Development and Validation of a Nomogram for Predicting the Risk of Adverse Cardiovascular Events in Patients with Coronary Artery Ectasia
Abstract
1. Introduction
2. Materials and Methods
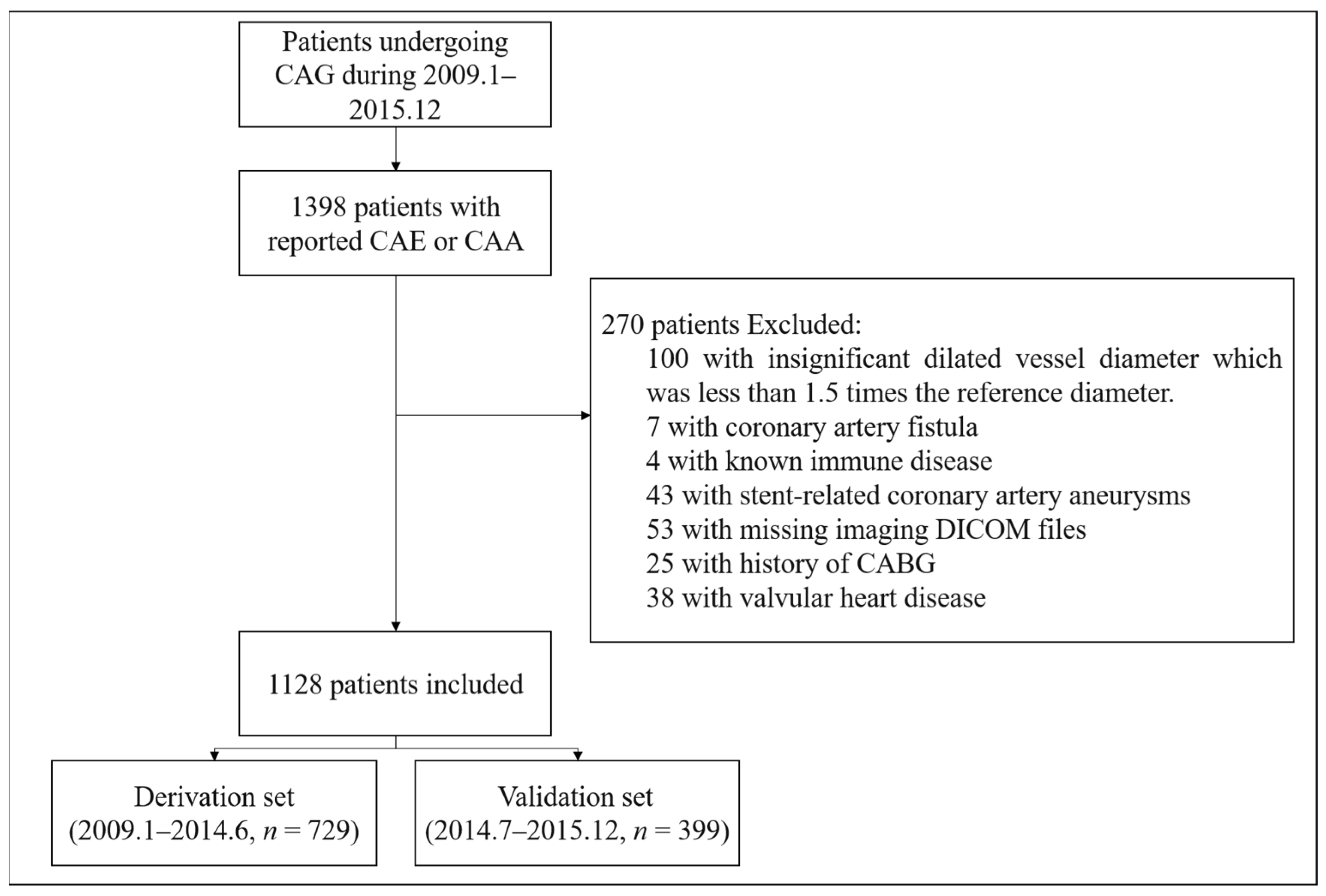
2.1. Study Population
2.2. Clinical Data Collection
2.3. Angiographic Evaluation and Quantitative Coronary Angiography (QCA)
2.4. Outcomes and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Clinical Features and Characteristics
3.2. Coronary Angiography Evaluation
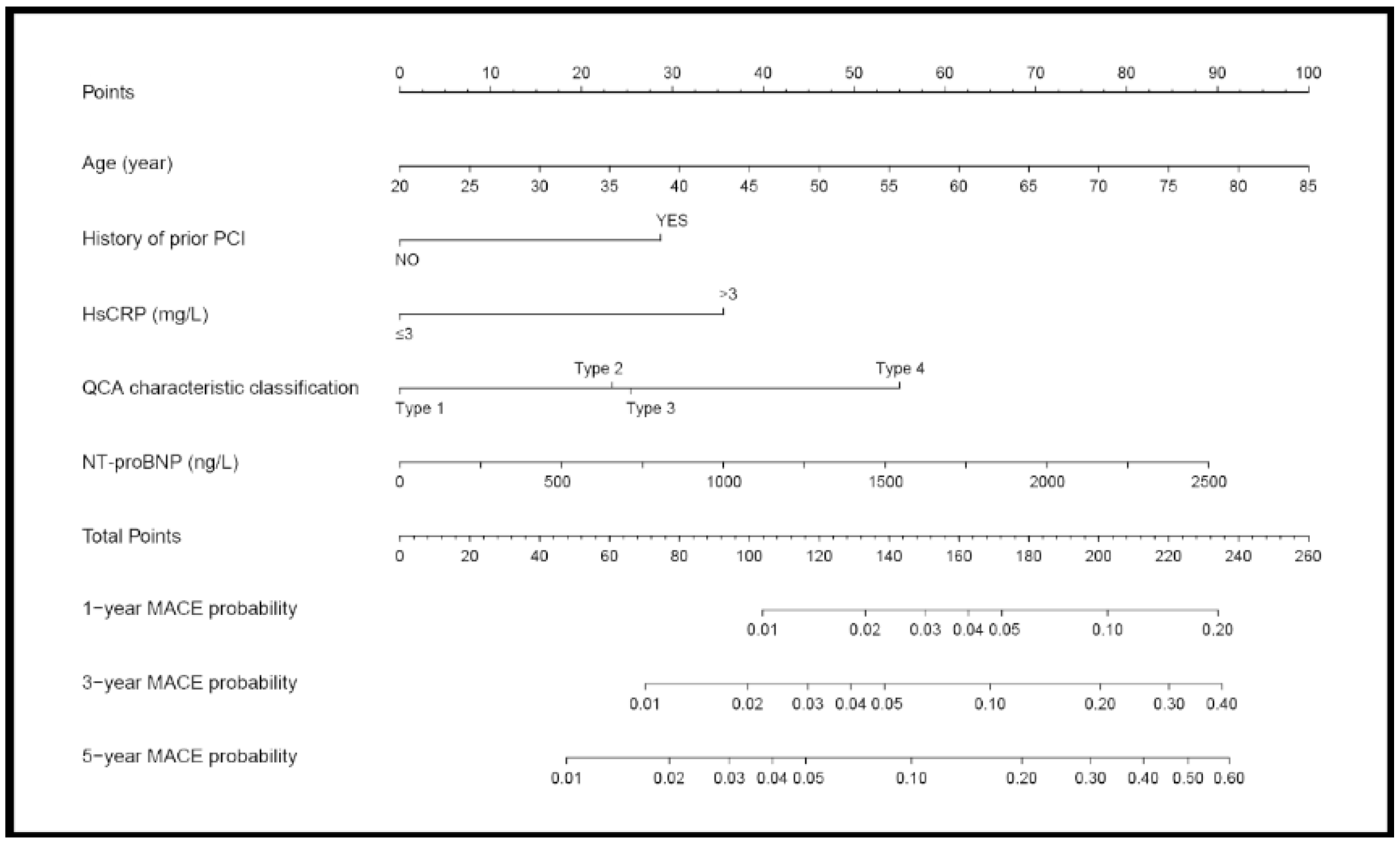
3.3. Nomogram Prediction of MACE
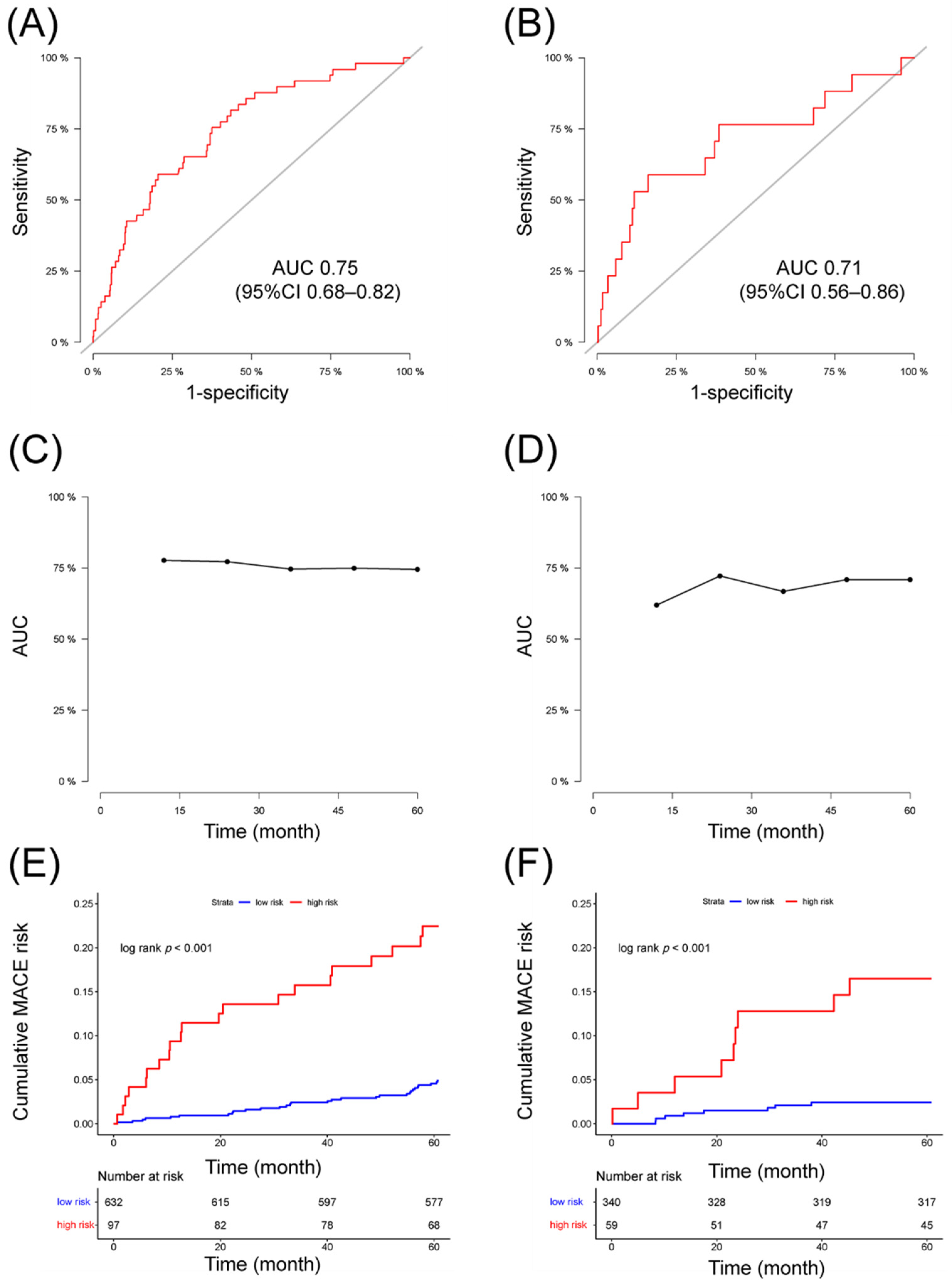
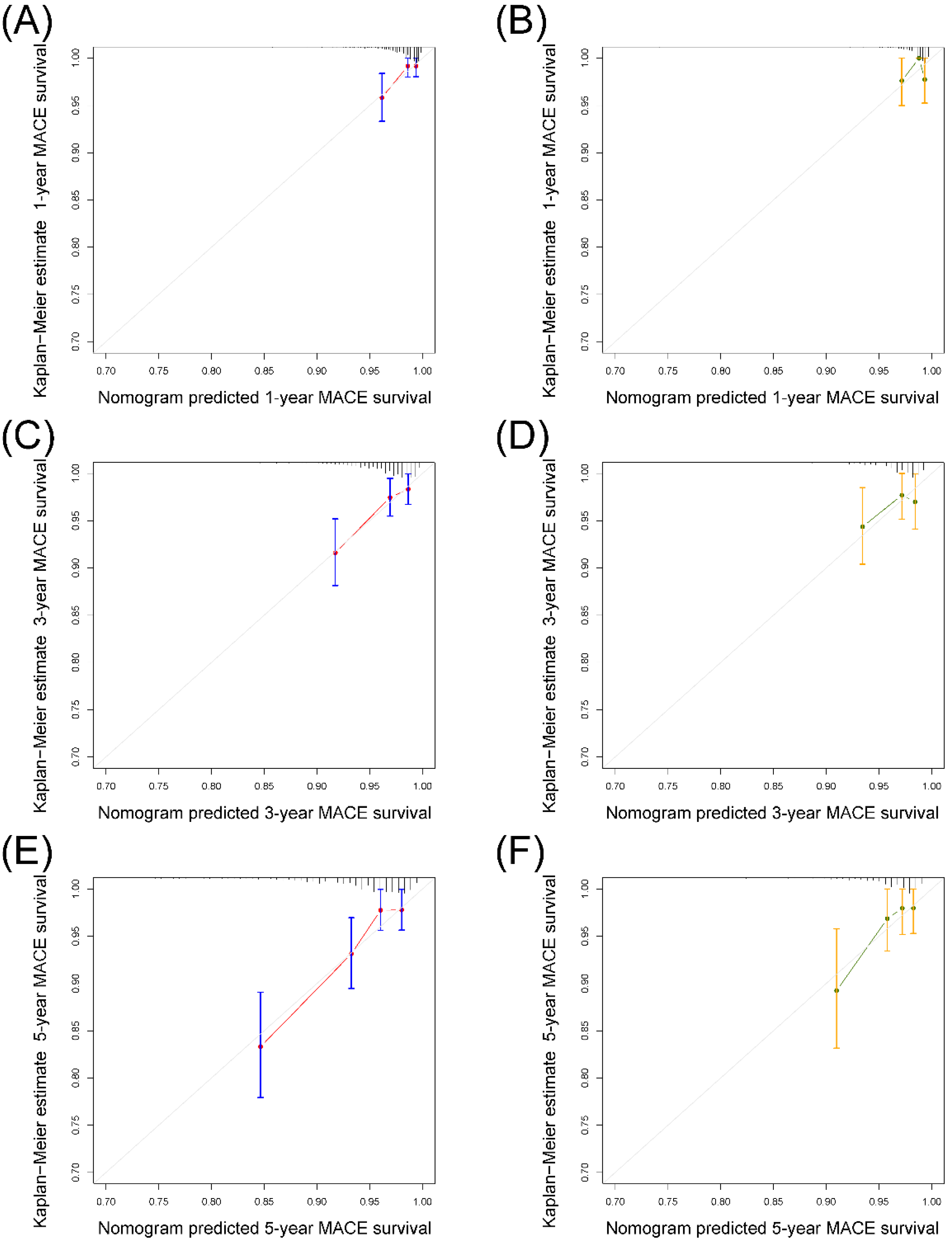
3.4. Validation of the Nomogram
3.5. Severity of Coronary Stenosis and 5-Year MACE
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variables | p Value | Hazard Ratio (95% CI) |
|---|---|---|
| Continuous Variable | ||
| Age | 0.011 | 1.04 (1.01–1.06) |
| BMI | 0.981 | 1.00 (0.97–1.03) |
| LVEF | 0.107 | 0.98 (0.95–1.01) |
| LVID | 0.143 | 1.03 (0.99–1.08) |
| D-dimer | 0.616 | 1.09 (0.77–1.54) |
| Serum creatinine | 0.512 | 1.00 (0.99–1.02) |
| eGFR | 0.966 | 1.00 (0.99–1.01) |
| TC | 0.847 | 1.02 (0.81–1.29) |
| TG | 0.578 | 0.92 (0.68–1.24) |
| LDL-C | 0.867 | 0.98 (0.73–1.30) |
| HDL-C | 0.750 | 1.18 (0.43–3.27) |
| NT-proBNP (increase of 500 ng/mL) | 0.001 | 1.77 (1.25–2.52) |
| hsCRP | 0.007 | 1.08 (1.02–1.15) |
| ESR | 0.608 | 1.00 (0.99–1.02) |
| SYNTAX score | 0.399 | 1.01 (0.99–1.04) |
| MAXD | 0.736 | 1.01 (0.95–1.08) |
| MAXD ratio | 0.833 | 1.03 (0.81–1.30) |
| MAXL | 0.165 | 1.01 (1.00–1.01) |
| MAXL ratio | 0.061 | 2.14 (0.97–4.74) |
| Categorical Variable | ||
| Male | 0.263 | 1.69 (0.67–4.26) |
| MI at enrollment | 0.815 | 1.09 (0.54–2.17) |
| Prior MI | 0.188 | 1.47 (0.83–2.61) |
| Prior PCI | 0.116 | 1.59 (0.89–2.85) |
| Diabetes | 0.668 | 1.15 (0.61–2.15) |
| Hypertension | 0.951 | 1.02 (0.57–1.82) |
| Dyslipidemia | 0.964 | 0.99 (0.56–1.74) |
| Peripheral arterial disease | 0.795 | 1.30 (0.18–9.41) |
