Non-Traditional Cardiovascular Risk Factors: Tailored Assessment and Clinical Implications
Abstract
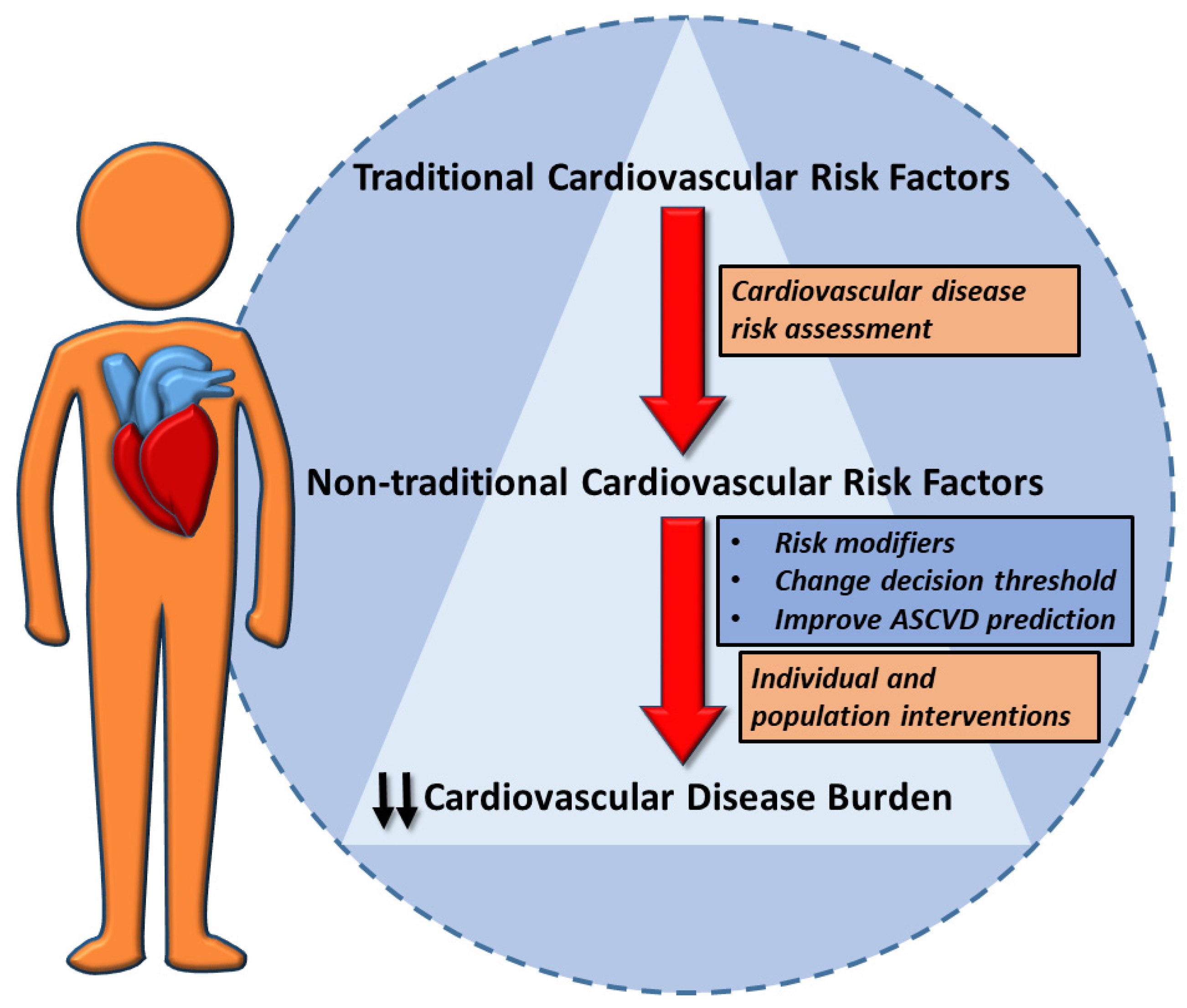
1. Introduction
2. New Biomarkers
3. Cancers and Their Therapies
4. Chronic Kidney Disease
5. Chronic Obstructive Pulmonary Disease
6. Environmental Exposure
7. Chronic Inflammatory Diseases
8. Infections and Gut Microbiota
9. Sleep Disorders and Cardiovascular Risk
10. Sex-Specific Conditions
11. Mental Disorders
12. Psychosocial Factors
13. Migraine with Aura
14. Discussion
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, J. Risk Assessment for Cardiovascular Disease With Nontraditional Risk Factors. JAMA 2018, 320, 316. [Google Scholar] [CrossRef]
- Lin, J.S.; Evans, C.V.; Johnson, E.; Redmond, N.; Coppola, E.L.; Smith, N. Nontraditional Risk Factors in Cardiovascular Disease Risk Assessment: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 320, 281–297. [Google Scholar] [CrossRef]
- Tragomalou, A.; Paltoglou, G.; Manou, M.; Kostopoulos, I.V.; Loukopoulou, S.; Binou, M.; Tsitsilonis, O.E.; Bacopoulou, F.; Kassari, P.; Papadopoulou, M.; et al. Non-Traditional Cardiovascular Risk Factors in Adolescents with Obesity and Metabolic Syndrome May Predict Future Cardiovascular Disease. Nutrients 2023, 15, 4342. [Google Scholar] [CrossRef] [PubMed]
- Whayne, T.F. Non-Traditional Cardiovascular Risk Markers in the Era of Established Major Risk Factors and Multiple Guidelines. Curr. Vasc. Pharmacol. 2019, 17, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Wilhelm, M.; Abreu, A.; Adami, P.E.; Ambrosetti, M.; Antonopoulou, M.; Biffi, A.; Cavarretta, E.; D’Ascenzi, F.; Gibson, I.; Grobbee, D.E.; et al. EAPC Core Curriculum for Preventive Cardiology. Eur. J. Prev. Cardiol. 2022, 29, 251–274. [Google Scholar] [CrossRef]
- Duarte Lau, F.; Giugliano, R.P. Lipoprotein(a) and its Significance in Cardiovascular Disease: A Review. JAMA Cardiol. 2022, 7, 760–769. [Google Scholar] [CrossRef]
- Di Fusco, S.A.; Arca, M.; Scicchitano, P.; Alonzo, A.; Perone, F.; Gulizia, M.M.; Gabrielli, D.; Oliva, F.; Imperoli, G.; Colivicchi, F. Lipoprotein(a): A risk factor for atherosclerosis and an emerging therapeutic target. Heart 2022, 109, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Erqou, S.; Kaptoge, S.; Perry, P.L.; Di Angelantonio, E.; Thompson, A.; White, I.R.; Marcovina, S.M.; Collins, R.; Thompson, S.G.; Danesh, J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009, 302, 412–423. [Google Scholar] [CrossRef]
- Di Fusco, S.A.; Maggioni, A.P.; Bernelli, C.; Perone, F.; De Marzo, V.; Conte, E.; Musella, F.; Uccello, G.; Luca, L.; Gabrielli, D.; et al. Inclisiran: A New Pharmacological Approach for Hypercholesterolemia. Rev. Cardiovasc. Med. 2022, 23, 375. [Google Scholar] [CrossRef]
- Tsimikas, S. A Test in Context: Lipoprotein(a): Diagnosis, Prognosis, Controversies, and Emerging Therapies. J. Am. Coll. Cardiol. 2017, 69, 692–711. [Google Scholar] [CrossRef] [PubMed]
- Kamstrup, P.R.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J. Am. Coll. Cardiol. 2014, 63, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Cannon, C.P.; Morrow, D.; Rifai, N.; Rose, L.M.; McCabe, C.H.; Pfeffer, M.A.; Braunwald, E. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005, 352, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Clearfield, M.; Downs, J.R.; Weis, S.E.; Miles, J.S.; Gotto, A.M., Jr. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N. Engl. J. Med. 2001, 344, 1959–1965. [Google Scholar] [CrossRef]
- Cooke, J.; Butler, C.; Hopstaken, R.; Dryden, M.S.; McNulty, C.; Hurding, S.; Moore, M.; Livermore, D.M. Narrative review of primary care point-of-care testing (POCT) and antibacterial use in respiratory tract infection (RTI). BMJ Open Respir. Res. 2015, 2, e000086. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Paneni, F.; Sabouret, P. Homocysteine: A futile comeback or a promising tool for the risk assessment of hypertensive patients? Eur. J. Prev. Cardiol. 2024, 31, 1090–1091. [Google Scholar] [CrossRef]
