Venous Hemodynamic Dysfunction and Recurrent Miscarriage: Case Series and Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
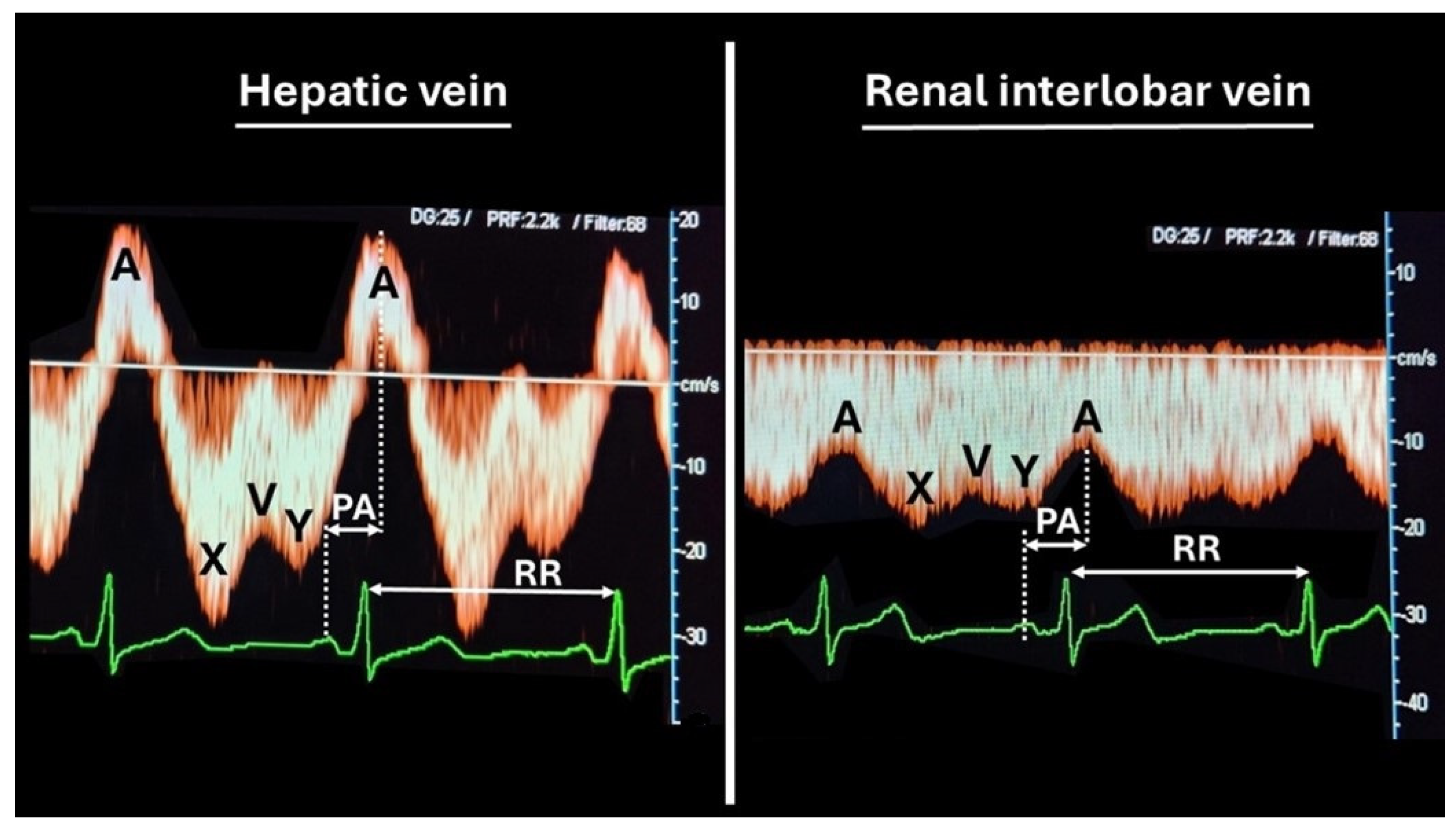
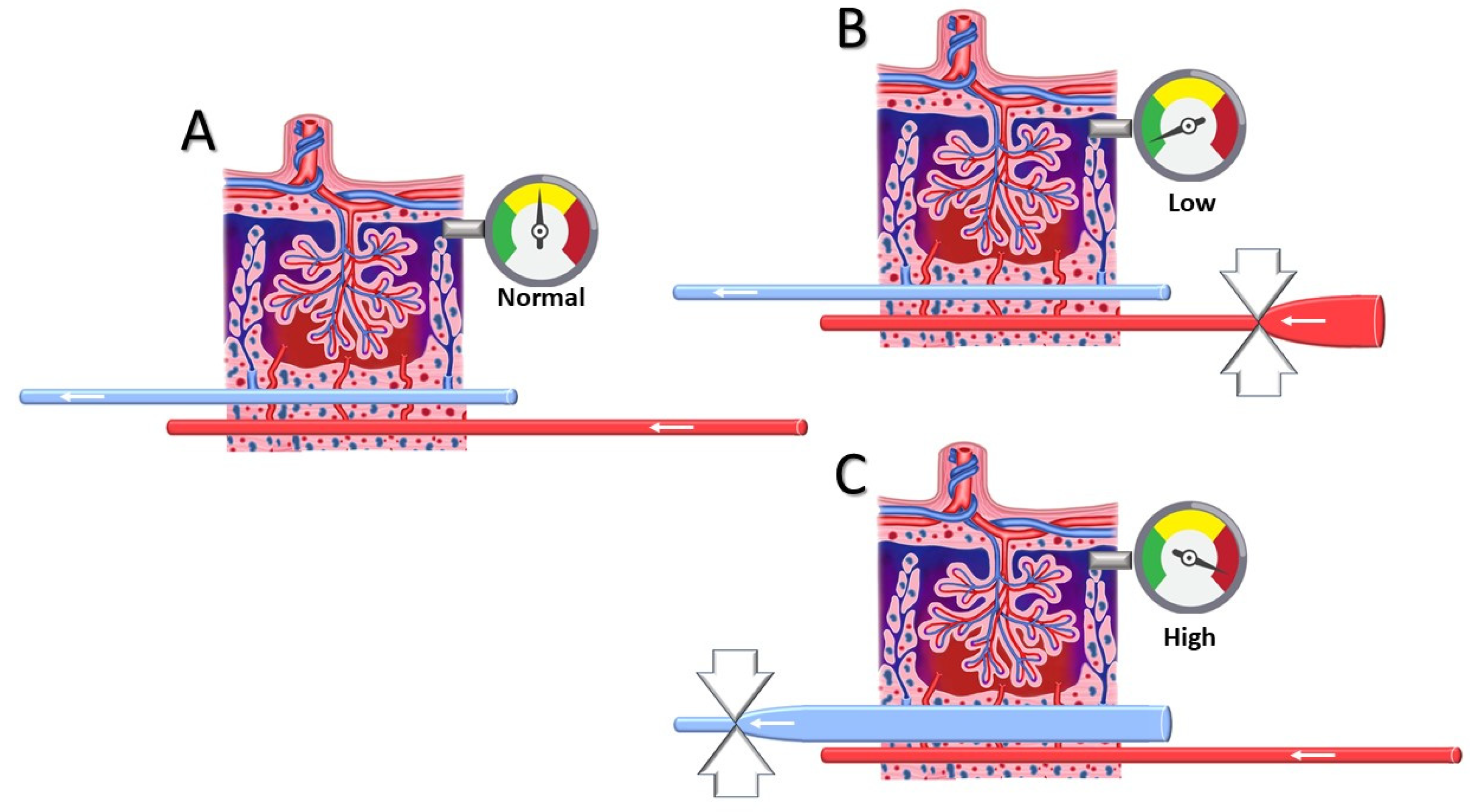
2.2. Venous Doppler Sonography
2.3. Literature Search
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ESHRE Guideline Group on RPL; Bender Atik, R.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Mcheik, S.; Peramo, B.; Quenby, S.; et al. ESHRE guideline: Recurrent pregnancy loss: An update in 2022. Hum. Reprod. Open 2023, 2023, hoad002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Habets, D.H.J.; Schiffer, V.M.M.M.; Kraneburg, L.P.A.; de Krom, F.J.W.; Gürtekin, I.; van Bree, B.E.; van Golde, R.J.T.; Wieten, L.; Spaanderman, M.E.A.; Al-Nasiry, S. Preconceptional evaluation of women with recurrent pregnancy loss: The additional value of assessing vascular and metabolic status. BMC Pregnancy Childbirth 2022, 22, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donckers, J.; Scholten, R.R.; Oyen, W.J.; Hopman, M.T.; Lotgering, F.K.; Spaanderman, M.E. Unexplained first trimester recurrent pregnancy loss and low venous reserves. Hum. Reprod. 2012, 27, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- Habara, T.; Nakatsuka, M.; Konishi, H.; Asagiri, K.; Noguchi, S.; Kudo, T. Elevated blood flow resistance in uterine arteries of women with unexplained recurrent pregnancy loss. Hum. Reprod. 2002, 17, 190–194. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Pires, C.R.; Moron, A.F.; Araujo, J.E.; Traina, E.; Mattar, R. Doppler assessment of uterine blood flow in recurrent pregnancy loss. Int. J. Gynaecol. Obstet. 2007, 98, 115–119. [Google Scholar] [CrossRef]
- Gyselaers, W.; Peeters, L. Physiological implications of arteriovenous anastomoses and venous hemodynamic dysfunction in early gestational uterine circulation: A review. J. Matern. Fetal Neonatal Med. 2013, 26, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Mesens, T.; Tomsin, K.; Oben, J.; Staelens, A.; Gyselaers, W. Maternal venous hemodynamics assessment for prediction of preeclampsia should be longitudinal. J. Matern. Fetal Neonatal Med. 2014, 28, 311–315. [Google Scholar] [CrossRef]
