The Value of Fetuin-A as a Predictor to Identify Takotsubo Patients at Risk of Cardiovascular Events
Abstract
1. Introduction
2. Methods
2.1. Patients and Controls
2.2. Blood Samples
2.3. Biomarker Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Correlation of Biomarkers with Clinical Characteristics
3.3. Biomarkers as Indicators of Cardiac Decompensation within the Acute Phase of TTC
3.4. Biomarkers as Predictors for Arrhythmia within the Acute Phase of TTC
3.5. Biomarkers, as Indicators for All Cause Complications
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sachdev, E.; Bairey Merz, C.N.; Mehta, P.K. Takotsubo Cardiomyopathy. Eur. J. Cardiol. 2015, 10, 25–30. [Google Scholar] [CrossRef]
- Wedekind, H.; Möller, K.; Scholz, K.H. Tako-Tsubo-Kardiomyopathie. Herz 2006, 31, 339–346. [Google Scholar] [CrossRef]
- Shams, Y.; Tornvall, P. Epidemiology, pathogenesis, and management of takotsubo syndrome. Clin. Auton. Res. 2017, 28, 53–65. [Google Scholar] [CrossRef]
- Pelliccia, F.; Kaski, J.C.; Crea, F.; Camici, P.G. Pathophysiology of Takotsubo Syndrome. Circulation 2017, 135, 2426–2441. [Google Scholar] [CrossRef]
- Roshanzamir, S.; Showkathali, R. Takotsubo cardiomyopathy a short review. Curr. Cardiol. Rev. 2013, 9, 191–196. [Google Scholar] [CrossRef]
- Santoro, F.; Costantino, M.D.; Guastafierro, F.; Triggiani, G.; Ferraretti, A.; Tarantino, N.; Saguner, A.; Di Biase, M.; Brunetti, N.D. Inflammatory patterns in Takotsubo cardiomyopathy and acute coronary syndrome: A propensity score matched analysis. Atherosclerosis 2018, 274, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, J.; Joshaghani, H.-R.; Akaberi, A.; Hesari, Z.; Ghanbari, R.; Mohammadzadeh, M.; Golshan, A.-R. Potential Correlation Between Circulating Fetuin-A and Pentraxin-3 With Biochemical Parameters of Calcification in Hemodialysis Patients. Arch. Iran. Med. 2017, 20, 752–755. [Google Scholar] [PubMed]
- Sun, Z.-L.; Xie, Q.-Y.; Guo, G.-L.; Ma, K.; Huang, Y.-Y. Serum Fetuin-A Levels in Patients with Cardiovascular Disease: A Meta-Analysis. Biomed. Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Feistritzer, H.-J.; Klug, G.; Reinstadler, S.J.; Gröber, M.-T.; Mair, J.; Kirchmair, R.; Henninger, B.; Franz, W.-M.; Metzler, B. Fetuin-A is related to infarct size, left ventricular function and remodelling after acute STEMI. Open Hear. 2015, 2, e000244. [Google Scholar] [CrossRef] [PubMed]
