Copeptin Levels Are Independent from Mild Therapeutic Hypothermia but Do Not Predict Infarct Size in Patients Presenting with ST-Segment Elevation Myocardial Infarction
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Endpoints
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Copeptin and Troponin T Levels during the Treatment
4. Discussion
4.1. Copeptin as Ubiquitous Biomarker for Circulatory Stress
4.2. Kinetics
4.3. Infarct Size
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Observatory Data—Top 10 Causes of Death. Available online: https://www.who.int/gho/mortality_burden_disease/causes_death/top_10/en/ (accessed on 24 April 2020).
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [Green Version]
- Noc, M.; Erlinge, D.; Neskovic, A.N.; Kafedzic, S.; Merkely, B.; Zima, E.; Fister, M.; Petrovic, M.; Čanković, M.; Veress, G.; et al. COOL AMI EU pilot trial: A multicentre, prospective, randomised controlled trial to assess cooling as an adjunctive therapy to percutaneous intervention in patients with acute myocardial infarction. EuroIntervention J. Eur. Collab. Work Group Interv. Cardiol. Eur. Soc. Cardiol. 2017, 13, e531–e539. [Google Scholar] [CrossRef] [Green Version]
- Erlinge, D.; Götberg, M.; Lang, I.; Holzer, M.; Noc, M.; Clemmensen, P.; Jensen, U.; Metzler, B.; James, S.; Bøtker, H.E.; et al. Rapid Endovascular Catheter Core Cooling Combined With Cold Saline as an Adjunct to Percutaneous Coronary Intervention for the Treatment of Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2014, 63, 1857–1865. [Google Scholar] [CrossRef] [Green Version]
- Nichol, G.; Strickland, W.; Shavelle, D.; Maehara, A.; Ben-Yehuda, O.; Genereux, P.; Dressler, O.; Parvataneni, R.; Nichols, M.; McPherson, J.; et al. Prospective, Multicenter, Randomized, Controlled Pilot Trial of Peritoneal Hypothermia in Patients With ST-Segment— Elevation Myocardial Infarction. Circ. Cardiovasc. Interv. 2015, 8, e001965. [Google Scholar] [CrossRef] [Green Version]
- Testori, C.; Beitzke, D.; Mangold, A.; Sterz, F.; Loewe, C.; Weiser, C.; Scherz, T.; Herkner, H.; Lang, I. Out-of-hospital initiation of hypothermia in ST-segment elevation myocardial infarction: A randomised trial. Heart 2018, 105, 531–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Crit. Care 2017, 21, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolov, N.M.; Cunningham, A.J. Mild Therapeutic Hypothermia to Improve the Neurologic Outcome after Cardiac Arrest. N. Engl. J. Med. 2002, 346, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Lascarrou, J.-B.; Merdji, H.; Le Gouge, A.; Colin, G.; Grillet, G.; Girardie, P.; Coupez, E.; Dequin, P.-F.; Cariou, A.; Boulain, T.; et al. Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm. N. Engl. J. Med. 2019, 381, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Soar, J.; Cariou, A.; Cronberg, T.; Moulaert, V.R.M.; Deakin, C.D.; Bottiger, B.W.; Friberg, H.; Sunde, K.; Sandroni, C. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 2015, 95, 202–222. [Google Scholar] [CrossRef]