- Humphrey, L.L.; Fu, R.; Rogers, K.; Freeman, M.; Helfand, M. Homocysteine level and coronary heart disease incidence: A systematic review and meta-analysis. Mayo Clin. Proc. 2008, 83, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Mason, A.M.; Carter, P.; Burgess, S.; Larsson, S.C. Homocysteine, B vitamins, and cardiovascular disease: A Mendelian randomization study. BMC Med. 2021, 19, 97. [Google Scholar] [CrossRef]
- Ma, Y.; Peng, D.; Liu, C.; Huang, C.; Luo, J. Serum high concentrations of homocysteine and low levels of folic acid and vitamin B(12) are significantly correlated with the categories of coronary artery diseases. BMC Cardiovasc. Disord. 2017, 17, 37. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Perone, F.; Zamora Auñon, P.; Rodríguez, L.; Vinal, D.; Caro-Codon, J.; Pertejo, A.; Martínez Marín, V.; Espinosa, E.; López-Fernández, T. Cardiac monitoring during trastuzumab therapy in metastatic breast cancer: Early incidence of cardiac dysfunction. Monaldi Arch. Chest Dis. 2022, 92. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Lenihan, D. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline Summary. J. Oncol. Pract. 2017, 13, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Nasir, K.; Cainzos-Achirica, M. Role of coronary artery calcium score in the primary prevention of cardiovascular disease. BMJ 2021, 373, n776. [Google Scholar] [CrossRef]
- Perone, F.; Bernardi, M.; Redheuil, A.; Mafrica, D.; Conte, E.; Spadafora, L.; Ecarnot, F.; Tokgozoglu, L.; Santos-Gallego, C.G.; Kaiser, S.E.; et al. Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions. J. Clin. Med. 2023, 12, 5563. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Chen, S.; Tang, R.; Liu, B. Current Understanding of Cardiovascular Calcification in Patients with Chronic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 10225. [Google Scholar] [CrossRef]
- Suh, S.H.; Kim, S.W. Dyslipidemia in Patients with Chronic Kidney Disease: An Updated Overview. Diabetes Metab. J. 2023, 47, 612–629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Volgman, A.S.; Koschinsky, M.L.; Mehta, A.; Rosenson, R.S. Genetics and Pathophysiological Mechanisms of Lipoprotein(a)-Associated Cardiovascular Risk. J. Am. Heart Assoc. 2024, 13, e033654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levin, A.; Tonelli, M.; Bonventre, J.; Coresh, J.; Donner, J.A.; Fogo, A.B.; Fox, C.S.; Gansevoort, R.T.; Heerspink, H.J.L.; Jardine, M.; et al. Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 2017, 390, 1888–1917. [Google Scholar] [CrossRef] [PubMed]
- Mende, C.W. Chronic Kidney Disease and SGLT2 Inhibitors: A Review of the Evolving Treatment Landscape. Adv. Ther. 2022, 39, 148–164. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022, 45, 3075–3090. [Google Scholar] [CrossRef] [PubMed]
- Polman, R.; Hurst, J.R.; Uysal, O.F.; Mandal, S.; Linz, D.; Simons, S. Cardiovascular disease and risk in COPD: A state of the art review. Expert Rev. Cardiovasc. Ther. 2024, 22, 177–191. [Google Scholar] [CrossRef]
- Finkelstein, J.; Cha, E.; Scharf, S.M. Chronic obstructive pulmonary disease as an independent risk factor for cardiovascular morbidity. Int. J. Chronic Obstr. Pulm. Dis. 2009, 4, 337–349. [Google Scholar] [CrossRef]
- Hawkins, N.M.; Nordon, C.; Rhodes, K.; Talukdar, M.; McMullen, S.; Ekwaru, P.; Pham, T.; Randhawa, A.K.; Sin, D.D. Heightened long-term cardiovascular risks after exacerbation of chronic obstructive pulmonary disease. Heart 2024, 110, 702–709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Lucas-Ramos, P.; Izquierdo-Alonso, J.L.; Rodriguez-Gonzalez Moro, J.M.; Frances, J.F.; Lozano, P.V.; Bellón-Cano, J.M. Chronic obstructive pulmonary disease as a cardiovascular risk factor. Results of a case-control study (CONSISTE study). Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 679–686. [Google Scholar] [CrossRef]
- Wang, M.T.; Liou, J.T.; Lin, C.W.; Tsai, C.L.; Wang, Y.H.; Hsu, Y.J.; Lai, J.H. Association of Cardiovascular Risk With Inhaled Long-Acting Bronchodilators in Patients With Chronic Obstructive Pulmonary Disease: A Nested Case-Control Study. JAMA Intern. Med. 2018, 178, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Rochester, C.L.; Holland, A.E. Pulmonary Rehabilitation and Improved Survival for Patients With COPD. JAMA 2020, 323, 1783–1785. [Google Scholar] [CrossRef]
