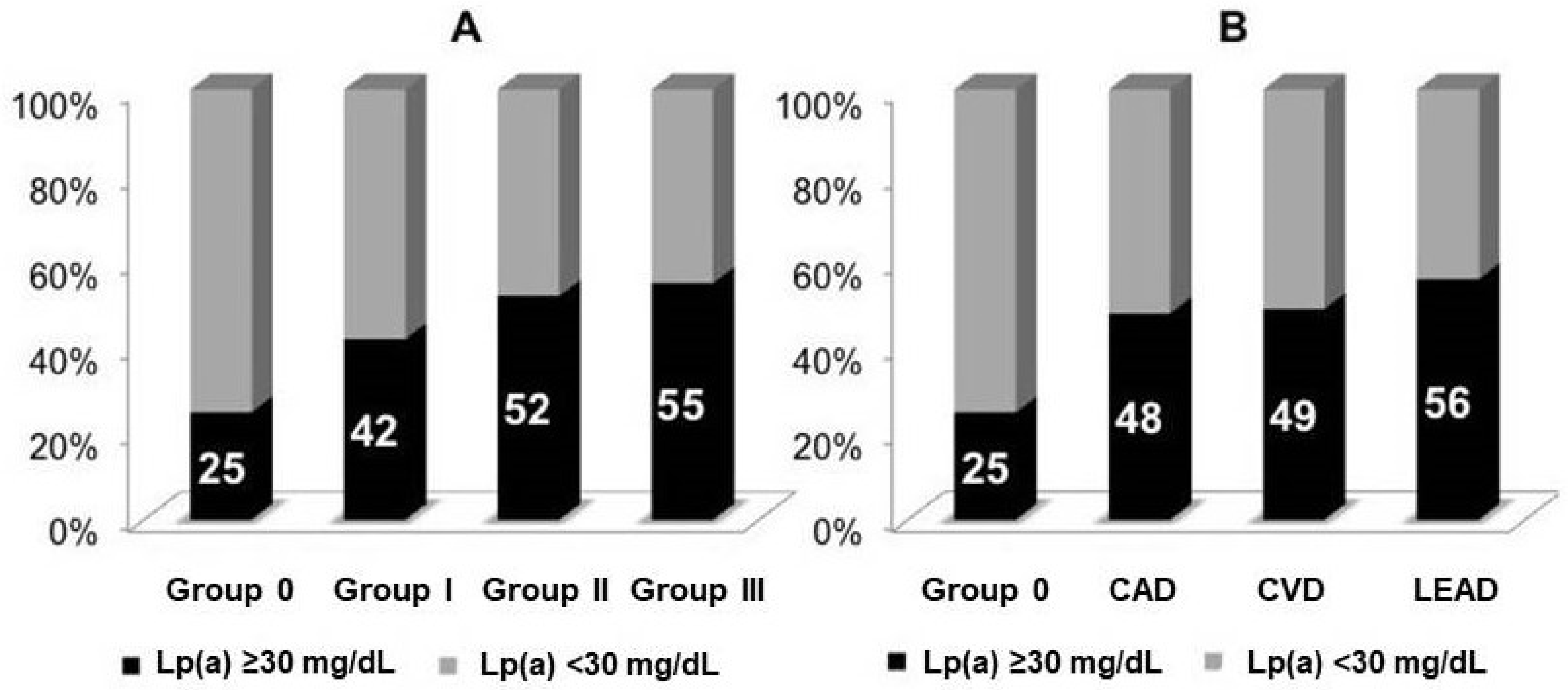

| Parameters | Group 0 n = 339 | Group I n = 470 | Group II n = 315 | Group III n = 164 |
|---|---|---|---|---|
| Age, years | 57 (48; 64) | 59 (51; 65) * | 66 (59; 74) ** | 69 (64; 76) ** |
| Male sex | 151 (44%) | 370 (78%) ** | 236 (74%) ** | 138 (84%) ** |
| Hypertension | 195 (57%) | 339 (72%) ** | 273 (86%) ** | 148 (90%) ** |
| Smoking | 94 (27%) | 251 (53%) ** | 164 (52%) ** | 101 (61%) ** |
| Type 2 diabetes | 49 (14%) | 74 (15%) | 99 (31%) ** | 61 (37%) ** |
| Family history | 80 (24%) | 126 (27%) | 80 (25%) | 45 (27%) |
| TC, mmol/L | 6.0 (5.1; 6.9) | 5.6 (4.4; 6.8) ** | 4.6 (3.7; 6.0) ** | 4.4 (3.9; 5.2) ** |
| TG, mmol/L | 1.8 (1.3; 2.4) | 1.7 (1.2; 2.4) | 1.5 (1.1; 2.1) ** | 1.4 (1.1; 2.0) ** |
| HDL-C, mmol/L | 1.2 (1.1; 1.5) | 1.1 (1.0; 1.3) ** | 1.1 (0.9; 1.3) ** | 1.1 (1.0; 1.3) ** |
| LDL-C, mmol/L | 3.9 (3.0; 4.7) | 3.4 (2.4; 4.5) ** | 2.7 (2.0; 3.7) ** | 2.5 (2.1; 3.1) ** |
| LDL-Ccorrected, mmol/L | 3.7 (2.8; 4.4) | 3.1 (2.1; 4.2) ** | 2.2 (1.6; 3.4) ** | 2.2 (1.7; 2.8) ** |
| Statin therapy | 79 (23%) | 381 (81%) ** | 286 (91%) ** | 154 (94%) ** |
| CRP, mg/L | 3.9 (1.2; 8.9) | 3.5 (1.7; 7.7) | 6.8 (4.0; 15.5) ** | 5.5 (2.3; 9.3) |
| CICs, lab units | 309 (250; 372) | 254 (216; 310) ** | 241 (204; 279) ** | 235 (192; 264) ** |
| Neutrophil-to-lymphocyte ratio | 1.4 (1.1; 2.1) | 1.7 (1.4; 2.3) ** | 1.9 (1.5; 2.4) ** | 1.8 (1.5; 2.6) ** |
| Platelet-to-lymphocyte ratio | 99.0 (83.6; 123.5) | 102.3 (77.7; 124.6) | 102.4 (78.9; 125.4) | 102.9 (80.6; 123.3) |
| Lymphocyte-to-monocyte ratio | 4.3 (3.7; 5.7) | 3.9 (3.0; 5.0) * | 3.6 (2.9; 4.6) ** | 3.8 (3.0; 4.9) ** |
| Absolute monocytes, ×109/L | 0.49 (0.43; 0.65) | 0.55 (0.46; 0.68) * | 0.60 (0.49; 0.71) ** | 0.56 (0.46; 0.70)* |
| Absolute neutrophils, ×109/L | 3.2 (2.5; 4.2) | 3.8 (3.1; 4.8) ** | 4.3 (3.4; 5.2) ** | 4.1 (3.2; 5.0) ** |
