Percutaneous Coronary Intervention for Left Main Disease in High Bleeding Risk: Outcomes from a Subanalysis of the Delta 2 Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definitions and Endpoints
2.3. Statistical Analysis
3. Results
4. Discussion
- The prevalence of HBR is high among patients undergoing LM PCI.
- HBR patients experienced worse in-hospital clinical outcomes (all-cause mortality, myocardial infarction, and acute kidney injury), whereas the incidence of procedural outcomes (TLR, TVR, definite or probable stent thrombosis) was low and comparable between the two groups.
- The composite primary study objective occurred three times more frequently in the HBR population and was mainly driven by all-cause death according to adjusted Cox regression analysis; the procedural outcomes were durable and comparable between the two populations.
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Alasnag, M.; Yaqoub, L.; Saati, A.; Al-Shaibi, K. Left Main Coronary Artery Interventions. Interv. Cardiol. Rev. 2019, 14, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Chieffo, A.; Meliga, E.; Latib, A.; Park, S.-J.; Onuma, Y.; Capranzano, P.; Valgimigli, M.; Jegere, S.; Makkar, R.R.; Palacios, I.F.; et al. Drug-eluting stent for left main coronary artery disease. The DELTA registry: A multicenter registry evaluating percutaneous coronary intervention versus coronary artery bypass grafting for left main treatment. JACC Cardiovasc. Interv. 2012, 5, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.A.; Tamez, H.; Abbott, J.D.; Moussa, I.D.; Messenger, J.C.; Waldo, S.W.; Kennedy, K.F.; Masoudi, F.A.; Yeh, R.W. Contemporary Use and Trends in Unprotected Left Main Coronary Artery Percutaneous Coronary Intervention in the United States: An Analysis of the National Cardiovascular Data Registry Research to Practice Initiative. JAMA Cardiol. 2019, 4, 100–109. [Google Scholar] [CrossRef]
- Brennan, J.M.; Dai, D.; Patel, M.R.; Rao, S.V.; Armstrong, E.J.; Messenger, J.C.; Curtis, J.P.; Shunk, K.A.; Anstrom, K.J.; Eisenstein, E.L.; et al. Characteristics and long-term outcomes of percutaneous revascularization of unprotected left main coronary artery stenosis in the United States: A report from the National Cardiovascular Data Registry, 2004 to 2008. J. Am. Coll. Cardiol. 2012, 59, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef]