- Bhatnagar, A. Environmental Determinants of Cardiovascular Disease. Circ. Res. 2017, 121, 162–180. [Google Scholar] [CrossRef]
- Hadley, M.B.; Henderson, S.B.; Brauer, M.; Vedanthan, R. Protecting Cardiovascular Health From Wildfire Smoke. Circulation 2022, 146, 788–801. [Google Scholar] [CrossRef]
- Kaziród-Wolski, K.; Sielski, J.; Jóźwiak, M.; Wolska, M.; Bernardi, M.; Spadafora, L.; Biondi-Zoccai, G.; Siudak, Z.; Versaci, F. Does PM 2.5 and PM 10-associated heavy metals affect short-term and long-term survival after out-of-hospital cardiac arrest? Four-year study based on regional registry. Minerva Med. 2023, 115, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Al-Kindi, S.G.; Brook, R.D. Air Pollution and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2054–2070. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Hahad, O.; Daiber, A.; Landrigan, P.J. Soil and water pollution and human health: What should cardiologists worry about? Cardiovasc. Res. 2023, 119, 440–449. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]
- Wu, D.; Feng, Y.; Wang, R.; Jiang, J.; Guan, Q.; Yang, X.; Wei, H.; Xia, Y.; Luo, Y. Pigment microparticles and microplastics found in human thrombi based on Raman spectral evidence. J. Adv. Res. 2023, 49, 141–150. [Google Scholar] [CrossRef]
- Huynh, K. Presence of microplastics in carotid plaques linked to cardiovascular events. Nat. Rev. Cardiol. 2024, 21, 279. [Google Scholar] [CrossRef]
- Harris, E. Plastic in Arteries Tied With Higher Risk of Cardiovascular Problems. JAMA 2024, 331, 1354. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Gori, T.; Babisch, W.; Basner, M. Cardiovascular effects of environmental noise exposure. Eur. Heart J. 2014, 35, 829–836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tobías, A.; Recio, A.; Díaz, J.; Linares, C. Noise levels and cardiovascular mortality: A case-crossover analysis. Eur. J. Prev. Cardiol. 2015, 22, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, N.; Nkeh-Chungag, B.N. Impact of Indoor Air Pollutants on the Cardiovascular Health Outcomes of Older Adults: Systematic Review. Clin. Interv. Aging 2024, 19, 1629–1639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conrad, N.; Verbeke, G.; Molenberghs, G.; Goetschalckx, L.; Callender, T.; Cambridge, G.; Mason, J.C.; Rahimi, K.; McMurray, J.J.V.; Verbakel, J.Y. Autoimmune diseases and cardiovascular risk: A population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet 2022, 400, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Saramet, E.E.; Pomȋrleanu, C.; Maştaleru, A.; Oancea, A.; Cojocaru, D.-C.; Russu, M.; Negru, R.D.; Ancuța, C. Autonomic Dysfunction and Cardiovascular Risk in Patients with Rheumatoid Arthritis: Can Heart Rate Variability Analysis Contribute to a Better Evaluation of the Cardiovascular Profile of a Patient? J. Clin. Med. 2023, 12, 7736. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.K.; Jacobsson, L.; Bengtsson, K.; Askling, J. Is ankylosing spondylitis a risk factor for cardiovascular disease, and how do these risks compare with those in rheumatoid arthritis? Ann. Rheum. Dis. 2017, 76, 364–370. [Google Scholar] [CrossRef]
- Liu, L.; Cui, S.; Liu, M.; Huo, X.; Zhang, G.; Wang, N. Psoriasis Increased the Risk of Adverse Cardiovascular Outcomes: A New Systematic Review and Meta-Analysis of Cohort Study. Front. Cardiovasc. Med. 2022, 9, 829709. [Google Scholar] [CrossRef]
- Gao, N.; Kong, M.; Li, X.; Zhu, X.; Wei, D.; Ni, M.; Wang, Y.; Hong, Z.; Dong, A. The Association Between Psoriasis and Risk of Cardiovascular Disease: A Mendelian Randomization Analysis. Front. Immunol. 2022, 13, 918224. [Google Scholar] [CrossRef]
- Nada, H.; Elakhrass, A.; Ahmad, N.; Refaat, M. Psoriasis: Is it a risk factor for cardiovascular diseases? J. Dermatol. Treat. 2022, 33, 3154–3159. [Google Scholar] [CrossRef]
