Coconuts and Health: Different Chain Lengths of Saturated Fats Require Different Consideration
Abstract
1. Introduction
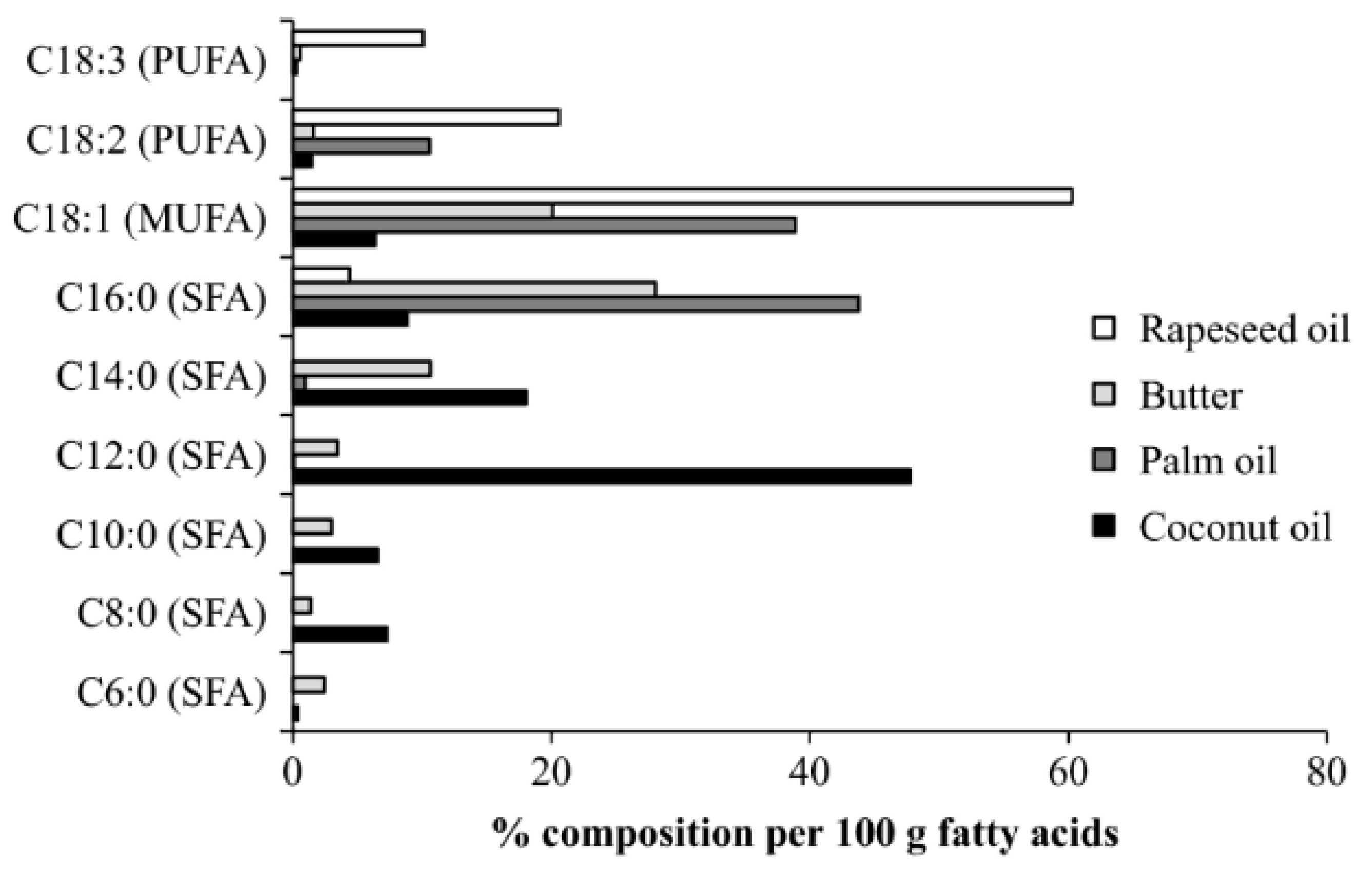
2. Coconut Oil
3. Cardiovascular Risk Factors
3.1. Saturated Fat and CVD/CHD
3.2. Type of Fat
4. Alzheimer’s Disease
5. Antimicrobial/Antiviral
6. Conclusions
Funding
Conflicts of Interest
References
- Keys, A.; Anderson, J.T.; Grande, F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet 1957, 273, 959–966. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 5, Cd011737. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ 2018, 361, k2139. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, N.; Chiu, S.; Williams, P.T.; M King, S.; Krauss, R.M. Effects of red meat, white meat, and nonmeat protein sources on atherogenic lipoprotein measures in the context of low compared with high saturated fat intake: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Krauss, R.M. All low-density lipoprotein particles are not created equal. Am. Heart Assoc. 2014, 34, 959–961. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, 563–595. [Google Scholar] [CrossRef]
- Central Committee for Medical and Community Program of the American Heart Association. Dietary fat and its relation to heart attacks and strokes. Report by the Central Committee for Medical and Community Program of the American Heart Association. JAMA 1961, 175, 389–391. [Google Scholar] [CrossRef]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, 76–99. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary linoleic acid and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Brouwer, I.A.; Mensink, R.P.; Diekman, C.; Hornstra, G. Fats in Foods: Current Evidence for Dietary Advice. Ann. Nutr. Metab. 2018, 72, 248–254. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Zhu, Y.; Tsai, M.Y.; Sun, Q.; Hinkle, S.N.; Rawal, S.; Mendola, P.; Ferrara, A.; Albert, P.S.; Zhang, C. A prospective and longitudinal study of plasma phospholipid saturated fatty acid profile in relation to cardiometabolic biomarkers and the risk of gestational diabetes. Am. J. Clin. Nutr. 2018, 107, 1017–1026. [Google Scholar] [CrossRef]
- Micha, R.; Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef]
- Boateng, L.; Ansong, R.; Owusu, W.B.; Steiner-Asiedu, M. Coconut oil and palm oil’s role in nutrition, health and national development: A review. Ghana Med. J. 2016, 50, 189–196. [Google Scholar]
- Barter, P.; Genest, J. HDL cholesterol and ASCVD risk stratification: A debate. Atherosclerosis 2019, 283, 7–12. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Medeiros, D.M. Nutrition: Real People, Real Choices; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Ascherio, A.; Colditz, G.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am. J. Clin. Nutr. 1999, 70, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated Fats Compared With Unsaturated Fats and Sources of Carbohydrates in Relation to Risk of Coronary Heart Disease: A Prospective Cohort Study. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Elevitch, C.R. Cocos nucifera (coconut). Species Profiles Pac. Isl. Agrofor. 2006, 2, 1–27. [Google Scholar]
- Krishna, A.G.; Gaurav, R.; Singh, B.A.; Kumar, P.P.; Preeti, C. Coconut oil: Chemistry, production and its applications—A review. Indian Coconut J. 2010, 53, 15–27. [Google Scholar]
- Nevin, K.; Rajamohan, T. Virgin coconut oil supplemented diet increases the antioxidant status in rats. Food Chem. 2006, 99, 260–266. [Google Scholar] [CrossRef]