- Valgimigli, M.; Costa, F.; Lokhnygina, Y.; Clare, R.M.; Wallentin, L.; Moliterno, D.J.; Armstrong, P.W.; White, H.D.; Held, C.; Aylward, P.E.; et al. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: Lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur. Heart J. 2017, 38, 804–810. [Google Scholar] [CrossRef]
- Urban, P.; Mehran, R.; Colleran, R.; Angiolillo, D.J.; Byrne, R.A.; Capodanno, D.; Cuisset, T.; Cutlip, D.; Eerdmans, P.; Eikelboom, J.; et al. Defining High Bleeding Risk in Patients Undergoing Percutaneous Coronary Intervention. Circulation 2019, 140, 240–261. [Google Scholar] [CrossRef]
- Chiarito, M.; Kini, A.; Roumeliotis, A.; Cao, D.; Power, D.; Sartori, S.; Reisman, A.; Zhang, Z.; Mtisi, T.; Nicolas, J.; et al. Prevalence and Impact of High Bleeding Risk in Patients Undergoing Left Main Artery Disease PCI. JACC Cardiovasc. Interv. 2021, 14, 2447–2457. [Google Scholar] [CrossRef]
- Kang, J.; Yun, J.; Park, K.W.; Park, M.; Park, S.; Hwang, D.; Han, J.-K.; Yang, H.-M.; Kang, H.-J.; Koo, B.-K.; et al. Long-term outcomes of high bleeding risk patients undergoing percutaneous coronary intervention: A Korean nationwide registry. Eur. Heart J. 2024, 45, 3721–3731. [Google Scholar] [CrossRef]
- Kim, A.M.; Park, J.H.; Cho, S.; Kang, S.; Yoon, T.H.; Kim, Y. Factors associated with the rates of coronary artery bypass graft and percutaneous coronary intervention. BMC Cardiovasc. Disord. 2019, 19, 275. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Tanaka, A.; Erglis, A.; Morice, M.C.; Van Mieghem, N.M.; Meliga, E.; D’ascenzo, F.; Stefanini, G.G.; Capodanno, D.; Chieffo, A. New-Generation Drug-Eluting Stents for Left Main In-Stent Restenosis: The DELTA-2 Registry. JACC Cardiovasc. Interv. 2018, 11, 2438–2440. [Google Scholar] [CrossRef]
- Bovill, E.G.; Terrin, M.L.; Stump, D.C.; Berke, A.D.; Frederick, M.; Collen, D.; Feit, F.; Gore, J.M.; Hillis, L.D.; Lambrew, C.T.; et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI), Phase II Trial. Ann. Intern. Med. 1991, 115, 256–265. [Google Scholar] [CrossRef]