- Czubkowski, P.; Osiecki, M.; Szymanska, E.; Kierkus, J. The risk of cardiovascular complications in inflammatory bowel disease. Clin. Exp. Med. 2020, 20, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Nunez, P.; Garcia Mateo, S.; Quera, R.; Gomollon, F. Inflammatory bowel disease and the risk of cardiovascular diseases. Gastroenterol. Hepatol. 2021, 44, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kullo, I.J.; Pardi, D.S.; Loftus, E.V., Jr. Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 26–35. [Google Scholar] [CrossRef]
- Biondi, R.B.; Salmazo, P.S.; Bazan, S.G.Z.; Hueb, J.C.; de Paiva, S.A.R.; Sassaki, L.Y. Cardiovascular Risk in Individuals with Inflammatory Bowel Disease. Clin. Exp. Gastroenterol. 2020, 13, 107–113. [Google Scholar] [CrossRef]
- Castro Aguilar-Tablada, T.; Navarro-Alarcon, M.; Quesada Granados, J.; Samaniego Sanchez, C.; Rufian-Henares, J.A.; Nogueras-Lopez, F. Ulcerative Colitis and Crohn’s Disease Are Associated with Decreased Serum Selenium Concentrations and Increased Cardiovascular Risk. Nutrients 2016, 8, 780. [Google Scholar] [CrossRef]
- Sipilä, P.N.; Lindbohm, J.V.; Batty, G.D.; Heikkilä, N.; Vahtera, J.; Suominen, S.; Väänänen, A.; Koskinen, A.; Nyberg, S.T.; Meri, S.; et al. Severe Infection and Risk of Cardiovascular Disease: A Multicohort Study. Circulation 2023, 147, 1582–1593. [Google Scholar] [CrossRef]
- Atzeni, F.; Nucera, V.; Galloway, J.; Zoltan, S.; Nurmohamed, M. Cardiovascular risk in ankylosing spondylitis and the effect of anti-TNF drugs: A narrative review. Expert Opin. Biol. Ther. 2020, 20, 517–524. [Google Scholar] [CrossRef]
- Warren-Gash, C.; Smeeth, L.; Hayward, A.C. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: A systematic review. Lancet Infect. Dis. 2009, 9, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Medina, V.F.; Alvarez, K.N.; Weissfeld, L.A.; Angus, D.C.; Chirinos, J.A.; Chang, C.-C.H.; Newman, A.; Loehr, L.; Folsom, A.R.; Elkind, M.S.; et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015, 313, 264–274. [Google Scholar] [CrossRef]
- Nishimura, N.; Fukuda, H. Risk of cardiovascular events leading to hospitalisation after Streptococcus pneumoniae infection: A retrospective cohort LIFE Study. BMJ Open 2022, 12, e059713. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Deng, L.; Wang, L.; Zhong, W.; Hong, J.; Chen, L.; Yang, J.; Huang, B.; Xiao, X. Cardiovascular involvement in Epstein-Barr virus infection. Front. Immunol. 2023, 14, 1188330. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Affandi, J.; Waters, S.; Price, P. Human Cytomegalovirus Infection and Cardiovascular Disease: Current Perspectives. Viral Immunol. 2023, 36, 13–24. [Google Scholar] [CrossRef]
- Sun, L.; Zheng, H.; Qiu, M.; Hao, S.; Liu, X.; Zhu, X.; Cai, X.; Huang, Y. Helicobacter pylori infection and risk of cardiovascular disease. Helicobacter 2023, 28, e12967. [Google Scholar] [CrossRef]
- Poyatos, P.; Luque, N.; Sabater, G.; Eizaguirre, S.; Bonnin, M.; Orriols, R.; Tura-Ceide, O. Endothelial dysfunction and cardiovascular risk in post-COVID-19 patients after 6- and 12-months SARS-CoV-2 infection. Infection 2024, 52, 1269–1285. [Google Scholar] [CrossRef]
- So-Armah, K.; A Benjamin, L.; Bloomfield, G.S.; Feinstein, M.J.; Hsue, P.; Njuguna, B.; Freiberg, M.S. HIV and cardiovascular disease. Lancet HIV 2020, 7, e279–e293. [Google Scholar] [CrossRef] [PubMed]
- Tsampasian, V.; Bäck, M.; Bernardi, M.; Cavarretta, E.; Dębski, M.; Gati, S.; Hansen, D.; Kränkel, N.; Koskinas, K.; Niebauer, J.; et al. Cardiovascular disease as part of Long COVID: A systematic review. Eur. J. Prev. Cardiol. 2024; epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hassan, D.; Zahra, T.; Kanaan, G.; Khan, M.U.; Mushtaq, K.; Nashwan, A.J.; Hamid, P.F. The Impact of Gut Microbiome Constitution to Reduce Cardiovascular Disease Risk: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101459. [Google Scholar] [CrossRef]