- Dande, A.S.; Sena, S.F.; Wasserman, H.S.; Warshofsky, M.K.; Belsky, J.L. Prevalence and Consequences of Vitamin D Insufficiency in Women With Takotsubo Cardiomyopathy. J. Clin. Endocrinol. Metab. 2013, 98, E872–E876. [Google Scholar] [CrossRef][Green Version]
- Looi, J.-L.; Lee, M.; Grey, C.; Webster, M.; To, A.; Kerr, A.J. Seasonal variation in Takotsubo syndrome compared with myocardial infarction: ANZACS-QI 16. New Zealand Med. J. 2018, 131, 21–29. [Google Scholar] [PubMed]
- Nef, H.M.; Möllmann, H.; Troidl, C.; Kostin, S.; Voss, S.; Hilpert, P.; Behrens, C.B.; Rolf, A.; Rixe, J.; Weber, M.; et al. Abnormalities in intracellular Ca2+ regulation contribute to the pathomechanism of Tako-Tsubo cardiomyopathy. Eur. Heart J. 2009, 30, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Sun, M.; Sader, S. Matrix metalloproteinases in cardiovascular disease. Can. J. Cardiol. 2006, 22, 25B–30B. [Google Scholar] [CrossRef]
- Peeters, S.A.; Engelen, L.; Buijs, J.; Jorsal, A.; Parving, H.-H.; Tarnow, L.; Rossing, P.; Schalkwijk, C.G.; Stehouwer, C.D.A. Plasma matrix metalloproteinases are associated with incident cardiovascular disease and all-cause mortality in patients with type 1 diabetes: A 12-year follow-up study. Cardiovasc. Diabetol. 2017, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Essa, E.M.; Zile, M.R.; Stroud, R.E.; Rice, A.; Gumina, R.J.; Leier, C.V.; Spinale, F.G. Changes in plasma profiles of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in stress-induced cardiomyopathy. J. Card. Fail. 2012, 18, 487–492. [Google Scholar] [CrossRef][Green Version]
- Parkkonen, O.; Nieminen, M.T.; Vesterinen, P.; Tervahartiala, T.; Perola, M.; Salomaa, V.; Jousilahti, P.; Sorsa, T.; Pussinen, P.J.; Sinisalo, J. Low MMP-8/TIMP-1 reflects left ventricle impairment in takotsubo cardiomyopathy and high TIMP-1 may help to differentiate it from acute coronary syndrome. PLoS ONE 2017, 12, e0173371. [Google Scholar] [CrossRef]
- Nicholls, S.; Hazen, S.L. Myeloperoxidase and Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2005, 25, 1102–1111. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase – A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Templin, C.; Cammann, V.L.; Hahad, O. Takotsubo Syndrome: Impact of endothelial dysfunction and oxidative stress. Free. Radic. Biol. Med. 2021, 169, 216–223. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, S.; Teng, X.; Duan, X.; Chen, Y.; Wu, Y. Hydrogen sulfide attenuates cardiac injury in takotsubo cardiomyopathy by alleviating oxidative stress. Nitric Oxide 2017, 67, 10–25. [Google Scholar] [CrossRef]