| Family history of CAD | 0.513 | 1.27 (0.62–2.61) |
| Current smoker | 0.828 | 1.07 (0.60–1.89) |
| SYNTAX Score Level | - | - |
| low (≤22) | - | - |
| mid (>22 and ≤32) | 0.211 | 0.58 (0.25–1.36) |
| high (>32) | 0.788 | 1.17 (0.36–3.79) |
| Severe CAD (LM or 3-vessels disease) | 0.332 | 1.31 (0.76–2.27) |
| LM ectasia | 0.621 | 1.24 (0.53–2.91) |
| LAD ectasia | 0.982 | 1.01 (0.58–1.76) |
| LCX ectasia | 0.628 | 0.87 (0.48–1.55) |
| RCA ectasia | 0.697 | 1.12 (0.63–1.99) |
| Diffuse dilation (MAXL ratio ≥ 1/3) | 0.022 | 2.02 (1.11–3.69) |
| Markis Classification | - | - |
| Type I | - | - |
| Type II | 0.179 | 4.00 (0.53–30.29) |
| Type III | 0.160 | 4.22 (0.57–31.53) |
| Type IV | 0.485 | 2.05 (0.27–15.48) |
| Contrast agent stasis | 0.267 | 1.39 (0.78–2.49) |
| Thrombus in dilated segment | 0.996 | 0.00 (0.00–Inf) |
| Calcification in dilated segment | 0.500 | 0.61 (0.15–2.53) |
| LVEF < 50% | 0.155 | 1.78 (0.80–3.96) |
| LVID > 55 mm | 0.342 | 1.40 (0.70–2.79) |
| HsCRP > 3 mg/L | 0.002 | 2.36 (1.36–4.09) |
| D-dimer > 0.5 mg/L | 0.145 | 1.67 (0.84–3.34) |
| MAXD ≥ 5 mm | 0.016 | 2.27 (1.16–4.42) |
| QCA Characteristic Classification | - | - |
| Type 1 | - | - |
| Type 2 | 0.341 | 1.78 (0.54–5.83) |
| Type 3 | 0.278 | 1.80 (0.62–5.18) |
| Type 4 | 0.007 | 3.31 (1.38–7.92) |

References
- Swaye, P.S.; Fisher, L.D.; Litwin, P.; Vignola, P.A.; Judkins, M.P.; Kemp, H.G.; Mudd, J.G.; Gosselin, A.J. Aneurysmal coronary artery disease. Circulation 1983, 67, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Kawsara, A.; Nunez Gil, I.J.; Alqahtani, F.; Moreland, J.; Rihal, C.S.; Alkhouli, M. Management of Coronary Artery Aneurysms. JACC Cardiovasc. Interv. 2018, 11, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Kataoka, Y.; Noguchi, T.; Shibata, T.; Nakashima, T.; Kawakami, S.; Nakao, K.; Fujino, M.; Nagai, T.; Kanaya, T.; et al. Coronary Artery Ectasia Predicts Future Cardiac Events in Patients with Acute Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2350–2355. [Google Scholar] [CrossRef] [PubMed]