| Absolute lymphocytes, ×109/L | 2.2 (1.8; 2.7) | 2.2 (1.7; 2.6) | 2.2 (1.8; 2.7) | 2.2 (1.7; 2.7) |
| WBC, ×109/L | 6.4 (5.2; 7.6) | 6.7 (5.8; 8.0) * | 7.3 (6.2; 8.8) ** | 6.9 (6.0; 8.4) ** |
| Parameters | Group 0 n = 339 | Group I n = 470 | Group II n = 315 | Group III n = 164 |
|---|---|---|---|---|
| Age ≥ 64 years | 1 | 1.6 (1.2–2.4) * | 4.1 (2.7–6.2) * | 8.7 (4.9–15.6) * |
| Male sex | 1 | 4.6 (3.2–6.6) * | 4.8 (3.1–7.6) * | 10.2 (5.0–20.8) * |
| Hypertension | 1 | 2.2 (1.5–3.1) * | 4.1 (2.6–6.5) * | 6.8 (3.3–14.0) * |
| Type 2 diabetes | 1 | 1.1 (0.7–1.8) | 2.8 (1.7–4.5) * | 3.3 (1.8–6.2) * |
| Smoking | 1 | 1.9 (1.4–2.7) * | 2.3 (1.5–3.5) * | 3.1 (1.7–5.6) * |
| Lp(a) ≥ 30 mg/dL | 1 | 2.3 (1.7–3.3) * | 3.5 (2.3–5.2) * | 6.1 (3.4–10.9) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tmoyan, N.A.; Afanasieva, O.I.; Ezhov, M.V.; Klesareva, E.A.; Balakhonova, T.V.; Pokrovsky, S.N. Lipoprotein(a), Immunity, and Inflammation in Polyvascular Atherosclerotic Disease. J. Cardiovasc. Dev. Dis. 2021, 8, 11. https://doi.org/10.3390/jcdd8020011
Tmoyan NA, Afanasieva OI, Ezhov MV, Klesareva EA, Balakhonova TV, Pokrovsky SN. Lipoprotein(a), Immunity, and Inflammation in Polyvascular Atherosclerotic Disease. Journal of Cardiovascular Development and Disease. 2021; 8(2):11. https://doi.org/10.3390/jcdd8020011
Chicago/Turabian StyleTmoyan, Narek A., Olga I. Afanasieva, Marat V. Ezhov, Elena A. Klesareva, Tatiana V. Balakhonova, and Sergei N. Pokrovsky. 2021. "Lipoprotein(a), Immunity, and Inflammation in Polyvascular Atherosclerotic Disease" Journal of Cardiovascular Development and Disease 8, no. 2: 11. https://doi.org/10.3390/jcdd8020011
APA StyleTmoyan, N. A., Afanasieva, O. I., Ezhov, M. V., Klesareva, E. A., Balakhonova, T. V., & Pokrovsky, S. N. (2021). Lipoprotein(a), Immunity, and Inflammation in Polyvascular Atherosclerotic Disease. Journal of Cardiovascular Development and Disease, 8(2), 11. https://doi.org/10.3390/jcdd8020011