- Miftode, R.-S.; Şerban, I.-L.; Timpau, A.-S.; Miftode, I.-L.; Ion, A.; Buburuz, A.-M.; Costache, A.-D.; Costache, I.-I. Syndecan-1: A Review on Its Role in Heart Failure and Chronic Liver Disease Patients’ Assessment. Cardiol. Res. Pr. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Lunde, I.G.; Herum, K.M.; Carlson, C.C.; Christensen, G. Syndecans in heart fibrosis. Cell Tissue Res. 2016, 365, 539–552. [Google Scholar] [CrossRef]
- Daub, S.; Lutgens, E.; Münzel, T.; Daiber, A. CD40/CD40L and Related Signaling Pathways in Cardiovascular Health and Disease—The Pros and Cons for Cardioprotection. Int. J. Mol. Sci. 2020, 21, 8533. [Google Scholar] [CrossRef]
- Pamukcu, B.; Lip, G.Y.H.; Snezhitskiy, V.; Shantsila, E. The CD40-CD40L system in cardiovascular disease. Ann. Med. 2011, 43, 331–340. [Google Scholar] [CrossRef]
- Scally, C.; Abbas, H.; Ahearn, T.; Srinivasan, J.; Mezincescu, A.; Rudd, A.; Spath, N.; Yucel-Finn, A.; Yuecel, R.; Oldroyd, K.; et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2019, 139, 1581–1592. [Google Scholar] [CrossRef]
- Sattler, S.; Couch, L.S.; Harding, S.E. Takotsubo syndrome: Latest addition to the expanding family of immune-mediated diseases? JACC Basic Transl. Sci. 2018, 3, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Scantlebury, D.; Prasad, A. Diagnosis of Takotsubo Cardiomyopathy. Circ. J. 2014, 78, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Komamura, K. Takotsubo cardiomyopathy: Pathophysiology, diagnosis and treatment. World, J. Cardiol. 2014, 6, 602–609. [Google Scholar] [CrossRef]
- Kurowski, V.; Radke, P.W.; Schunkert, H.; Burgdorf, C. Patient care in the acute phase of stress induced cardiomyopathy (Tako-Tsubo cardiomyopathy)--and thereafter? Herz 2010, 35, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Hefner, J.; Csef, H.; Frantz, S.; Glatter, N.; Warrings, B. Recurrent Tako-Tsubo cardiomyopathy (TTC) in a pre-menopausal woman: Late sequelae of a traumatic event? BMC Cardiovasc. Disord. 2015, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Uribarri, A.; Núñez-Gil, I.J.; Conty, D.A.; Vedia, O.; Almendro-Delia, M.; Cambra, A.D.; Martin-Garcia, A.C.; Barrionuevo-Sánchez, M.; Martínez-Sellés, M.; Raposeiras-Roubín, S.; et al. Short- and Long-Term Prognosis of Patients With Takotsubo Syndrome Based on Different Triggers: Importance of the Physical Nature. J. Am. Hear. Assoc. 2019, 8, e013701. [Google Scholar] [CrossRef]