- Cutlip, D.E.; Windecker, S.; Mehran, R.; Boam, A.; Cohen, D.J.; van Es, G.-A.; Steg, P.G.; Morel, M.-A.; Mauri, L.; Vranckx, P.; et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 2007, 115, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Morice, M.-C.; Serruys, P.W.; Kappetein, A.P.; Feldman, T.E.; Ståhle, E.; Colombo, A.; Mack, M.J.; Holmes, D.R.; Choi, J.W.; Ruzyllo, W.; et al. Five-year outcomes in patients with left main disease treated with either percutaneous coronary intervention or coronary artery bypass grafting in the synergy between percutaneous coronary intervention with taxus and cardiac surgery trial. Circulation 2014, 129, 2388–2394. [Google Scholar] [CrossRef]
- Stone, G.W.; Kappetein, A.P.; Sabik, J.F.; Pocock, S.J.; Morice, M.-C.; Puskas, J.; Kandzari, D.E.; Karmpaliotis, D.; Brown, W.M.; Lembo, N.J.; et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N. Engl. J. Med. 2019, 381, 1820–1830. [Google Scholar] [CrossRef]
- Ong, A.T.; Serruys, P.W.; Mohr, F.W.; Morice, M.-C.; Kappetein, A.P.; Holmes, D.R.; Mack, M.J.; Brand, M.v.D.; Morel, M.-A.; van Es, G.-A.; et al. The SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) study: Design, rationale, and run-in phase. Am. Heart J. 2006, 151, 1194–1204. [Google Scholar] [CrossRef]
- Stone, G.W.; Sabik, J.F.; Serruys, P.W.; Simonton, C.A.; Généreux, P.; Puskas, J.; Kandzari, D.E.; Morice, M.-C.; Lembo, N.; Brown, W.M.I.; et al. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N. Engl. J. Med. 2016, 375, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Alaour, B.; Onwordi, E.; Khan, A.; Menexi, C.; Carta, S.; Strike, P.; Griffiths, H.; Anantharam, B.; Hobson, A.; Dana, A. Outcome of left main stem percutaneous coronary intervention in a UK nonsurgical center: A 5-year clinical experience. Catheter. Cardiovasc. Interv. 2022, 99, 601–606. [Google Scholar] [CrossRef]
- Chandrasekhar, J.; Baber, U.; Sartori, S.; Aquino, M.; Tomey, M.; Kruckoff, M.; Moliterno, D.; Henry, T.D.; Weisz, G.; Gibson, C.M.; et al. Patterns and associations between DAPT cessation and 2-year clinical outcomes in left main/proximal LAD versus other PCI: Results from the Patterns of Non-Adherence to Dual Antiplatelet Therapy in Stented Patients (PARIS) registry. Int. J. Cardiol. 2017, 243, 132–139. [Google Scholar] [CrossRef]