- Prins, F.M.; Collij, V.; E Groot, H.; Björk, J.R.; Swarte, J.C.; Andreu-Sánchez, S.; Jansen, B.H.; Fu, J.; Harmsen, H.J.M.; Zhernakova, A.; et al. The gut microbiome across the cardiovascular risk spectrum. Eur. J. Prev. Cardiol. 2023, 31, 935–944. [Google Scholar] [CrossRef]
- Rashid, S.; Sado, A.I.M.; Afzal, M.S.; Ahmed, A.M.; Almaalouli, B.; Waheed, T.M.; Abid, R.M.; Majumder, K.M.; Kumar, V.; Tejwaney, U.P.D.; et al. Role of gut microbiota in cardiovascular diseases—A comprehensive review. Ann. Med. Surg. 2023, 86, 1483–1489. [Google Scholar] [CrossRef]
- Belli, M.; Barone, L.; Longo, S.; Prandi, F.R.; Lecis, D.; Mollace, R.; Margonato, D.; Muscoli, S.; Sergi, D.; Federici, M.; et al. Gut Microbiota Composition and Cardiovascular Disease: A Potential New Therapeutic Target? Int. J. Mol. Sci. 2023, 24, 11971. [Google Scholar] [CrossRef]
- Sarode, R.; Nikam, P.P. The Impact of Sleep Disorders on Cardiovascular Health: Mechanisms and Interventions. Cureus 2023, 15, e49703. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Germany, R.; Lozier, M.R.; Schweitzer, M.D.; Kosseifi, S.; Anand, R. Central sleep apnea and atrial fibrillation: A review on pathophysiological mechanisms and therapeutic implications. Int. J. Cardiol. Heart Vasc. 2020, 30, 100527. [Google Scholar] [CrossRef]
- Hou, X.-Z.; Li, Y.-S.; Wu, Q.; Lv, Q.-Y.; Yang, Y.-T.; Li, L.-L.; Ye, X.-J.; Yang, C.-Y.; Wang, M.-S.; Lv, Y.-F.; et al. Association of sleep characteristics with cardiovascular disease risk in adults over 40 years of age: A cross-sectional survey. Front. Cardiovasc. Med. 2024, 11, 1308592. [Google Scholar] [CrossRef] [PubMed]
- Solano-Pérez, E.; Coso, C.; Romero-Peralta, S.; Castillo-García, M.; López-Monzoni, S.; Ortigado, A.; Mediano, O. New Approaches to the Management of Cardiovascular Risk Associated with Sleep Respiratory Disorders in Pediatric Patients. Biomedicines 2024, 12, 411. [Google Scholar] [CrossRef]
- Tong, J.; Yu, Q.; Li, Y.; Du, J.; Qiu, J. Obstructive sleep apnea and cardiovascular events in acute coronary syndrome: A meta-analysis. Coron. Artery Dis. 2023, 34, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Howarth, N.E. Sleep and cardiovascular disease. Emerg. Top. Life Sci. 2023, 7, 457–466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torquati, L.; Mielke, G.I.; Brown, W.J.; Kolbe-Alexander, T. Shift work and the risk of cardiovascular disease. A systematic review and meta-analysis including dose-response relationship. Scand. J. Work. Environ. Health 2018, 44, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Millett, E.R.C.; Peters, S.A.E.; Woodward, M. Sex differences in risk factors for myocardial infarction: Cohort study of UK Biobank participants. BMJ 2018, 363, k4247. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef]
- Petrov, G.; Dworatzek, E.; Schulze, T.M.; Dandel, M.; Kararigas, G.; Mahmoodzadeh, S.; Knosalla, C.; Hetzer, R.; Regitz-Zagrosek, V. Maladaptive remodeling is associated with impaired survival in women but not in men after aortic valve replacement. JACC Cardiovasc. Imaging 2014, 7, 1073–1080. [Google Scholar] [CrossRef]
- Mureddu, G.F. How much does hypertension in pregnancy affect the risk of future cardiovascular events? Eur. Heart J. Suppl. 2023, 25, B111–B113. [Google Scholar] [CrossRef] [PubMed]
- Hallum, S.; Basit, S.; Kamper-Jørgensen, M.; Sehested, T.S.G.; A Boyd, H. Risk and trajectory of premature ischaemic cardiovascular disease in women with a history of pre-eclampsia: A nationwide register-based study. Eur. J. Prev. Cardiol. 2023, 30, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef]
- Osibogun, O.; Ogunmoroti, O.; Michos, E.D. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends Cardiovasc. Med. 2020, 30, 399–404. [Google Scholar] [CrossRef]