- Santoro, F.; Mallardi, A.; Leopizzi, A.; Vitale, E.; Zimotti, T.; Ieva, R.; Iacoviello, M.; Brunetti, N.D. Neoplastic markers in Takotsubo syndrome. Results from a prospective registry. Eur. Heart, J. 2020, 41, ehaa946-3295. [Google Scholar] [CrossRef]
- Santoro, F.; Guastafierro, F.; Zimotti, T.; Mallardi, A.; Leopizzi, A.; Cannone, M.; Biase, M.D.; Brunetti, N.D. Neutrophil/lymphocyte ratio predicts in-hospital complications in Takotsubo syndrome. Results from a prospective multi-center registry. Clin. Cardiol. 2020, 43, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Tarantino, N.; Ferraretti, A.; Ieva, R.; Musaico, F.; Guastafierro, F.; Di Martino, L.; Di Biase, M.; Brunetti, N.D. Serum interleukin 6 and 10 levels in Takotsubo cardiomyopathy: Increased admission levels may predict adverse events at follow-up. Atherosclerosis 2016, 254, 28–34. [Google Scholar] [CrossRef]
- Gore, M.O.; de Lemos, J.A. Cardiac Troponins and the Future of Precision Medicine. Circ. Cardiovasc. Interv. 2016, 9, e004031. [Google Scholar] [CrossRef]
- Falcone, C.; Lucibello, S.; Mazzucchelli, I.; Bozzini, S.; D’Angelo, A.; Schirinzi, S.; Totaro, R.; Bondesan, M.; Pelissero, G. Galectin-3 Plasma Levels and Coronary Artery Disease: A New Possible Biomarker of Acute Coronary Syndrome. Int. J. Immunopathol. Pharmacol. 2011, 24, 905–913. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, S.; Poveda, J.; Navarro-García, J.A.; González-Lafuente, L.; Rodríguez-Sánchez, E.; Ruilope, L.M.; Ruiz-Hurtado, G. An Overview of FGF-23 as a Novel Candidate Biomarker of Cardiovascular Risk. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef]
- Franco-Peláez, J.A.; Martín-Reyes, R.; Pello-Lázaro, A.M.; Aceña, Á.; Lorenzo, Ó.; Martín-Ventura, J.L.; Blanco-Colio, L.; González-Casaus, M.L.; Hernández-González, I.; Carda, R.; et al. Monocyte Chemoattractant Protein-1 Is an Independent Predictor of Coronary Artery Ectasia in Patients with Acute Coronary Syndrome. J. Clin. Med. 2020, 9, 3037. [Google Scholar] [CrossRef]
- Conradi, P.M.; van Loon, R.B.; Handoko, M.L. Dynamic left ventricular outflow tract obstruction in Takotsubo cardiomyopathy resulting in cardiogenic shock. BMJ Case Rep. 2021, 14, e240010. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.F.; Asirvatham, S.J.; Francis, J. Arrhythmia occurrence with takotsubo cardiomyopathy: A literature review. Europace 2010, 13, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Möller, C.; Eitel, C.; Thiele, H.; Eitel, I.; Stiermaier, T. Ventricular arrhythmias in patients with Takotsubo syndrome. J. Arrhythmia 2018, 34, 369–375. [Google Scholar] [CrossRef]