- Chang, C.C.; Onuma, Y.; Lesiak, M.; Merkulov, E.; Anderson, R.; Kretov, E.; Barragan, P.; Oldroyd, K.G.; van Geuns, R.-J. Optical Coherence Tomography Assessment for Percutaneous Coronary Intervention of the Left Main Artery: The IDEAL-LM Trial. JACC Cardiovasc. Interv. 2020, 13, 401–402. [Google Scholar] [CrossRef] [PubMed]
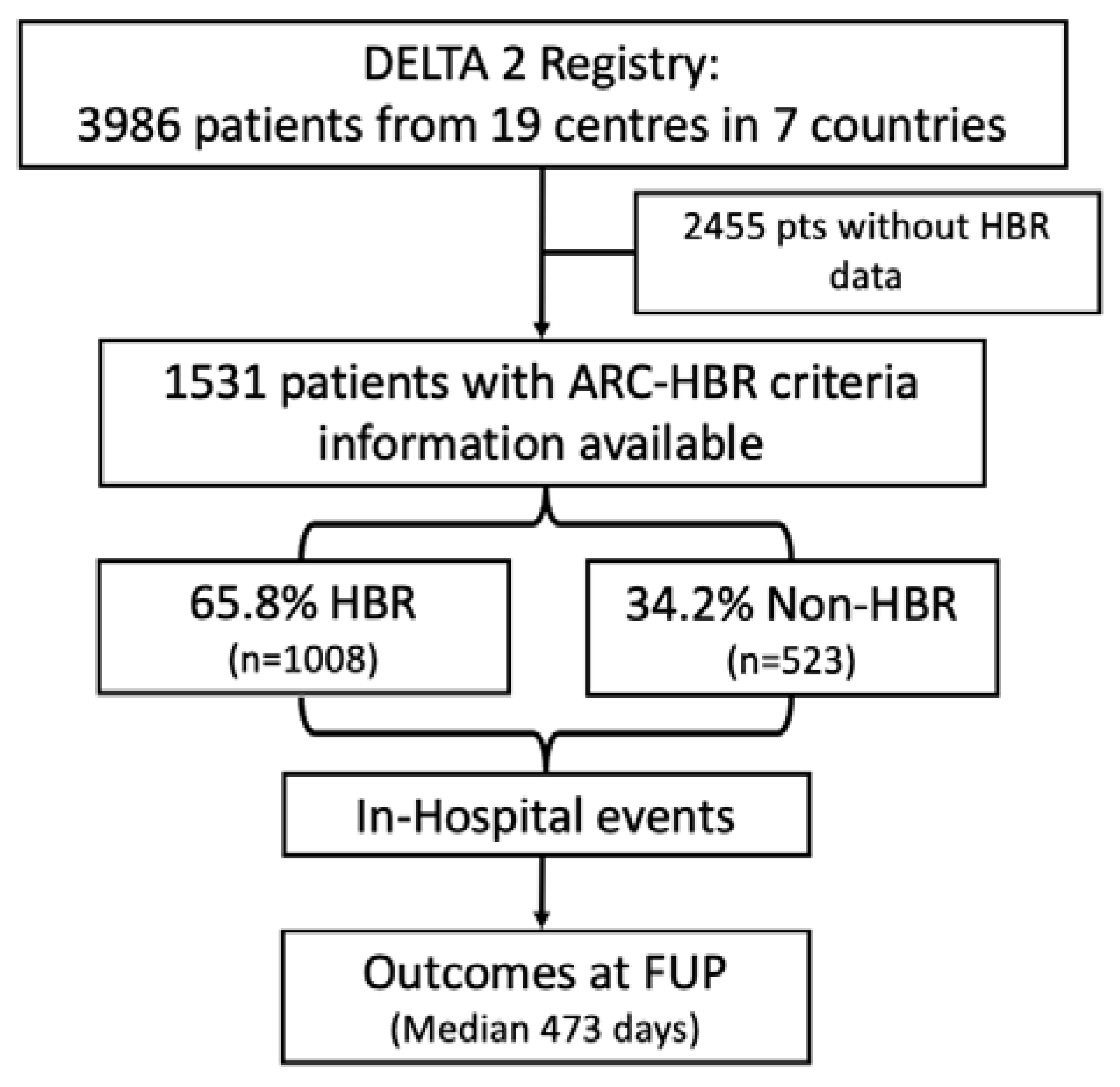

| Overall n = 1531 | HBR n = 1008 (65.8) | Non-HBR n = 523 (34.2) | p-Value | |
|---|---|---|---|---|
| Baseline Characteristics | ||||
| Age | 71.1 ± 10.8 | 73.7 ±10.8 | 66.1 ± 9.1 | <0.001 |
| Age > 75 | 626 (40.9) | 555 (55.1) | 71 (13.6) | <0.001 |
| Male | 1114 (72.8) | 680 (67.5) | 434 (83.0) | <0.001 |
| BMI kg/m2 | 26.4 ± 5.3 | 26.1 ± 5.0 | 26.9 ± 5.8 | 0.338 |
| Current smoker | 230 (15.0) | 126 (12.5) | 104 (19.9) | <0.001 |
| Hypertension | 1251 (81.7) | 804 (79.8) | 447 (85.5) | 0.006 |
| Diabetes mellitus | 588 (38.4) | 380 (37.7) | 208 (39.8) | 0.429 |
| Insulin dependent | 163 (10.6) | 125 (12.4) | 38 (7.3) | 0.002 |
| Chronic kidney disease | 614 (40.6) | 549 (55.4) | 65 (12.4) | <0.001 |
| Severe CKD | 118 (8.1) | 118 (12.6) | - | <0.001 |
| Hemodialysis | 61 (5.2) | 60 (9.2) | 1 (0.2) | <0.001 |
| Moderate anemia | 211 (15.7) | 143 (17.4) | 68 (13.0) | 0.032 |
| Severe anemia | 536 (39.8) | 536 (65.0) | - | <0.001 |
| CHF at presentation | 102 (15.0) | 94 (17.5) | 8 (5.6) | <0.001 |
| Previous MI | 468 (30.6) | 333 (33.1) | 135 (25.8) | 0.003 |
| Previous PCI | 578 (37.8) | 363 (36.0) | 215 (41.1) | 0.053 |
| Previous CABG | 88 (5.8) | 68 (6.8) | 20 (3.8) | 0.020 |
| Family history of CAD | 352 (23.9) | 222 (23.3) | 130 (24.9) | 0.508 |
| Peripheral arterial disease | 298 (19.7) | 238 (24.1) | 60 (11.5) | <0.001 |
| Cerebrovascular disease | 214 (14.0) | 198 (19.7) | 16 (3.1) | <0.001 |
| COPD | 212 (14.7) | 183 (19.0) | 29 (6.0) | <0.001 |
| Clinical Presentation | ||||
| Stable/silent ischemia | 895 (58.5) | 512 (50.8) | 383 (73.2) | <0.001 |
| Unstable angina | 274 (17.9) | 175 (17.4) | 99 (18.9) | 0.448 |