- Nevin, K.; Rajamohan, T. Beneficial effects of virgin coconut oil on lipid parameters and in vitro LDL oxidation. Clin. Biochem. 2004, 37, 830–835. [Google Scholar] [CrossRef]
- Lima, E.B.; Sousa, C.N.; Meneses, L.N.; Ximenes, N.C.; Santos Junior, M.A.; Vasconcelos, G.S.; Lima, N.B.; Patrocinio, M.C.; Macedo, D.; Vasconcelos, S.M. Cocos nucifera (L.) (Arecaceae): A phytochemical and pharmacological review. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. Biol. 2015, 48, 953–964. [Google Scholar] [CrossRef]
- Nair, D. Global Scenario on Sustainable Coconut Development. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Padang, West Sumatra Province, Indonesia, 9–30 October 2018; p. 012006. [Google Scholar]
- Fernando, W.M.A.D.B.; Martins, I.J.; Goozee, K.; Brennan, C.S.; Jayasena, V.; Martins, R.N. The role of dietary coconut for the prevention and treatment of Alzheimer’s disease: Potential mechanisms of action. Br. J. Nutr. 2015, 114, 1–14. [Google Scholar] [CrossRef]
- Bach, A.; Babayan, V. Medium-chain triglycerides: An update. Am. J. Clin. Nutr. 1982, 36, 950–962. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Dayrit, F.M. Lauric acid is a medium-chain fatty acid, coconut oil is a medium-chain triglyceride. Philipp. J. Sci. 2014, 143, 157–166. [Google Scholar]
- Dayrit, F.M.; Buenafe, O.E.; Chainani, E.T.; de Vera, I.M. Analysis of monoglycerides, diglycerides, sterols, and free fatty acids in coconut (Cocos nucifera L.) oil by 31P NMR spectroscopy. J. Agric. Food Chem. 2008, 56, 5765–5769. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Stanner, S. Coconut oil—A nutty idea? Nutr. Bull. 2016, 41, 42–54. [Google Scholar] [CrossRef]
- Kannaian, U.P.N.; Edwin, J.B.; Rajagopal, V.; Shankar, S.N.; Srinivasan, B. Phytochemical composition and antioxidant activity of coconut cotyledon. Heliyon 2020, 6, 03411. [Google Scholar]
- Teng, M.; Zhao, Y.J.; Khoo, A.L.; Yeo, T.C.; Yong, Q.W.; Lim, B.P. Impact of coconut oil consumption on cardiovascular health: A systematic review and meta-analysis. Nutr. Rev. 2020, 78, 249–259. [Google Scholar] [CrossRef]
- Eyres, L.; Eyres, M.F.; Chisholm, A.; Brown, R.C. Coconut oil consumption and cardiovascular risk factors in humans. Nutr. Rev. 2016, 74, 267–280. [Google Scholar] [CrossRef]
- Bremer, J. Carnitine—metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480. [Google Scholar] [CrossRef]
- Swift, L.L.; Hill, J.; Peters, J.; Greene, H. Medium-chain fatty acids: Evidence for incorporation into chylomicron triglycerides in humans. Am. J. Clin. Nutr. 1990, 52, 834–836. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Ascherio, A.; Rimm, E.B.; Giovannucci, E.L.; Spiegelman, D.; Stampfer, M.; Willett, W.C. Dietary fat and risk of coronary heart disease in men: Cohort follow up study in the United States. BMJ 1996, 313, 84–90. [Google Scholar] [CrossRef]
- Praagman, J.; de Jonge, E.A.; Kiefte-de Jong, J.C.; Beulens, J.W.; Sluijs, I.; Schoufour, J.D.; Hofman, A.; van der Schouw, Y.T.; Franco, O.H. Dietary Saturated Fatty Acids and Coronary Heart Disease Risk in a Dutch Middle-Aged and Elderly Population. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2011–2018. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, 419–428. [Google Scholar] [CrossRef]