- Gyselaers, W. Hemodynamic pathways of gestational hypertension and preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S988–S1005. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2012, 98, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Gyselaers, W.; Staelens, A.; Mesens, T.; Tomsin, K.; Oben, J.; Vonck, S.; Verresen, L.; Molenberghs, G. Maternal venous Doppler characteristics are abnormal in pre-eclampsia but not in gestational hypertension. Ultrasound Obstet. Gynecol. 2015, 45, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Gyselaers, W. Maternal Venous Hemodynamic Dysfunction in Proteinuric Gestational Hypertension: Evidence and Implications. J. Clin. Med. 2019, 8, 335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gyselaers, W.; Vonck, S.; Staelens, A.S.; Lanssens, D.; Tomsin, K.; Oben, J.; Dreesen, P.; Bruckers, L. Gestational hypertensive disorders show unique patterns of circulatory deterioration with ongoing pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R210–R221. [Google Scholar] [CrossRef] [PubMed]
- Gyselaers, W.; Vonck, S.; Staelens, A.S.; Lanssens, D.; Tomsin, K.; Oben, J.; Dreesen, P.; Bruckers, L. Body fluid volume homeostasis is abnormal in pregnancies complicated with hypertension and/or poor fetal growth. PLoS ONE 2018, 13, e0206257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomsin, K.; Vriens, A.; Mesens, T.; Gyselaers, W. Non-invasive cardiovascular profiling using combined electrocardiogram-Doppler ultrasonography and impedance cardiography: An experimental approach. Clin. Exp. Pharmacol. Physiol. 2013, 40, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Goemaes, R.; Fomenko, E.; Laubach, M.; De Coen, K.; Roelens, K.; Bogaerts, A. Perinatale gezondheid in Vlaanderen—Jaar 2023; Studiecentrum voor Perinatale Epidemiologie: Brussels, Belgium, 2024. [Google Scholar]
- Finan, R.R.; Tamim, H.; Ameen, G.; Sharida, H.E.; Rashid, M.; Almawi, W.Y. Prevalence of factor V G1691A (factor V-Leiden) and prothrombin G20210A gene mutations in a recurrent miscarriage population. Am. J. Hematol. 2002, 71, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Pranikoff, J.; Aregawi, D.; Haque, M.; Zhu, B.; Tracy, T.; Wang, P. The factor V Leiden mutation, high factor VIII, and high plasminogen activator inhibitor activity: Etiologies for sporadic miscarriage. Metabolism 2005, 54, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Aardenburg, R.; Spaanderman, M.E.; Courtar, D.A.; van Eijndhoven, H.W.; de Leeuw, P.W.; Peeters, L.L. A subnormal plasma volume in formerly preeclamptic women is associated with a low venous capacitance. J. Soc. Gynecol. Investig. 