- Baman, T.S.; Cole, J.H.; Devireddy, C.M.; Sperling, L.S. Risk factors and outcomes in patients with coronary artery aneurysms. Am. J. Cardiol. 2004, 93, 1549–1551. [Google Scholar] [CrossRef] [PubMed]
- Sianos, G.; Morel, M.A.; Kappetein, A.P.; Morice, M.C.; Colombo, A.; Dawkins, K.; van den Brand, M.; Van Dyck, N.; Russell, M.E.; Mohr, F.W.; et al. The SYNTAX Score: An angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005, 1, 219–227. [Google Scholar] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Farooq, V.; van Klaveren, D.; Steyerberg, E.W.; Meliga, E.; Vergouwe, Y.; Chieffo, A.; Kappetein, A.P.; Colombo, A.; Holmes, D.R., Jr.; Mack, M.; et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: Development and validation of SYNTAX score II. Lancet 2013, 381, 639–650. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, J.; Wang, H.; Yin, D.; Song, W.; Dou, K. Diffuse coronary artery dilation predicted worse long-term outcomes in patients with coronary artery Ectasia. Int. J. Cardiol. 2020, 319, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kruger, D.; Stierle, U.; Herrmann, G.; Simon, R.; Sheikhzadeh, A. Exercise-induced myocardial ischemia in isolated coronary artery ectasias and aneurysms (“dilated coronopathy”). J. Am. Coll. Cardiol. 1999, 34, 1461–1470. [Google Scholar] [CrossRef]
- Ma, Y.C.; Zuo, L.; Chen, J.H.; Luo, Q.; Yu, X.Q.; Li, Y.; Xu, J.S.; Huang, S.M.; Wang, L.N.; Huang, W.; et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tang, J.; Liu, X.; Qiu, J.; Ye, Z.; Lai, Y.; Yao, Y.; Li, J.; Wang, X.; Liu, X. Coronary Artery Aneurysm Differs from Coronary Artery Ectasia: Angiographic Characteristics and Cardiovascular Risk Factor Analysis in Patients Referred for Coronary Angiography. Angiology 2017, 68, 823–830. [Google Scholar] [CrossRef]
- Lu, T.P.; Chuang, N.C.; Cheng, C.Y.; Hsu, C.A.; Wang, Y.C.; Lin, Y.H.; Lee, J.K.; Wu, C.K.; Hwang, J.J.; Lin, L.Y.; et al. Genome-wide methylation profiles in coronary artery ectasia. Clin. Sci. 2017, 131, 583–594. [Google Scholar] [CrossRef] [PubMed]