- Ho, F.K.; Gray, S.R.; Welsh, P.; Petermann-Rocha, F.; Foster, H.; Waddell, H.; Anderson, J.; Lyall, D.; Sattar, N.; Gill, J.M. Associations of fat and carbohydrate intake with cardiovascular disease and mortality: Prospective cohort study of UK Biobank participants. BMJ 2020, 368. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; World Health Organization. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Khaw, K.T.; Sharp, S.J.; Finikarides, L.; Afzal, I.; Lentjes, M.; Luben, R.; Forouhi, N.G. Randomised trial of coconut oil, olive oil or butter on blood lipids and other cardiovascular risk factors in healthy men and women. BMJ Open 2018, 8, 020167. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.A.; Moreira, A.S.; de Oliveira, G.M.; Raggio Luiz, R.; Rosa, G. A Coconut extra virgin oil rich diet increases HDL cholesterol and decrease waist circumference and body mass in coronary artery disease patients. Nutr. Hosp. 2015, 32, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Feranil, A.B.; Duazo, P.L.; Kuzawa, C.W.; Adair, L.S. Coconut oil is associated with a beneficial lipid profile in pre-menopausal women in the Philippines. Asia Pac. J. Clin. Nutr. 2011, 20, 190–195. [Google Scholar] [PubMed]
- Cox, C.; Mann, J.; Sutherland, W.; Chisholm, A.; Skeaff, M. Effects of coconut oil, butter, and safflower oil on lipids and lipoproteins in persons with moderately elevated cholesterol levels. J. Lipid Res. 1995, 36, 1787–1795. [Google Scholar] [CrossRef]
- Neelakantan, N.; Seah, J.Y.H.; van Dam, R.M. The Effect of Coconut Oil Consumption on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Clinical Trials. Circulation 2020, 141, 803–814. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Bogensberger, B.; Benčič, A.; Knüppel, S.; Boeing, H.; Hoffmann, G. Effects of oils and solid fats on blood lipids: A systematic review and network meta-analysis. J. Lipid Res. 2018, 59, 1771–1782. [Google Scholar] [CrossRef]
- Patterson, C. World Alzheimer Report 2018: The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International (ADI): London, UK, 2018; pp. 32–36. [Google Scholar]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef]
- Chatterjee, P.; Fernando, M.; Fernando, B.; Dias, C.B.; Shah, T.; Silva, R.; Williams, S.; Pedrini, S.; Hillebrandt, H.; Goozee, K.; et al. Potential of coconut oil and medium chain triglycerides in the prevention and treatment of Alzheimer’s disease. Mech. Ageing Dev. 2020, 186, 111209. [Google Scholar] [CrossRef]
- Newport, M. What If There Was a Cure for Alzheimer’s Disease and No One Knew?—A Case Study by Dr. Mary Newport; Foundation for Alternative and Integrative Medicine: Loveland, CO, USA, 2008. [Google Scholar]
- De la Rubia Ortí, J.E.; García-Pardo, M.P.; Drehmer, E.; Sancho Cantus, D.; Julián Rochina, M.; Aguilar, M.A.; Hu Yang, I. Improvement of main cognitive functions in patients with alzheimer’s disease after treatment with coconut oil enriched mediterranean diet: A pilot study. J. Alzheimer’s Dis. 2018, 65, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Doty, L. Coconut oil for Alzheimer’s disease. Clin. Pract. 2012, 1, 12–17. [Google Scholar]
- Taylor, M.K.; Sullivan, D.K.; Mahnken, J.D.; Burns, J.M.; Swerdlow, R.H. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Dangelo, K.; Couch, S.C.; Benoit, S.C.; Clegg, D.J. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol. Aging 2012, 33, 425.e19–425.e427. [Google Scholar] [CrossRef]