2005, 12, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Krabbendam, I.; Janssen, B.J.; Van Dijk, A.P.; Jongsma, H.W.; Oyen, W.J.; Lotgering, F.K.; Spaanderman, M.E. The relation between venous reserve capacity and low plasma volume. Reprod. Sci. 2008, 15, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Krabbendam, I.; Courtar, D.A.; Janssen, B.J.; Aardenburg, R.; Peeters, L.L.; Spaanderman, M.E. Blunted autonomic response to volume expansion in formerly preeclamptic women with low plasma volume. Reprod. Sci. 2009, 16, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Aardenburg, R.; Spaanderman, M.E.; van Eijndhoven, H.W.; de Leeuw, P.W.; Peeters, L.L. Formerly preeclamptic women with a subnormal plasma volume are unable to maintain a rise in stroke volume during moderate exercise. J. Soc. Gynecol. Investig. 2005, 12, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Aardenburg, R.; Spaanderman, M.E.; van Eijndhoven, H.W.; de Leeuw, P.W.; Peeters, L.L. A low plasma volume in formerly preeclamptic women predisposes to the recurrence of hypertensive complications in the next pregnancy. J. Soc. Gynecol. Investig. 2006, 13, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Spaanderman, M.E.; Willekes, C.; Hoeks, A.P.; Ekhart, T.H.; Aardenburg, R.; Courtar, D.A.; Van Eijndhoven, H.W.; Peeters, L.L. Maternal nonpregnant vascular function correlates with subsequent fetal growth. Am. J. Obstet. Gynecol. 2005, 192, 504–512. [Google Scholar] [CrossRef] [PubMed]
- He, N.; van Iperen, L.; de Jong, D.; Szuhai, K.; Helmerhorst, F.M.; van der Westerlaken, L.A.; Chuva de Sousa Lopes, S.M. Human Extravillous Trophoblasts Penetrate Decidual Veins and Lymphatics Before Remodeling Spiral Arteries During Early Pregnancy. PLoS ONE 2017, 12, e0169849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moser, G.; Weiss, G.; Sundl, M.; Gauster, M.; Siwetz, M.; Lang-Olip, I.; Huppertz, B. Extravillous trophoblasts invade more than uterine arteries: Evidence for the invasion of uterine veins. Histochem. Cell Biol. 2017, 147, 353–366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Windsperger, K.; Dekan, S.; Pils, S.; Golletz, C.; Kunihs, V.; Fiala, C.; Kristiansen, G.; Knöfler, M.; Pollheimer, J. Extravillous trophoblast invasion of venous as well as lymphatic vessels is altered in idiopathic, recurrent, spontaneous abortions. Hum. Reprod. 