- ElGuindy, M.S.; ElGuindy, A.M. Aneurysmal coronary artery disease: An overview. Glob. Cardiol. Sci. Pract. 2017, 2017, e201726. [Google Scholar] [CrossRef] [PubMed]
- Markis, J.E.; Joffe, C.D.; Cohn, P.F.; Feen, D.J.; Herman, M.V.; Gorlin, R. Clinical significance of coronary arterial ectasia. Am. J. Cardiol. 1976, 37, 217–222. [Google Scholar] [CrossRef]
- Camp, R.L.; Dolled-Filhart, M.; Rimm, D.L. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 2004, 10, 7252–7259. [Google Scholar] [CrossRef]
- Weiser, M.R.; Landmann, R.G.; Kattan, M.W.; Gonen, M.; Shia, J.; Chou, J.; Paty, P.B.; Guillem, J.G.; Temple, L.K.; Schrag, D.; et al. Individualized prediction of colon cancer recurrence using a nomogram. J. Clin. Oncol. 2008, 26, 380–385. [Google Scholar] [CrossRef]
- Hartaigh, B.O.; Gransar, H.; Callister, T.; Shaw, L.J.; Schulman-Marcus, J.; Stuijfzand, W.J.; Valenti, V.; Cho, I.; Szymonifka, J.; Lin, F.Y.; et al. Development and Validation of a Simple-to-Use Nomogram for Predicting 5-, 10-, and 15-Year Survival in Asymptomatic Adults Undergoing Coronary Artery Calcium Scoring. JACC Cardiovasc. Imaging 2018, 11, 450–458. [Google Scholar] [CrossRef]
- Androulakis, A.E.; Katsaros, A.A.; Kartalis, A.N.; Stougiannos, P.N.; Andrikopoulos, G.K.; Triantafyllidi, E.I.; Pantazis, A.A.; Stefanadis, C.I.; Kallikazaros, I.E. Varicose veins are common in patients with coronary artery ectasia. Just a coincidence or a systemic deficit of the vascular wall? Eur. J. Vasc. Endovasc. Surg. 2004, 27, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Warisawa, T.; Naganuma, T.; Tomizawa, N.; Fujino, Y.; Ishiguro, H.; Tahara, S.; Kurita, N.; Nojo, T.; Nakamura, S.; Nakamura, S. High prevalence of coronary artery events and non-coronary events in patients with coronary artery aneurysm in the observational group. Int. J. Cardiol. Heart Vasc. 2016, 10, 29–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Papadakis, M.C.; Leontiadis, E.; Manginas, A.; Voudris, V.; Pavlides, G.; Karatasakis, G.; Foussas, S.G.; Mihalis, A.S.; Cokkinos, D.V. Frequency of coronary artery ectasia in patients undergoing surgery for ascending aortic aneurysms. Am. J. Cardiol. 2004, 94, 1433–1435. [Google Scholar] [CrossRef]