- Dae, M.W.; Gao, D.W.; Sessler, D.I.; Chair, K.; Stillson, C.A. Effect of endovascular cooling on myocardial temperature, infarct size, and cardiac output in human-sized pigs. Am. J. Physiol. Circ. Physiol. 2002, 282, H1584–H1591. [Google Scholar] [CrossRef]
- Götberg, M.; Van Der Pals, J.; Olivecrona, G.K.; Götberg, M.; Koul, S.; Erlinge, D. Mild hypothermia reduces acute mortality and improves hemodynamic outcome in a cardiogenic shock pig model. Resuscitation 2010, 81, 1190–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hale, S.L.; Dave, R.H.; Kloner, R.A. Regional hypothermia reduces myocardial necrosis even when instituted after the onset of ischemia. Basic Res. Cardiol. 1997, 92, 351–357. [Google Scholar] [CrossRef]
- Testori, C.; Sterz, F.; Delle-Karth, G.; Malzer, R.; Holzer, M.; Stratil, P.; Stöckl, M.; Weiser, C.; Van Tulder, R.; Gangl, C.; et al. Strategic target temperature management in myocardial infarction—a feasibility trial. Heart 2013, 99, 1663–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ-Crain, M. Vasopressin and Copeptin in health and disease. Rev. Endocr. Metab. Disord. 2019, 20, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Assay for the Measurement of Copeptin, a Stable Peptide Derived from the Precursor of Vasopressin. Clin. Chem. 2006, 52, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raskovalova, T.; Twerenbold, R.; Collinson, P.O.; Keller, T.; Bouvaist, H.; Folli, C.; Giavarina, D.; Lotze, U.; Eggers, K.M.; Dupuy, A.M.; et al. Diagnostic accuracy of combined cardiac troponin and copeptin assessment for early rule-out of myocardial infarction: A systematic review and meta-analysis. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Broessner, G.; Hasslacher, J.; Beer, R.; Lackner, P.; Lehner, G.F.; Harler, U.; Schiefecker, A.; Helbok, R.; Pfausler, B.; Hammerer-Lercher, A.; et al. Outcome prediction and temperature dependency of MR-proANP and Copeptin in comatose resuscitated patients. Resuscitation 2015, 89, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Salvo, F.; Luppi, F.; Lucchesi, D.M.; Canovi, S.; Franchini, S.; Polese, A.; Santi, F.; Trabucco, L.; Fasano, T.; Ferrari, A.M. Serum Copeptin levels in the emergency department predict major clinical outcomes in adult trauma patients. BMC Emerg. Med. 2020, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.-S.; Kim, H.J.; Chun, H.-J.; Kim, J.M.; Yi, H.-J.; Cheong, J.-H.; Kim, C.-H.; Oh, S.-J.; Ko, Y.; Kim, Y.-S.; et al. Prognostic role of copeptin after stroke: A systematic review and meta-analysis of observational studies. Sci. Rep. 2015, 5, srep11665. [Google Scholar] [CrossRef]