2017, 32, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B. Traditional and New Routes of Trophoblast Invasion and Their Implications for Pregnancy Diseases. Int. J. Mol. Sci. 2019, 21, 289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gyselaers, W. Origins of abnormal placentation: Why maternal veins must not be forgotten. Am. J. Obstet. Gynecol. 2023, 228, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Moffett, A. Trophoblast remodeling of the uterine veins and maternal placental blood flow. Am. J. Obstet. Gynecol. 2023, 229, 704–705. [Google Scholar] [CrossRef] [PubMed]
- Gyselaers, W. For drainage of decidual blood, the maternal venous compartment operates as 1 functional unit with the heart and the microcirculation: A response. Am. J. Obstet. Gynecol. 2023, 229, 705. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, E.S.; Brownbill, P.; Jones, N.W.; Abrahams, V.M.; Baker, P.N.; Sibley, C.P.; Crocker, I.P. Utero-placental haemodynamics in the pathogenesis of pre-eclampsia. Placenta 2009, 30, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, L.; Han, X. The Effect of a Subsequent Pregnancy After Ovarian Vein Embolization in Patients with Infertility Caused by Pelvic Congestion Syndrome. Acad. Radiol. 2019, 26, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Ticconi, C.; Pietropolli, A.; Specchia, M.; Nicastri, E.; Chiaramonte, C.; Piccione, E.; Scambia, G.; Di Simone, N. Pregnancy-Related Complications in Women with Recurrent Pregnancy Loss: A Prospective Cohort Study. J. Clin. Med. 2020, 9, 2833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Liu, X.; Rao, L.; Ma, R.; Wu, W.; Chen, C.; Lin, Y. Adverse obstetric and perinatal outcomes of patients with history of recurrent miscarriage: A retrospective cohort study. Fertil. Steril. 