- Balderston, J.R.; Giri, J.; Kolansky, D.M.; Bavaria, J.E.; Gertz, Z.M. Coronary artery aneurysms associated with ascending aortic aneurysms and abdominal aortic aneurysms: Pathophysiologic implications. Catheter. Cardiovasc. Interv. 2015, 85, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Hall, C. Essential biochemistry and physiology of (NT-pro)BNP. Eur. J. Heart Fail. 2004, 6, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, M.; Perez-Reviriego, A.A.; Castellano-Martinez, A.; Cascales-Poyatos, H.M. N-terminal probrain natriuretic peptide as biomarker for diagnosis of Kawasaki disease. Biomark. Med. 2019, 13, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, L.; Dionne, A.; Meloche-Dumas, L.; Fournier, A.; Dahdah, N. Echocardiographic Parameters During and Beyond Onset of Kawasaki Disease Correlate with Onset Serum N-Terminal pro-Brain Natriuretic Peptide (NT-proBNP). Pediatr. Cardiol. 2020, 41, 947–954. [Google Scholar] [CrossRef]
- Struthers, A.; Lang, C. The potential to improve primary prevention in the future by using BNP/N-BNP as an indicator of silent ‘pancardiac’ target organ damage: BNP/N-BNP could become for the heart what microalbuminuria is for the kidney. Eur. Heart J. 2007, 28, 1678–1682. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; You, S.; Wang, H.; Yin, D.; Dou, K.; Song, W. Independent Prognostic Value of High-sensitivity C-reactive Protein in Patients with Coronary Artery Ectasia. Chin. Med. J. 2016, 129, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Mohlenkamp, S.; Lehmann, N.; Moebus, S.; Schmermund, A.; Dragano, N.; Stang, A.; Siegrist, J.; Mann, K.; Jockel, K.H.; Erbel, R.; et al. Quantification of coronary atherosclerosis and inflammation to predict coronary events and all-cause mortality. J. Am. Coll. Cardiol. 