- Singh, T.; Khan, H.; Gamble, D.T.; Scally, C.; Newby, D.E.; Dawson, D. Takotsubo Syndrome: Pathophysiology, Emerging Concepts, and Clinical Implications. Circulation 2022, 145, 1002–1019, Erratum in Circulation 2022, 145, e1053. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montorsi, P.; Montorsi, F.; Schulman, C.C. Is erectile dysfunction the "tip of the iceberg" of a systemic vascular disorder? Eur. Urol. 2003, 44, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.V.; Terentes-Printzios, D.G.; Ioakeimidis, N.K.; Aznaouridis, K.A.; Stefanadis, C.I. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: A systematic review and meta-analysis of cohort studies. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Solomon, H.; Man, J.W.; Wierzbicki, A.S.; Jackson, G. Relation of erectile dysfunction to angiographic coronary artery disease. Am. J. Cardiol. 2003, 91, 230–231. [Google Scholar] [CrossRef]
- Montorsi, P.; Ravagnani, P.M.; Galli, S.; Rotatori, F.; Veglia, F.; Briganti, A.; Salonia, A.; Dehò, F.; Rigatti, P.; Montorsi, F.; et al. Association between erectile dysfunction and coronary artery disease. Role of coronary clinical presentation and extent of coronary vessels involvement: The COBRA trial. Eur. Heart J. 2006, 27, 2632–2639. [Google Scholar] [CrossRef]
- Christou, G.A.; Christou, K.A.; Nikas, D.N.; Goudevenos, J.A. Acute myocardial infarction in a young bodybuilder taking anabolic androgenic steroids: A case report and critical review of the literature. Eur. J. Prev. Cardiol. 2016, 23, 1785–1796. [Google Scholar] [CrossRef] [PubMed]
- Christou, G.A.; Christou, M.A.; Žiberna, L.; Christou, K.A. Indirect clinical markers for the detection of anabolic steroid abuse beyond the conventional doping control in athletes. Eur. J. Sport Sci. 2019, 19, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Starace, F.; Mungai, F.; Baccari, F.; Galeazzi, G.M. Excess mortality in people with mental illness: Findings from a Northern Italy psychiatric case register. Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 249–257. [Google Scholar] [CrossRef]
- Scott, K.M.; de Jonge, P.; Alonso, J.; Viana, M.C.; Liu, Z.; O’Neill, S.; Aguilar-Gaxiola, S.; Bruffaerts, R.; Caldas-De-Almeida, J.M.; Stein, D.J.; et al. Associations between DSM-IV mental disorders and subsequent heart disease onset: Beyond depression. Int. J. Cardiol. 2013, 168, 5293–5299. [Google Scholar] [CrossRef]
- Vaccarino, V.; Badimon, L.; Bremner, J.D.; Cenko, E.; Cubedo, J.; Dorobantu, M.; Duncker, D.J.; Koller, A.; Manfrini, O.; Milicic, D.; et al. Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. Eur. Heart J. 2019, 41, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Albus, C.; Waller, C.; Fritzsche, K.; Gunold, H.; Haass, M.; Hamann, B.; Herrmann-Lingen, C. Significance of psychosocial factors in cardiology: Update 2018: Position paper of the German Cardiac Society. Clin. Res. Cardiol. 2019, 108, 1175–1196. [Google Scholar] [CrossRef]
- Duflou, J. Psychostimulant use disorder and the heart. Addiction 2020, 115, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Tawakol, A.; Ishai, A.; AP Takx, R.; Figueroa, A.L.; Ali, A.; Kaiser, Y.; A Truong, Q.; Solomon, C.J.; Calcagno, C.; Mani, V.; et al. Relation between resting amygdalar activity and cardiovascular events: A longitudinal and cohort study. Lancet 2017, 389, 834–845. [Google Scholar] [CrossRef]
- Osborne, M.T.; Ishai, A.; Hammad, B.; Tung, B.; Wang, Y.; Baruch, A.; Fayad, Z.A.; Giles, J.T.; Lo, J.; Shin, L.M.; et al. Amygdalar activity predicts future incident diabetes independently of adiposity. Psychoneuroendocrinology 2019, 100, 32–40. [Google Scholar] [CrossRef]
- Grippo, A.J.; Johnson, A.K. Stress, depression and cardiovascular dysregulation: A review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 2009, 12, 1–21. [Google Scholar] [CrossRef]
- Valtorta, N.K.; Kanaan, M.; Gilbody, S.; Ronzi, S.; Hanratty, B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: Systematic review and meta-analysis of longitudinal observational studies. Heart 2016, 102, 1009–1016. [Google Scholar] [CrossRef]
- Kivimäki, M.; Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 2018, 15, 215–229. [Google Scholar] [CrossRef]