- Deshmukh, A.; Kumar, G.; Pant, S.; Rihal, C.; Murugiah, K.; Mehta, J.L. Prevalence of Takotsubo cardiomyopathy in the United States. Am. Hear. J. 2012, 164, 66–71. [Google Scholar] [CrossRef]
- Jesel, L.; Berthon, C.; Messas, N.; Lim, H.S.; Girardey, M.; Marzak, H.; Marchandot, B.; Trinh, A.; Ohlmann, P.; Morel, O. Atrial arrhythmias in Takotsubo cardiomyopathy: Incidence, predictive factors, and prognosis. Ep Eur. 2019, 21, 298–305. [Google Scholar] [CrossRef]
- Pelargonio, G.; La Rosa, G.; Di Stasio, E.; Narducci, M.L.; Rocco, E.; Angelini, A.; Pinnacchio, G.; Bencardino, G.; Perna, F.; Comerci, G.; et al. Ventricular arrhythmias in Takotsubo Syndrome: Incidence, predictors and clinical outcomes. J. Cardiovasc. Med. 2020, 22, 180–189. [Google Scholar] [CrossRef]
- Deo, M.; Weinberg, S.H.; Boyle, P.M. Calcium Dynamics and Cardiac Arrhythmia. Clin. Med. Insights: Cardiol. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Treu, F.; Dybkova, N.; Jung, P.; Li, Y.; Huebscher, D.; Maurer, W.; Hasenfuss, G.; Voigt, N.; Sossalla, S.; Wollnik, B.; et al. Genetic variants in calcium regulatory cardiac genes and their contribution to Takotsubo syndrome. Eur. Hear. J. 2020, 41. [Google Scholar] [CrossRef]

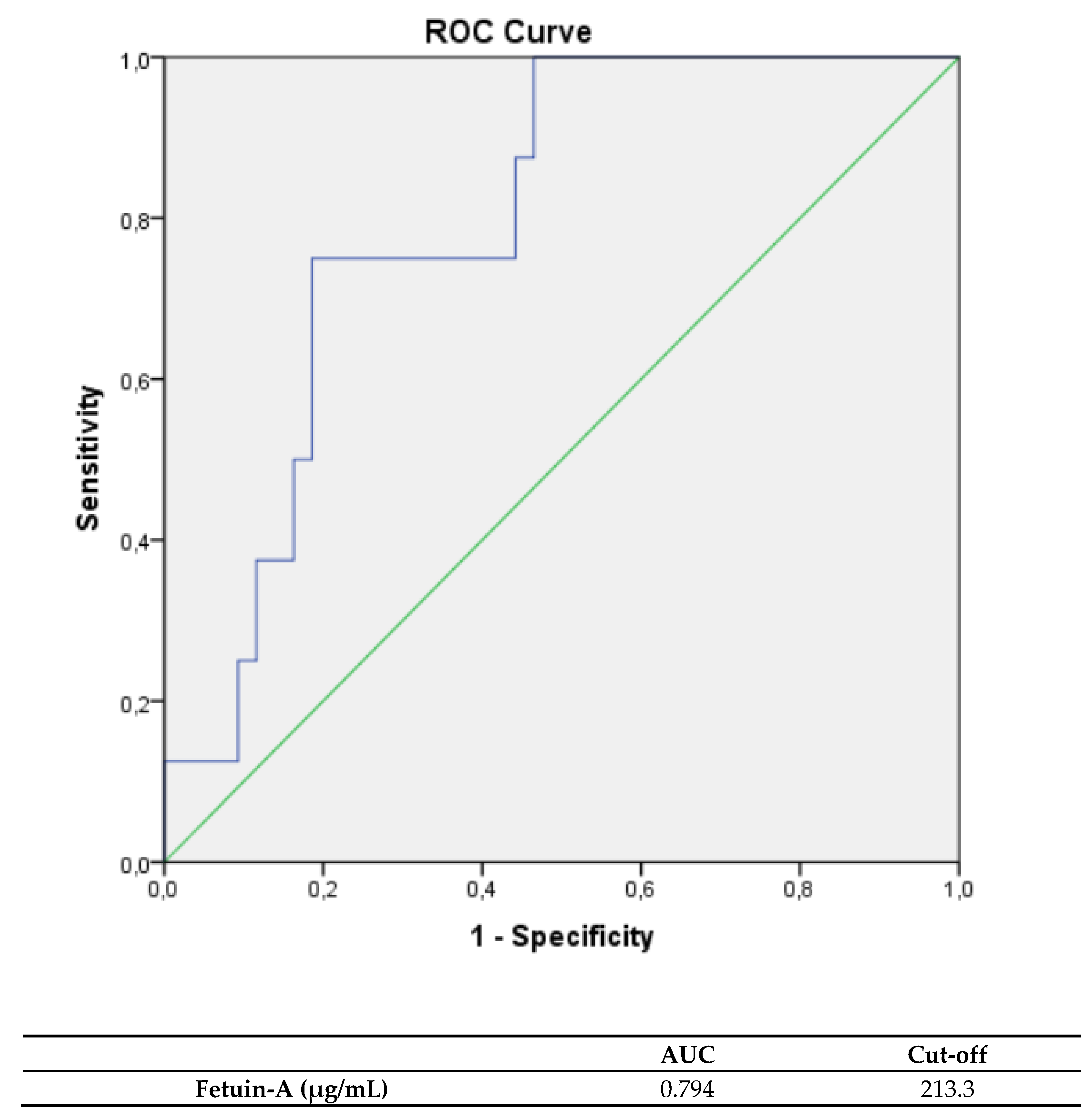
| TTC | ||
|---|---|---|
| Median | IQR | |
| Age (years) | 74.0 | 62.0–78.0 |
| BMI (kg/m2) | 24.7 | 21.8–29.2 |
| EF (%) | 40.0 | 35.0–46.0 |
| Creatinine (µmol/L) | 64.2 | 59.8–79.2 |
| LDL (mg/dL) | 90.0 | 75.0–122.0 |
| CRP (mg/L) | 0.4 | 0.2–0.9 |
| HbA1c (%) | 5.4 | 5.2–5.8 |
| QTc (ms) | 458.0 | 439–491 |
| Heart rate (bpm) | 78.0 | 71.0–90.0 |
| (hs) Troponin (pg/mL) | 162.0 | 53.0-395.0 |
| Pro-BNP (pg/mL) | 2866.0 | 664.6–4919.8 |
| Fetuin-A (µg/mL) | 203.2 | 189.3–216.5 |
| MMP-2 (ng/mL) | 111.1 | 93.9–127.5 |
| MPO (pg/mL) | 408461.5 | 189870.6–840546.0 |
| Syndecan-1 (pg/mL) | 598.3 | 387.5–911.3 |
| CD40-L (pg/mL) | 397.7 | 290.1–784.4 |
| Arrhythmia (n) | 8/51 | |
| Cardiac decompensation (n) | 19/51 | |
| All cause complications (n) | 23/51 | |
| Smoking | 15/51 (29.4%) | |
| Hypertension | 38/51 (74.5%) | |
| Sex (female) | 48/51 (94.1%) |
| Fetuin-A | CD40L | MMP-2 | Syndecan-1 | MPO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rs | p | rs | p | rs | p | rs | p | rs | p | |
| Age (y) | −0.215 | 0.130 | −0.095 | 0.506 | 0.243 | 0.086 | 0.159 | 0.264 | 0.120 | 0.367 |