2011, 57, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Bielas, H.; Meister-Langraf, R.E.; Schmid, J.P.; Barth, J.; Znoj, H.; Schnyder, U.; Princip, M.; von Kanel, R. Acute stress disorder and C-reactive protein in patients with acute myocardial infarction. Eur. J. Prev. Cardiol. 2018, 25, 298–305. [Google Scholar] [CrossRef]
- Ridker, P.M. Clinician’s Guide to Reducing Inflammation to Reduce Atherothrombotic Risk: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 3320–3331. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, P.; Claessen, B.E.; Kalkman, D.N.; Aquino, M.; Sorrentino, S.; Giustino, G.; Farhan, S.; Vogel, B.; Sartori, S.; Montalescot, G.; et al. Residual Inflammatory Risk in Patients with Low LDL Cholesterol Levels Undergoing Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2019, 73, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Rha, S.W.; Wani, S.P.; Oh, D.J. Parallel stenting using two sirolimus-eluting stents in an ectatic coronary artery stenosis. Heart 2007, 93, 976. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, P.; Stanojevic, D.; Drees, T.; Fritzlen, J.; Haghnegahdar, M.; McCullough, M.; Barua, R.; Mehta, A.; Hockstad, E.; Wiley, M.; et al. Prognostic significance, angiographic characteristics and impact of antithrombotic and anticoagulant therapy on outcomes in high versus low grade coronary artery ectasia: A long-term follow-up study. Catheter. Cardiovasc. Interv. 2019, 93, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Q.J.; Li, X.L.; Guo, Y.L.; Zhu, C.G.; Wang, X.W.; Xu, B.; Gao, R.L.; Li, J.J. Prognostic Value of Coronary Artery Stenoses, Markis Class, and Ectasia Ratio in Patients with Coronary Artery Ectasia. Cardiology 2015, 131, 251–259. [Google Scholar] [CrossRef] [PubMed]

| Derivation Set | Validation Set | p-Value | |
|---|---|---|---|
| No. of patients | 729 | 399 | |
| Male | 618 (84.77) | 333 (83.46) | 0.621 |
| Age, yrs | 57.30 ± 10.86 | 56.96 ± 11.25 | 0.627 |
| BMI, kg/m2 | 26.42 [24.22, 28.70] | 26.63 [24.44, 29.39] | 0.272 |