| NSTEMI | 253 (16.5) | 228 (22.6) | 25 (4.8) | <0.001 |
| STEMI | 109 (7.1) | 93 (9.2) | 16 (3.1) | <0.001 |
| LVEF % | 52.6 ± 12.3 | 50.2 ± 13.0 | 55.6 ± 10.6 | <0.001 |
| Overall n = 1531 | HBR n = 1008 (65.8) | Non-HBR n = 523 (34.2) | HR (95% CI) | p-Value | |
|---|---|---|---|---|---|
| In-hospital death | 19 (1.2) | 18(1.8) | 1 (0.2) | 9.5 (1.3–71.3) | 0.029 |
| In-hospital MI | 61 (4.0) | 50 (5.0) | 11 (2.1) | 2.4 (1.3–4.7) | 0.009 |
| In-hospital CVA events | 6 (0.4) | 5 (0.5) | 1 (0.2) | 2.2 (0.3–22.3) | 0.383 |
| In-hospital TLR | 4 (0.3) | 3 (0.3) | 1 (0.2) | 1.6 (0.2–15.0) | 0.701 |
| In-hospital TVR | 7 (0.5) | 5 (0.5) | 2 (0.4) | 1.3 (0.3–6.7) | 0.755 |
| In-hospital MACCE | 76 (5.0) | 63 (6.3) | 13 (2.5) | 2.6 (1.4–4.8) | 0.002 |
| Definite/probable ST | 6 (0.4) | 5 (0.5) | 1 (0.2) | 2.2 (0.3–22.3) | 0.383 |
| In-hospital Infectious Complications | 1 (1.0) | 6 (1.6) | 1 (0.3) | 5.4 (0.7–45.2) | 0.119 |
| In-hospital AKI | 17 (1.1) | 15 (1.5) | 2 (0.4) | 6.6 (1.5–29) | 0.013 |
| A | Overall n = 1531 | HBR n = 1008 (65.8) | Non-HBR n = 523 (34.2) | p-Value |
|---|---|---|---|---|
| Medications at discharge | ||||
| Aspirin | 1513 (98.8) | 992 (98.4) | 521 (99.6) | 0.038 |
| Clopidogrel | 1263 (82.5) | 860 (85.3) | 403 (77.1) | <0.001 |
| Ticagrelor | 82 (5.4) | 48 (4.9) | 34 (6.5) | 0.180 |
| Prasugrel | 166 (11.0) | 92 (9.3) | 74 (14.2) | 0.004 |
| OAC | 92 (6.5) | 92 (10.3) | - | <0.001 |
| DAPT only | 1403 (91.6) | 890 (88.3) | 513 (98.9) | <0.001 |
| SAPT only | 17 (1.5) | 8 (1.3) | 9 (1.7) | 0.493 |
| DAPT + OAC | 83 (7.7) | 83 (8.2) | - | <0.001 |
| SAPT + OAC | 9 (0.6) | 9 (0.9) | - | 0.030 |
| Overall n = 1531 | HBR n = 1008 (65.8) | Non-HBR n = 523 (34.2) | HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | |
|---|---|---|---|---|---|---|---|
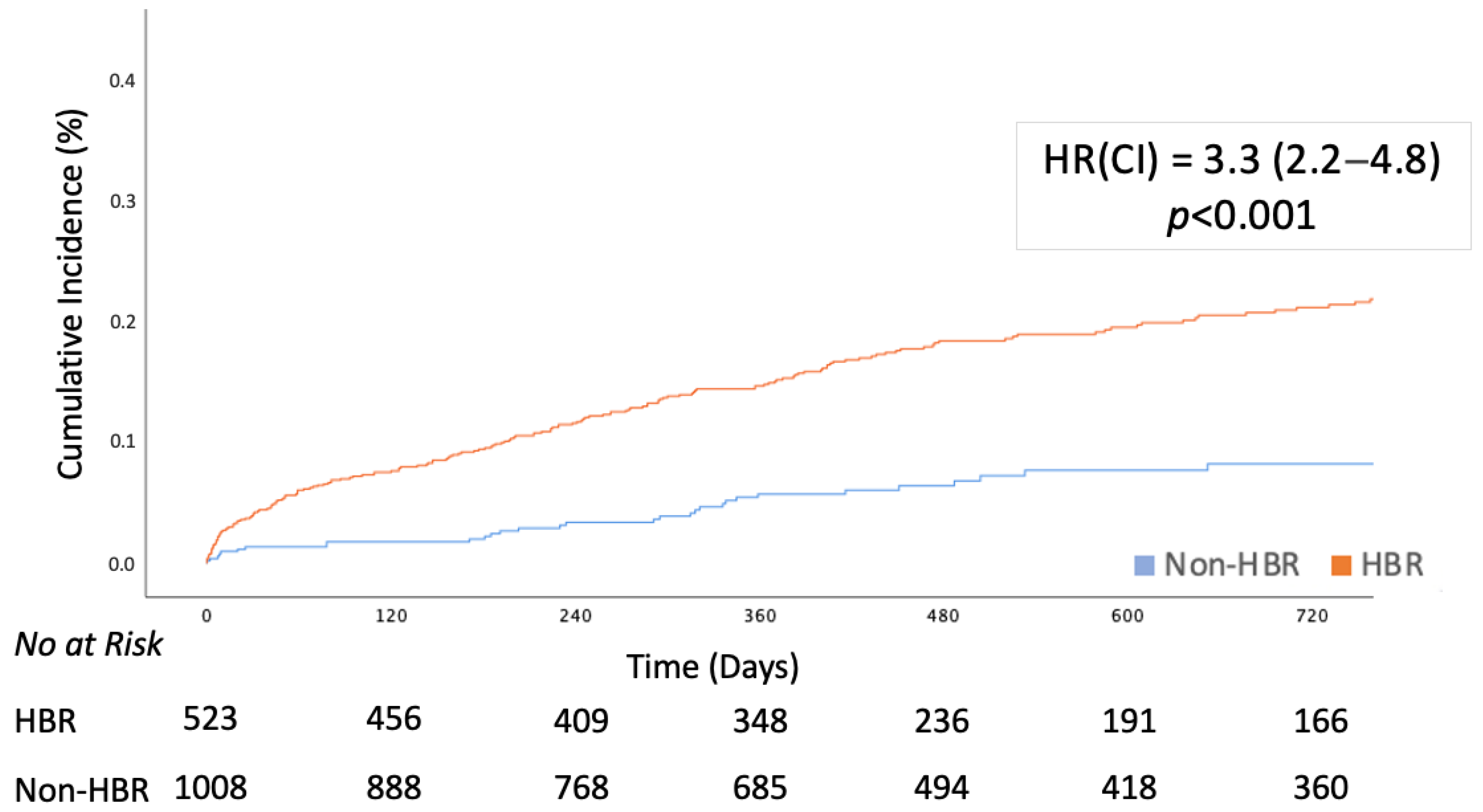
| Death or MI or CVA | 242 (15.8) | 210 (20.8) | 32 (6.1) | 3.3 (2.2–4.8) | <0.001 | 2.4 (1.6–3.4) | <0.001 |
| All-Cause Death | 187 (12.2) | 163 (16.2) | 24 (4.6) | 3.3 (2.2–5.1) | <0.001 | 2.4 (1.5–3.8) | <0.001 |
| Cardiovascular Death | 142 (9.3) | 124 (12.3) | 18 (3.4) | 3.2 (1.95–5.26) | <0.001 | 3.5 (1.1–11.1) | 0.038 |