- DebMandal, M.; Mandal, S. Coconut (Cocos nucifera L.: Arecaceae): In health promotion and disease prevention. Asian Pac. J. Trop. Med. 2011, 4, 241–247. [Google Scholar] [CrossRef]
- Kabara, J.J. The Pharmacological Effect of Lipids III: Role of Lipids in Cancer Research; The American Oil Chemists Society: Urbana, IL, USA, 1989; Volume 3. [Google Scholar]
- Oyi, A.; Onaolapo, J.; Obi, R. Formulation and antimicrobial studies of coconut (Cocos nucifera Linne) oil. Res. J. Appl. Sci. Eng. Technol. 2010, 2, 133–137. [Google Scholar]
- Verma, V.; Bhardwaj, A.; Rathi, S.; Raja, R. A potential antimicrobial agent from Cocos nucifera mesocarp extract; Development of a new generation antibiotic. Int. Res. J. Biol. Sci. 2012, 1, 48–54. [Google Scholar]
- Akinyele, T.A.; Okoh, O.O.; Akinpelu, D.A.; Okoh, A.I. In-vitro antibacterial properties of crude aqueous and n-hexane extracts of the husk of Cocos nucifera. Molecules 2011, 16, 2135–2145. [Google Scholar] [CrossRef]
- Tangwatcharin, P.; Khopaibool, P. Activity of virgin coconut oil, lauric acid or monolaurin in combination with lactic acid against Staphylococcus aureus. Southeast Asian J. Trop. Med. Public Health 2012, 43, 969–985. [Google Scholar]
- Shilling, M.; Matt, L.; Rubin, E.; Visitacion, M.P.; Haller, N.A.; Grey, S.F.; Woolverton, C.J. Antimicrobial effects of virgin coconut oil and its medium-chain fatty acids on Clostridium difficile. J. Med. Food 2013, 16, 1079–1085. [Google Scholar] [CrossRef]
- Yassen, L.T.; Khelkal, I.N. Effect of some fatty acids on virulence factors of Proteus mirabilis. Int. J. Adv. Biol. Res. 2015, 5, 108–117. [Google Scholar]
- Kohli, D.; Hugar, S.M.; Bhat, K.G.; Shah, P.P.; Mundada, M.V.; Badakar, C.M. Comparative evaluation of the antimicrobial susceptibility and cytotoxicity of husk extract of Cocos nucifera and chlorhexidine as irrigating solutions against Enterococcus faecalis, Prevotella intermedia and Porphyromonas gingivalis—An in-vitro study. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Projan, S.J.; Brown-Skrobot, S.; Schlievert, P.M.; Vandenesch, F.; Novick, R.P. Glycerol monolaurate inhibits the production of beta-lactamase, toxic shock toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J. Bacteriol. 1994, 176, 4204–4209. [Google Scholar] [CrossRef] [PubMed]
| Author | Type of Study | Purpose | Included | Conclusion |
|---|---|---|---|---|
| [11] | Meta-Analysis of prospective epidemiological studies | 21 studies related to the association of dietary saturated fat with CHD, stroke, CVD; CHD inclusive of stroke. | 5–23 years of follow-up of 347,747 subjects | No significant evidence that saturated fat is associated with increased risk of CHD or CVD |
| [41] | Epidemiological Cohort | Associations between consumption of carbohydrate, total fat, and each type of fat with cardiovascular disease and total mortality. | Dietary intake of 135,335 individuals aged 35–70 years, in 18 countries; median follow-up 74 years, | ↑ Carbohydrate intake associated with ↑ risk of total mortality; total fat and types of fat related to ↓ total mortality. Total fat and types of fat not associated with CVD, myocardial infarction, or cardiovascular disease mortality, saturated fat had an inverse association with stroke. |
| [22] | Prospective Cohort | Associations between intakes of individual SFAs and their food sources in relation to the risk of CHD. | 80,082 women aged 34–59 Nurse’s Health Study; 14-year follow up | Short- to medium-chain fats not associated with CHD risk; intake of longer chain = ↑ risk; ratio of PUFA to SFA inversely associated with CHD risk. |