- Krychtiuk, K.A.; Honeder, M.C.; Lenz, M.; Maurer, G.; Wojta, J.; Heinz, G.; Huber, K.; Speidl, W.S. Copeptin Predicts Mortality in Critically Ill Patients. PLoS ONE 2017, 12, e0170436. [Google Scholar] [CrossRef]
- Eitel, I.; Wang, J.; Stiermaier, T.; Fuernau, G.; Feistritzer, H.-J.; Joost, A.; Jobs, A.; Meusel, M.; Blodau, C.; Desch, S.; et al. Impact of Morphine Treatment on Infarct Size and Reperfusion Injury in Acute Reperfused ST-Elevation Myocardial Infarction. J. Clin. Med. 2020, 9, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doi, N.; Brown, C.H.; Cohen, H.D.; Leng, G.; A Russell, J. Effects of the endogenous opioid peptide, endomorphin 1, on supraoptic nucleus oxytocin and vasopressin neuronesin vivoandin vitro. Br. J. Pharmacol. 2001, 132, 1136–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pumford, K.; Russell, J.; Leng, G. Effects of the selective kappa-opioid agonist U50,488 upon the electrical activity of supraoptic neurones in morphine-tolerant and morphine-naive rats. Exp. Brain Res. 1993, 94, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Volpe, D.A.; Tobin, A.-M.; Mellon, R.D.; Katki, A.G.; Parker, R.J.; Colatsky, T.; Kropp, T.J.; Verbois, S.L. Uniform assessment and ranking of opioid Mu receptor binding constants for selected opioid drugs. Regul. Toxicol. Pharmacol. 2011, 59, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Hupf, H.; Grimm, D.; Riegger, G.A.J.; Schunkert, H. Evidence for a Vasopressin System in the Rat Heart. Circ. Res. 1999, 84, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Boeckel, J.-N.; Oppermann, J.; Anadol, R.; Fichtlscherer, S.; Zeiher, A.M.; Keller, T. Analyzing the Release of Copeptin from the Heart in Acute Myocardial Infarction Using a Transcoronary Gradient Model. Sci. Rep. 2016, 6, 20812. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.L.; Voors, A.A.; Zijlstra, F.; Hillege, H.L.; Struck, J.; Masson, S.; Vago, T.; Anker, S.D.; Heuvel, A.F.M.V.D.; Van Veldhuisen, D.J.; et al. Comparison of the temporal release pattern of copeptin with conventional biomarkers in acute myocardial infarction. Clin. Res. Cardiol. 2011, 100, 1069–1076. [Google Scholar] [CrossRef] [Green Version]