| MI | 68 (4.4) | 58 (5.8) | 10 (1.9) | 2.9 (1.5–5.7) | 0.002 | 2.1 (0.9–4.4) | 0.062 |
| CVA | 19 (1.2) | 17 (1.7) | 2 (0.4) | 4.2 (0.9–18.3) | 0.054 | 3.3 (1.2–23.6) | 0.029 |
| Definite/Probable ST | 30 (2.0) | 23 (2.3) | 7 (1.3) | 1.6 (0.7–4.0) | 0.22 | 1.0 (0.3–2.3) | 0.737 |
| TLR | 162 (10.6) | 104 (10.3) | 58 (11.1) | 0.9 (0.7–1.3) | 0.572 | 1.0 (0.7–1.5) | 0.805 |
| TVR | 265 (17.3) | 152 (15.1) | 113 (21.6) | 0.6 (0.5–0.8) | <0.001 | 0.8 (0.6–1.0) | 0.095 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botti, G.; Federico, F.; Meliga, E.; Daemen, J.; D’Ascenzo, F.; Capodanno, D.; Dumonteil, N.; Tchetche, D.; Van Mieghem, N.M.; Nakamura, S.; et al. Percutaneous Coronary Intervention for Left Main Disease in High Bleeding Risk: Outcomes from a Subanalysis of the Delta 2 Registry. J. Cardiovasc. Dev. Dis. 2025, 12, 179. https://doi.org/10.3390/jcdd12050179
Botti G, Federico F, Meliga E, Daemen J, D’Ascenzo F, Capodanno D, Dumonteil N, Tchetche D, Van Mieghem NM, Nakamura S, et al. Percutaneous Coronary Intervention for Left Main Disease in High Bleeding Risk: Outcomes from a Subanalysis of the Delta 2 Registry. Journal of Cardiovascular Development and Disease. 2025; 12(5):179. https://doi.org/10.3390/jcdd12050179
Chicago/Turabian StyleBotti, Giulia, Francesco Federico, Emanuele Meliga, Joost Daemen, Fabrizio D’Ascenzo, Davide Capodanno, Nicolas Dumonteil, Didier Tchetche, Nicolas M. Van Mieghem, Sunao Nakamura, and et al. 2025. "Percutaneous Coronary Intervention for Left Main Disease in High Bleeding Risk: Outcomes from a Subanalysis of the Delta 2 Registry" Journal of Cardiovascular Development and Disease 12, no. 5: 179. https://doi.org/10.3390/jcdd12050179
APA StyleBotti, G., Federico, F., Meliga, E., Daemen, J., D’Ascenzo, F., Capodanno, D., Dumonteil, N., Tchetche, D., Van Mieghem, N. M., Nakamura, S., Garot, P., Erglis, A., Vella, C., Tamburino, C., Morice, M. C., Mehran, R., Montorfano, M., & Chieffo, A. (2025). Percutaneous Coronary Intervention for Left Main Disease in High Bleeding Risk: Outcomes from a Subanalysis of the Delta 2 Registry. Journal of Cardiovascular Development and Disease, 12(5), 179. https://doi.org/10.3390/jcdd12050179