| Clinical presentation | 0.175 | ||
| Asymptomatic | 13 (1.78) | 11 (2.76) | |
| Stable angina | 240 (32.92) | 128 (32.08) | |
| Unstable angina | 333 (45.68) | 176 (44.11) | |
| NSTEMI | 35 (4.80) | 32 (8.02) | |
| STEMI | 98 (13.44) | 43 (10.78) | |
| Dyspnea | 3 (0.41) | 4 (1.00) | |
| Palpitation | 7 (0.96) | 5 (1.25) | |
| Prior MI | 201 (27.52) | 109 (27.32) | 0.983 |
| Prior PCI | 177 (24.28) | 97 (24.31) | 1.000 |
| Diabetes | 168 (23.05) | 95 (23.80) | 0.829 |
| Hypertension | 483 (66.26) | 267 (66.92) | 0.873 |
| Dyslipidemia | 457 (62.69) | 280 (70.18) | 0.014 |
| Peripheral arterial disease | 12 (1.65) | 10 (2.51) | 0.439 |
| Family history of CAD | 103 (14.13) | 92 (23.06) | <0.001 |
| Current smoker | 248 (34.02) | 146 (36.59) | 0.423 |
| Derivation Set | Validation Set | p-Value | |
|---|---|---|---|
| No. of patients | 729 | 399 | |
| Combined CAD | 0.584 | ||
| None | 69 (9.47) | 48 (12.03) | |
| 1-vessel disease | 134 (18.38) | 82 (20.55) | |
| 2-vessels disease | 197 (27.02) | 103 (25.81) | |
| 3-vessels disease | 260 (35.67) | 134 (33.58) | |
| LM disease | 3 (0.41) | 0 (0.00) | |
| LM + 1-vessel disease | 5 (0.69) | 3 (0.75) | |
| LM + 2-vessels disease | 11 (1.51) | 3 (0.75) | |
| LM + 3-vessels disease | 50 (6.86) | 26 (6.52) | |
| SYNTAX score | 14.50 [7.00, 21.50] | 14.00 [7.00, 21.25] | 0.674 |
| LM ectasia | 73 (10.01) | 58 (14.54) | 0.030 |
| LAD ectasia | 299 (41.02) | 174 (43.61) | 0.435 |
| LCX ectasia | 266 (36.49) | 164 (41.10) | 0.144 |
| RCA ectasia | 454 (62.28) | 232 (58.15) | 0.195 |
| diffuse dilation | 401 (55.01) | 194 (48.62) | 0.046 |
| Markis classification | 0.040 | ||
| Type I | 42 (5.76) | 30 (7.52) | |
| Type II | 161 (22.09) | 83 (20.80) | |
| Type III | 197 (27.02) | 81 (20.30) | |
| Type IV | 329 (45.13) | 205 (51.38) | |
| MAXD | 5.39 [4.59, 6.16] | 5.54 [4.69, 6.30] | 0.038 |
| MAXD ratio | 1.74 [1.59, 1.95] | 1.74 [1.58, 2.01] | 0.535 |
| MAXL | 31.07 [12.66, 62.39] | 28.68 [12.15, 56.15] | 0.190 |
| MAXL ratio | 0.36 [0.14, 0.69] | 0.31 [0.13, 0.61] | 0.090 |