| [14] | Meta-Analysis of observational studies | Associations between intake of saturated fat and trans unsaturated fat and all cause mortality, CVD and associated mortality, CHD and associated mortality, ischemic stroke, and type 2 diabetes. | 41 studies on saturated fats and health outcomes in prospective cohort studies published between 1981 and 2014; 67 data points; 20 studies with 28 data points for trans fats | Saturated fats are not associated with all cause mortality, CVD, CHD, ischemic stroke, or type 2 diabetes, but the evidence is heterogeneous with methodological limitations. Trans fats are associated with all cause mortality, total CHD, and CHD mortality. |
| [23] | Prospective Cohort | Associations of SFAs compared to PUFAs and carbohydrates to CHD risk. | 84,628 women 42,908 men 24–30 year follow up | ↑ Intake of PUFAs and whole grains = ↓ risk of CHD; replacing 5% of energy intake from SFAs with PUFAs, MUFAs, or carbohydrates from whole grains was associated with a 25%, 15%, and 9% ↓ risk of CHD. |
| [42] | Prospective Cohort | Association between fat intake and CHD. | 43,757 men aged 40 to 75 years; 6 year follow up | SFA intake not associated with CHD once corrected for fiber intake; ↑ linolenic acid = ↓ risk CHD. |
| [43] | Prospective Cohort | Association between dietary SFA and CHD depends on the food source, the carbon chain length of SFA, and the substituting macronutrient. | 4722 Dutch men and women > 55 years; 16.3 year follow up | ↑ Intake of palmitic acid ↑ risk; other SFAs are not associated with CHD; no effect of food source of SFA; replacement of SFA with animal protein ↑ risk; replacement with other macronutrients not associated with risk. |
| [44] | Prospective Cohort and Meta-Analysis | Association between carbohydrate intake and mortality. | 15,428 aged 55–64 years ARIC study; 25 year follow up | U-shaped relationship between carbohydrate intake and mortality; low carbohydrate intake with animal protein increased risk, while with high plant protein decreased risk. |
| [45] | Prospective population study | Association of macronutrient intake with all cause mortality and CVD, and the implications for dietary advice. | 195,658 adults 10.6 year follow up | Carbohydrate intake > 50% ↑ association with mortality; ↑ intake of MUFA, ↓ intake of PUFA, ↓ intake of SFA = ↓ risk of mortality. |
| [46] | Systematic Review and Meta-Analysis of RCTs | Assess the impact of phytosterol (PS) supplementation on serum Lp(a) and FFA concentration. | 12 effect sizes from 7 different studies | PS supplementation = ↓ in Lp(a) and FFA. |
| [37] | Systematic Review and Meta-Analysis | Examine the evidence surrounding coconut oil consumption and its impact on cardiovascular health. | 12 studies | Compared with plant oils and animal oils, coconut oil ↑ HDL-C by 0.57 mg/dL and 0.33 mg/dL. Coconut oil significantly ↑ LDL-C by 0.26 mg/dL compared with plant oils and ↓ LDL-C (48.1%) compared with animal oils. No effects on triglyceride. Better lipid profiles were demonstrated with the virgin form of coconut oil. |
| [49] | Prospective Cohort | Association between coconut oil intake and plasma lipid profiles. | 1896 Filipino women aged 35–69 years | In pre-menopausal women, dietary coconut oil use was associated with TC and HDL-C, not in post-menopausal women; coconut oil did not elevate TC, triglyceride levels, and TC/HDL. |