- Whitworth, J.A.; Williamson, P.M.; Mangos, G.; Kelly, J.J. Cardiovascular consequences of cortisol excess. Vasc. Health Risk Manag. 2005, 1, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Souverein, P.C.; Berard, A.; Van Staa, T.P.; Cooper, C.; Egberts, A.C.G.; Leufkens, H.G.M.; Walker, B.R. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart 2004, 90, 859–865. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation 2022, 146, e18–e43. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Cohen, B.E.; Commodore-Mensah, Y.; Fleury, J.; Huffman, J.C.; Khalid, U.; Labarthe, D.R.; Lavretsky, H.; Michos, E.D.; Spatz, E.S.; et al. Psychological Health, Well-Being, and the Mind-Heart-Body Connection: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e763–e783. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Mahmoud, A.N.; Mentias, A.; Elgendy, A.Y.; Qazi, A.; Barakat, A.F.; Saad, M.; Mohsen, A.; Abuzaid, A.; Mansoor, H.; Mojadidi, M.K.; et al. Migraine and the risk of cardiovascular and cerebrovascular events: A meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open 2018, 8, e020498. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.A. Genes, proteases, cortical spreading depression and migraine: Impact on pathophysiology and treatment. Funct. Neurol. 2007, 22, 133–136. [Google Scholar]
- Waeber, C.; Moskowitz, M.A. Migraine as an inflammatory disorder. Neurology 2005, 64 (Suppl. S2), S9–S15. [Google Scholar] [CrossRef]
- Kim, J.M.; Stewart, R.; Kang, H.J.; Kim, S.Y.; Kim, J.W.; Lee, H.J.; Lee, J.Y.; Kim, S.W.; Shin, I.S.; Kim, M.C.; et al. Long-term cardiac outcomes of depression screening, diagnosis and treatment in patients with acute coronary syndrome: The DEPACS study. Psychol. Med. 2020, 111, 964–974. [Google Scholar]
- Bibbins-Domingo, K.; Chertow, G.M.; Coxson, P.G.; Moran, A.; Lightwood, J.M.; Pletcher, M.J.; Goldman, L. Projected effect of dietary salt reductions on future cardiovascular disease. N. Engl. J. Med. 2010, 362, 590–599. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steptoe, A.; Kivimäki, M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 2012, 9, 360–370. [Google Scholar] [CrossRef] [PubMed]

| Risk Factor | Definition | Prevalence | Features | Prognostic Impact | Strategies for Management | Clinical Example |
|---|---|---|---|---|---|---|
| Chronic Kidney Disease | Condition characterized by progressive loss of kidney function. | Affects ~10% of adults globally. | Retention of uremic toxins, vascular calcification, chronic inflammation, and the presence of atherogenic lipoproteins. | Accelerates atherosclerosis and arterial stiffness and increases risk of heart failure and MI. | Control BP with ACE inhibitors/ARBs; manage lipids with statins; utilize SGLT2 inhibitors for renal and cardiac protection. | A 63-year-old man with CKD (eGFR < 45 mL/min/1.73 m2) presenting with MI. |
| COPD | Chronic inflammatory lung disease that causes obstructed airflow. | Affects 5–10% of adults worldwide. | Systemic inflammation, oxidative stress, and shared risk factors like smoking. | Increases risk of MI, stroke, and arrhythmias. | Manage exacerbations, use inhaled medications cautiously, and incorporate pulmonary rehabilitation to improve outcomes. | A 68-year-old woman with COPD exacerbations and atrial fibrillation. |
| Environmental Exposure | Exposure to pollutants like PM2.5, nitrogen dioxide, and microplastics. | Widespread; varies by geographic location and industrial activity. | Triggers oxidative stress, systemic inflammation, and endothelial dysfunction. | Linked to acute events like MI and stroke; chronic exposure increases risk of hypertension and heart failure. | Minimize outdoor activities during high pollution; advocate for clean energy policies; promote individual protective measures (e.g., masks and air purifiers). | A 45-year-old man exposed to heavy air pollution with elevated BP and plaque build-up on imaging. |
| Inflammatory Conditions | Autoimmune diseases like RA and SLE. | 1–2% of the population affected by RA; SLE affects 0.1%. | Chronic inflammation, immune dysregulation, and endothelial dysfunction. | Significantly increases cardiovascular mortality, including MI and stroke. | Optimize treatment of inflammation with disease-modifying antirheumatic drugs (DMARDs); regular cardiovascular monitoring and risk reduction strategies. | A 55-year-old woman with RA and recurrent chest pain diagnosed with coronary artery disease. |