| contrast agent stasis | 192 (26.34) | 100 (25.06) | 0.692 |
| thrombus in dilated segment | 14 (1.92) | 6 (1.50) | 0.786 |
| calcification in dilated segment | 47 (6.45) | 28 (7.02) | 0.808 |
| Variables | Hazard Ratio (95% CI) | p-Value |
|---|---|---|
| Age | 1.037 (1.010, 1.065) | 0.007 |
| History of prior PCI | 1.980 (1.091, 3.595) | 0.025 |
| NT-proBNP (Each increase of 500 ng/L) | 1.528 (1.031, 2.264) | 0.035 |
| Increased hsCRP(>3 mg/L) | 2.335 (1.325, 4.114) | 0.003 |
| QCA characteristic classification | - | 0.011 |
| Type 1 | - | - |
| Type 2 | 1.745 (0.520, 5.854) | 0.368 |
| Type 3 | 1.829 (0.632, 5.294) | 0.261 |
| Type 4 | 3.703 (1.543, 8.888) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.; Wang, Y.; Li, L.; Wang, H.; Song, C.; Yin, D.; Song, W.; Dou, K. Development and Validation of a Nomogram for Predicting the Risk of Adverse Cardiovascular Events in Patients with Coronary Artery Ectasia. J. Cardiovasc. Dev. Dis. 2021, 8, 186. https://doi.org/10.3390/jcdd8120186
Cai Z, Wang Y, Li L, Wang H, Song C, Yin D, Song W, Dou K. Development and Validation of a Nomogram for Predicting the Risk of Adverse Cardiovascular Events in Patients with Coronary Artery Ectasia. Journal of Cardiovascular Development and Disease. 2021; 8(12):186. https://doi.org/10.3390/jcdd8120186
Chicago/Turabian StyleCai, Zhongxing, Yintang Wang, Luqi Li, Haoyu Wang, Chenxi Song, Dong Yin, Weihua Song, and Kefei Dou. 2021. "Development and Validation of a Nomogram for Predicting the Risk of Adverse Cardiovascular Events in Patients with Coronary Artery Ectasia" Journal of Cardiovascular Development and Disease 8, no. 12: 186. https://doi.org/10.3390/jcdd8120186
APA StyleCai, Z., Wang, Y., Li, L., Wang, H., Song, C., Yin, D., Song, W., & Dou, K. (2021). Development and Validation of a Nomogram for Predicting the Risk of Adverse Cardiovascular Events in Patients with Coronary Artery Ectasia. Journal of Cardiovascular Development and Disease, 8(12), 186. https://doi.org/10.3390/jcdd8120186