| [47] | RCT | Compare changes in blood lipid profile, weight, fat distribution, and metabolic markers after four weeks of consumption of 50 g daily of extra virgin coconut oil, butter, or extra virgin olive oil. | 91 men and women | LDL-C significantly increased on butter compared with coconut oil and with olive oil, no differences in change of LDL-C in coconut oil compared with olive oil. Coconut oil significantly increased HDL-C compared with butter or olive oil. Butter significantly increased TC/HDL-C ratio and non-HDL-C compared with coconut oil, while coconut oil did not significantly differ from olive oil for TC/HDL-C and non-HDL-C. No significant differences in changes in weight, BMI, central adiposity, fasting blood glucose, and systolic or diastolic blood pressure in any group. |
| [50] | RCT cross over | Compare the effects of coconut oil, butter, and safflower oil on lipids and lipoproteins of moderately hypercholesterolemic subjects. | 13 men and 15 women with a plasma total cholesterol between 5.5 and 7.9 mmol/L and plasma triacylglycerols (TAG) less than 3 mmol/L consumed 50% of fat from butter, coconut oil, or safflower oil | Coconut oil and butter diets increased TC and LDL-C compared to safflower oil; the levels of both were significantly lower in the coconut oil than on the butter diet. |
| [51] | Systematic Review and Meta-Analysis | A systematic review of the effect of coconut oil consumption on blood lipids and other cardiovascular risk factors compared with other cooking oils using data from clinical trials. | 16 articles included in the meta-analysis | Coconut oil increased LDL-cholesterol by 10.47 mg/dL and HDL-cholesterol by 4.00 mg/dL compared with nontropical vegetable oils. Coconut oil consumption did not significantly affect markers of glycemia, inflammation, and adiposity as compared with nontropical vegetable oils. |
| [52] | Systematic Review and Network meta-Analysis | Compare the effects of different oils/solid fats on blood lipids. | 54 RCTs 2065 subjects included | Safflower oil ↓ in TC and LDL-C the most, followed by rapeseed oil and sunflower oil; soybean oil was the most effective oil to ↓ TG, followed by corn oil and palm oil; butter and lard were ranked worst for TC and LDL-C reduction; coconut oil was ranked best to ↑ HDL-C, followed by palm oil and beef fat. The NMA showed that all vegetable oils were more effective in reducing TC and LDL-C compared with butter. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hewlings, S. Coconuts and Health: Different Chain Lengths of Saturated Fats Require Different Consideration. J. Cardiovasc. Dev. Dis. 2020, 7, 59. https://doi.org/10.3390/jcdd7040059
Hewlings S. Coconuts and Health: Different Chain Lengths of Saturated Fats Require Different Consideration. Journal of Cardiovascular Development and Disease. 2020; 7(4):59. https://doi.org/10.3390/jcdd7040059
Chicago/Turabian StyleHewlings, Susan. 2020. "Coconuts and Health: Different Chain Lengths of Saturated Fats Require Different Consideration" Journal of Cardiovascular Development and Disease 7, no. 4: 59. https://doi.org/10.3390/jcdd7040059
APA StyleHewlings, S. (2020). Coconuts and Health: Different Chain Lengths of Saturated Fats Require Different Consideration. Journal of Cardiovascular Development and Disease, 7(4), 59. https://doi.org/10.3390/jcdd7040059