- Ananth, V.; Beig, J.R.; Tramboo, N.A.; Rasool, R.; Choh, N.A.; Bashir, S.; Rather, H.A.; Lone, A.A. Does plasma copeptin level at admission predict final infarct size in ST-elevation myocardial infarction. Int. J. Cardiol. 2016, 219, 326–330. [Google Scholar] [CrossRef]
- Reinstadler, S.J.; Klug, G.; Feistritzer, H.-J.; Mayr, A.; Harrasser, B.; Mair, J.; Bader, K.; Streil, K.; Hammerer-Lercher, A.; Esterhammer, R.; et al. Association of copeptin with myocardial infarct size and myocardial function after ST segment elevation myocardial infarction. Heart 2013, 99, 1525–1529. [Google Scholar] [CrossRef]
- Árnadóttir, Á.; Schoos, M.; Lønborg, J.; Ahtarovski, K.; Kelbæk, H.; Helqvist, S.; Høfsten, D.; Clemmensen, P.; Engstrøm, T.; Nepper-Christensen, L.; et al. Can copeptin and troponin T ratio predict final infarct size and myocardial salvage index in patients with ST-elevation myocardial infarction: A sub-study of the DANAMI-3 trial. Clin. Biochem. 2018, 59, 37–42. [Google Scholar] [CrossRef]
- Pedersen, C.K.; Stengaard, C.; Søndergaard, H.; Dodt, K.K.; Hjort, J.; Bøtker, M.T.; Terkelsen, C.J. A multicentre, randomized, controlled open-label trial to compare an Accelerated Rule-Out protocol using combined prehospital copeptin and in-hospital high sensitive troponin with standard rule-out in patients suspected of acute Myocardial Infarction—the AROMI trial. Trials 2018, 19, 683. [Google Scholar] [CrossRef] [Green Version]
| Hypothermia (n = 47) | Control (n = 52) | |
|---|---|---|
| Age, years (SD) | 58 (±10) | 56 (±10) |
| Female sex, n (%) | 10 (21) | 9 (17) |
| Hypertension, n (%) | 13 (28) | 23 (44) |
| Diabetes, n (%) | 5 (11) | 10 (19) |
| Dyslipidemia, n (%) | 10 (21) | 16 (31) |
| Current smoker, n (%) | 26 (55) | 29 (56) |
| Family history of CAD, n (%) | 12 (26) | 12 (23) |
| Obesity, n (%) | 10 (21) | 19 (37) |
| Body mass index (kg/m2), mean (SD) | 27.0 (±5.5) | 29.5 (±4.9) |
| Previous medication | ||
| Acetylsalicylic acid, n (%) | 1 (2) | 4 (8) |
| Beta blocker, n (%) | 4 (9) | 6 (12) |
| ACE-I/ARB, n (%) | 8 (17) | 9 (17) |
| Statin, n (%) * | 3 (6) | 11 (21) |
| Diuretics, n (%) | 1 (2) | 1 (2) |
| Initial sinus rhythm, n (%) | 44 (94) | 48 (92) |
| ECG diagnosis of anterior wall infarction, n (%) | 27 (57) | 24 (46) |
| Emergency therapy | ||
| Acetylsalicylic acid, n (%) | 46 (98) | 52 (100) |
| Heparin, n (%) | 46 (98) | 52 (100) |
| Prasugrel/Ticagrelor | 47 (100) | 51 (98) |
| Nitroglycerin, n (%) | 9 (19) | 12 (23) |
| Beta blocker, n (%) | 0 (0) | 3 (6) |
| Opioids, n (%) | 46 (98) | 49 (94) |
| Morphine, n (%) † | 11 (23) | 44 (85) |
| Meperidin, n (%) † | 42 (89) | 5 (10) |
| Infarct-related artery | ||
| Left anterior descending artery, n (%) | 28 (60) | 23 (44) |