2023, 120 Pt 2, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Townend, J.; Shetty, A.; Campbell, D.; Bhattacharya, S. Does miscarriage in an initial pregnancy lead to adverse obstetric and perinatal outcomes in the next continuing pregnancy? BJOG 2008, 115, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- van Oppenraaij, R.H.; Jauniaux, E.; Christiansen, O.B.; Horcajadas, J.A.; Farquharson, R.G.; Exalto, N.; ESHRE Special Interest Group for Early Pregnancy (SIGEP). Predicting adverse obstetric outcome after early pregnancy events and complications: A review. Hum. Reprod. Update 2009, 15, 409–421. [Google Scholar] [CrossRef] [PubMed]
- McNestry, C.; Killeen, S.L.; Crowley, R.K.; McAuliffe, F.M. Pregnancy complications and later life women’s health. Acta Obstet. Gynecol. Scand. 2023, 102, 523–531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peters, S.A.E.; Yang, L.; Guo, Y.; Chen, Y.; Bian, Z.; Tian, X.; Chang, L.; Zhang, S.; Liu, J.; Wang, T.; et al. Pregnancy, pregnancy loss, and the risk of cardiovascular disease in Chinese women: Findings from the China Kadoorie Biobank. BMC Med. 2017, 15, 148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asgharvahedi, F.; Gholizadeh, L.; Siabani, S. The risk of cardiovascular disease in women with a history of miscarriage and/or stillbirth. Health Care Women Int. 2019, 40, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Dreesen, P.; Volders, P.; Lanssens, D.; Nouwen, S.; Vrancken, B.; Janssen, F.; Eijnde, B.O.; Hansen, D.; Ceulemans, M.; Soubry, A.; et al. Preconception Physical Exercise Is Associated with Phenotype-Specific Cardiovascular Alterations in Women at Risk for Gestational Hypertensive Disorders. J. Clin. Med. 2024, 13, 4164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zoller, D.; Lüttgenau, J.; Steffen, S.; Bollwein, H. The effect of isosorbide dinitrate on uterine and ovarian blood flow in cycling and early pregnant mares: A pilot study. Theriogenology 2016, 85, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
| Population (n = 9) | |
|---|---|
| Age | 35 (28.5–37.5) |
| <30 | 1 (11.1%) |
| 30–35 | 4 (44.4%) |
| >35 | 4 (44.4%) |
| BMI | 26.6 (21.75–28.75) |
| 20–25 | 4 (44.4%) |
| 25–30 | 4 (44.4%) |
| >30 | 1 (11.1%) |
| Previous medical history | |
| Diabetes mellitus | 0 (0%) |
| Autoimmune hypothyroidism | 1 (11.1%) |
| Chronic hypertension | 1 (11.1%) |
| PID | 1 (11.1%) |
| Maternal low birth weight | 4 (44.4%) |
| Previous pregnancy complications | |
| Pre-eclampsia | 1 (11.1%) |