| BMI (kg/m^2) | 0.049 | 0.734 | −0.197 | 0.165 | -0.168 | 0.238 | 0.065 | 0.650 | −0.032 | 0.826 |
| EF (%) | −0.091 | 0.524 | 0.053 | 0.711 | 0.119 | 0.405 | −0.116 | 0.416 | 0.113 | 0.432 |
| Heart rate (bpm) | −0.099 | 0.8 | −0.039 | 0.786 | -0.177 | 0.214 | 0.091 | 0.527 | −0.157 | 0.272 |
| QTc (ms) | 0.183 | 0.200 | −0.121 | 0.397 | 0.260 | 0.065 | 0.135 | 0.343 | −0.103 | 0.471 |
| Creatinine (µmol/L) | 0.141 | 0.325 | −0.272 | 0.054 | 0.349 | 0.012 | 0.273 | 0.053 | −0.255 | 0.112 |
| CRP (mg/dL) | −0.012 | 0.931 | −0.299 | 0.033 | 0.198 | 0.165 | 0.061 | 0.671 | −0.141 | 0.322 |
| LDL (mg/dL) | −0.040 | 0.790 | 0.070 | 0.641 | -0.271 | 0.065 | −0.027 | 0.855 | 0.032 | 0.833 |
| HbA1c (%) | 0.052 | 0.771 | −0.284 | 0.104 | 0.382 | 0.026 | −0.056 | 0.753 | −0.307 | 0.077 |
| Cardiac decompensation (n) | −0.400 | 0.004 | −0.047 | 0.744 | 0.077 | 0.591 | 0.141 | 0.325 | 0.095 | 0.507 |
| Arrhythmia (n) | 0.370 | 0.008 | −0.013 | 0.929 | 0.011 | 0.939 | −0.137 | 0.336 | 0.440 | 0.759 |
| All-cause complications (n) | −0.268 | 0.058 | −0.060 | 0.675 | 0.040 | 0.780 | 0.055 | 0.702 | 0.007 | 0.963 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topf, A.; Mirna, M.; Bacher, N.; Paar, V.; Edlinger, C.; Motloch, L.J.; Gharibeh, S.; Bannehr, M.; Hoppe, U.C.; Lichtenauer, M. The Value of Fetuin-A as a Predictor to Identify Takotsubo Patients at Risk of Cardiovascular Events. J. Cardiovasc. Dev. Dis. 2021, 8, 127. https://doi.org/10.3390/jcdd8100127
Topf A, Mirna M, Bacher N, Paar V, Edlinger C, Motloch LJ, Gharibeh S, Bannehr M, Hoppe UC, Lichtenauer M. The Value of Fetuin-A as a Predictor to Identify Takotsubo Patients at Risk of Cardiovascular Events. Journal of Cardiovascular Development and Disease. 2021; 8(10):127. https://doi.org/10.3390/jcdd8100127
Chicago/Turabian StyleTopf, Albert, Moritz Mirna, Nina Bacher, Vera Paar, Christoph Edlinger, Lukas J. Motloch, Sarah Gharibeh, Marwin Bannehr, Uta C. Hoppe, and Michael Lichtenauer. 2021. "The Value of Fetuin-A as a Predictor to Identify Takotsubo Patients at Risk of Cardiovascular Events" Journal of Cardiovascular Development and Disease 8, no. 10: 127. https://doi.org/10.3390/jcdd8100127
APA StyleTopf, A., Mirna, M., Bacher, N., Paar, V., Edlinger, C., Motloch, L. J., Gharibeh, S., Bannehr, M., Hoppe, U. C., & Lichtenauer, M. (2021). The Value of Fetuin-A as a Predictor to Identify Takotsubo Patients at Risk of Cardiovascular Events. Journal of Cardiovascular Development and Disease, 8(10), 127. https://doi.org/10.3390/jcdd8100127