| Circumflex artery, n (%) | 3 (6) | 4 (8) |
| Right coronary artery, n (%) | 16 (34) | 25 (48) |
| Multivessel disease, n (%) | 22 (47) | 30 (58) |
| Initial TIMI grade flow 0/1, n (%) | 34 (72) | 42 (81) |
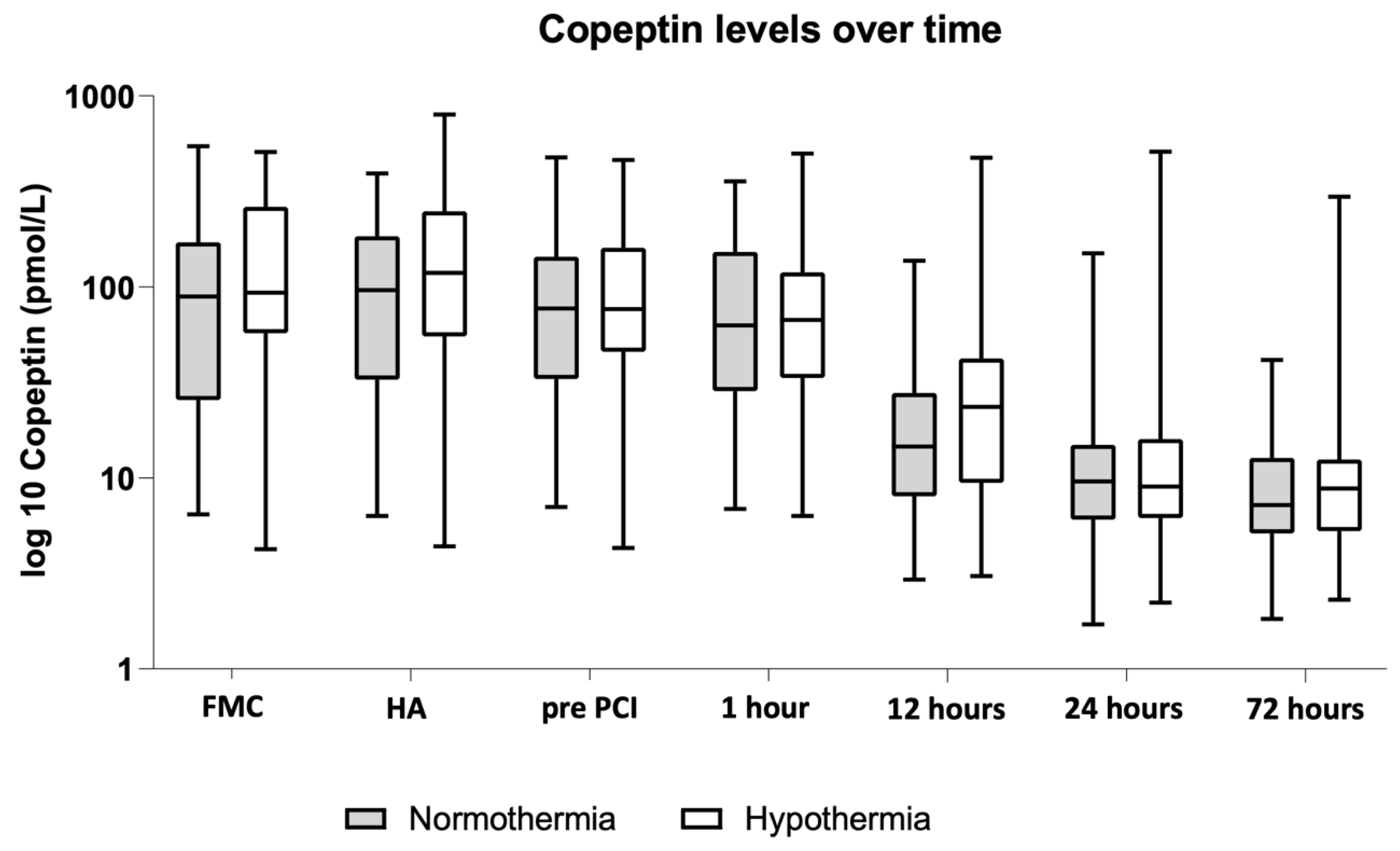
| Copeptin | Hypothermia (n = 47) | Control (n = 52) | p-Value |
|---|---|---|---|
| First medical contact | 101.3 (57.8–258.1) | 89.1 (25.6–170.9) | 0.216 |
| Hospital admission | 118.5 (55.1–248.6) | 96.2 (32.7–184.2) | 0.148 |
| Prior to pPCI | 76.5 (45.9–160.2) | 77.1 (33.1–143.6) | 0.353 |
| 1 h after pPCI | 67.2 (33.4–119.2) | 62.7 (28.5–152.0) | 0.636 |
| 12 h after pPCI | 23.5 (9.4–42.1) | 14.6 (8.0–27.8) | 0.157 |
| 24 h after pPCI | 9.0 (6.2–15.9) | 9.6 (6.1–14.9) | 0.710 |
| 72 h after pPCI | 8.8 (5.3–12.4) | 7.2 (5.1–12.7) | 0.742 |
| Troponin T | |||
| Hospital admission | 43 (18–103) | 24.5 (17–58.25) | 0.182 |
| Troponin peak level | 4480 (1750–6746) | 3610 (2112.5–6800) | 0.969 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller, M.; Beitzke, D.; Scherz, T.; Loewe, C.; Mangold, A.; Marculescu, R.; Poppe, M.; Sterz, F.; Herkner, H.; Lang, I.; et al. Copeptin Levels Are Independent from Mild Therapeutic Hypothermia but Do Not Predict Infarct Size in Patients Presenting with ST-Segment Elevation Myocardial Infarction. J. Cardiovasc. Dev. Dis. 2021, 8, 131. https://doi.org/10.3390/jcdd8100131
Mueller M, Beitzke D, Scherz T, Loewe C, Mangold A, Marculescu R, Poppe M, Sterz F, Herkner H, Lang I, et al. Copeptin Levels Are Independent from Mild Therapeutic Hypothermia but Do Not Predict Infarct Size in Patients Presenting with ST-Segment Elevation Myocardial Infarction. Journal of Cardiovascular Development and Disease. 2021; 8(10):131. https://doi.org/10.3390/jcdd8100131
Chicago/Turabian StyleMueller, Matthias, Dietrich Beitzke, Thomas Scherz, Christian Loewe, Andreas Mangold, Rodrig Marculescu, Michael Poppe, Fritz Sterz, Harald Herkner, Irene Lang, and et al. 2021. "Copeptin Levels Are Independent from Mild Therapeutic Hypothermia but Do Not Predict Infarct Size in Patients Presenting with ST-Segment Elevation Myocardial Infarction" Journal of Cardiovascular Development and Disease 8, no. 10: 131. https://doi.org/10.3390/jcdd8100131
APA StyleMueller, M., Beitzke, D., Scherz, T., Loewe, C., Mangold, A., Marculescu, R., Poppe, M., Sterz, F., Herkner, H., Lang, I., Testori, C., & Weiser, C. (2021). Copeptin Levels Are Independent from Mild Therapeutic Hypothermia but Do Not Predict Infarct Size in Patients Presenting with ST-Segment Elevation Myocardial Infarction. Journal of Cardiovascular Development and Disease, 8(10), 131. https://doi.org/10.3390/jcdd8100131