| Fetal growth restriction | 2 (22.22%) |
| IUFD | 2 (22.22%) |
| Investigation | |
| Negative | 6 (66.66%) |
| Thrombophilia | 2 (22.22%) |
| Refused | 1 (11.1%) |
| Liver | Right Kidney | Left Kidney | Result | ||||
|---|---|---|---|---|---|---|---|
| HVI | VPTT | RIVI | VPTT | RIVI | VPTT | ||
| Reference p25–p75 | 1.11–1.57 | 0.14–0.23 | 0.32–0.44 | 0.22–0.36 | 0.33–0.42 | 0.23–0.36 | |
| Patient 1 | 1.38 | 0.116 | 0.499 | 0.157 | 0.457 | 0.202 | Abnormal |
| Patient 2 | 0.955 | 0.36 | 0.258 | 0.398 | 0.327 | 0.438 | |
| Patient 3 | 1.527 | 0.175 | 0.569 | 0.182 | 0.289 | 0.331 | Abnormal |
| Patient 4 | 0.408 | 0.325 | 0.351 | 0.434 | 0.305 | 0.395 | |
| Patient 5 | 0.576 | 0.272 | 0.542 | 0.226 | 0.271 | 0.473 | Abnormal |
| Patient 6 | 1.279 | 0.088 | 0.566 | 0.076 | 0.572 | 0.126 | Abnormal |
| Liver | Right Kidney | Left Kidney | Result | ||||
|---|---|---|---|---|---|---|---|
| HVI | VPTT | RIVI | VPTT | RIVI | VPTT | ||
| Reference p25–p75 | 1.11–1.57 | 0.14–0.23 | 0.32–0.44 | 0.22–0.36 | 0.33–0.42 | 0.23–0.36 | |
| Patient 7 | 1.261 | 0.212 | 0.41 | 0.443 | 0.274 | 0.499 | |
| Patient 8 | 1.487 | 0.119 | 0.519 | 0.185 | 0.436 | 0.285 | Abnormal |
| Patient 9 | 1.461 | 0.151 | 0.498 | 0.132 | 0.476 | 0.239 | Abnormal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabattini, E.; Van Kerrebroeck, H.; Gyselaers, W. Venous Hemodynamic Dysfunction and Recurrent Miscarriage: Case Series and Literature Review. J. Cardiovasc. Dev. Dis. 2025, 12, 193. https://doi.org/10.3390/jcdd12050193
Sabattini E, Van Kerrebroeck H, Gyselaers W. Venous Hemodynamic Dysfunction and Recurrent Miscarriage: Case Series and Literature Review. Journal of Cardiovascular Development and Disease. 2025; 12(5):193. https://doi.org/10.3390/jcdd12050193
Chicago/Turabian StyleSabattini, Elisa, Helena Van Kerrebroeck, and Wilfried Gyselaers. 2025. "Venous Hemodynamic Dysfunction and Recurrent Miscarriage: Case Series and Literature Review" Journal of Cardiovascular Development and Disease 12, no. 5: 193. https://doi.org/10.3390/jcdd12050193
APA StyleSabattini, E., Van Kerrebroeck, H., & Gyselaers, W. (2025). Venous Hemodynamic Dysfunction and Recurrent Miscarriage: Case Series and Literature Review. Journal of Cardiovascular Development and Disease, 12(5), 193. https://doi.org/10.3390/jcdd12050193