| Lipoprotein(a) | Genetically determined lipoprotein variant associated with atherogenic and pro-thrombotic properties. | Elevated in 20–30% of the general population globally. | Promotes foam cell formation, oxidative LDL modification, and endothelial dysfunction. | Increases risk of MI, stroke, peripheral artery disease, and aortic stenosis. | Measure levels at least once; consider PCSK9 inhibitors or antisense therapies in high-risk patients. | A 58-year-old woman with premature MI and a family history of CAD showing elevated Lp(a). |
| Psychosocial Stress | Chronic or acute stressors impacting hypothalamic–pituitary–adrenal axis regulation. | Highly variable; common in lower socioeconomic groups. | Dysregulated cortisol levels, hyperlipidemia, and insulin resistance. | Elevated risk of heart failure, ischemic heart disease, and stroke. | Cognitive–behavioral therapy, stress management, and lifestyle modifications; consider treating comorbid mental health disorders. | A 40-year-old woman with a stressful job, insomnia, and new-onset hypertension. |
| Sleep Apnea (OSA) | Sleep disorder causing repetitive upper airway obstruction during sleep. | 10–20% of adults (higher in obese individuals). | Increased sympathetic tone, nocturnal BP spikes, and hypoxia. | Strongly linked to hypertension, atrial fibrillation, and recurrent major adverse cardiovascular events. | Lifestyle changes, weight loss, and continuous positive airway pressure therapy. | A 50-year-old obese man with daytime fatigue and poorly controlled hypertension. |
| hsCRP | Biomarker of systemic inflammation correlated with atherosclerosis and major adverse cardiovascular events (MACEs). | Elevated in many patients with CVD, especially those with metabolic syndrome. | Endothelial dysfunction, plaque instability, and increased thrombosis risk. | Associated with higher risk of myocardial infarction, stroke, and cardiovascular death. | Lifestyle modifications; statins and anti-inflammatory agents (e.g., colchicine) can lower hsCRP. | A 55-year-old with metabolic syndrome and hsCRP >2 mg/L at risk for future MI. |
| Noise Pollution | Chronic exposure to high-intensity sound levels (e.g., traffic and industrial noise) leading to physiological stress responses. | Urban areas, industrial zones, and high-traffic regions are most affected. | Increased stress hormone levels, endothelial dysfunction, and autonomic nervous system activation. | Higher risk of hypertension, atrial fibrillation, and cardiovascular events. | Soundproofing, noise regulations, public policy interventions, and personal protection measures. | A 48-year-old living near an airport with new-onset hypertension and stress-related arrhythmias. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perone, F.; Bernardi, M.; Spadafora, L.; Betti, M.; Cacciatore, S.; Saia, F.; Fogacci, F.; Jaiswal, V.; Asher, E.; Paneni, F.; et al. Non-Traditional Cardiovascular Risk Factors: Tailored Assessment and Clinical Implications. J. Cardiovasc. Dev. Dis. 2025, 12, 171. https://doi.org/10.3390/jcdd12050171
Perone F, Bernardi M, Spadafora L, Betti M, Cacciatore S, Saia F, Fogacci F, Jaiswal V, Asher E, Paneni F, et al. Non-Traditional Cardiovascular Risk Factors: Tailored Assessment and Clinical Implications. Journal of Cardiovascular Development and Disease. 2025; 12(5):171. https://doi.org/10.3390/jcdd12050171
Chicago/Turabian StylePerone, Francesco, Marco Bernardi, Luigi Spadafora, Matteo Betti, Stefano Cacciatore, Francesco Saia, Federica Fogacci, Vikash Jaiswal, Elad Asher, Francesco Paneni, and et al. 2025. "Non-Traditional Cardiovascular Risk Factors: Tailored Assessment and Clinical Implications" Journal of Cardiovascular Development and Disease 12, no. 5: 171. https://doi.org/10.3390/jcdd12050171
APA StylePerone, F., Bernardi, M., Spadafora, L., Betti, M., Cacciatore, S., Saia, F., Fogacci, F., Jaiswal, V., Asher, E., Paneni, F., De Rosa, S., Banach, M., Biondi Zoccai, G., & Sabouret, P. (2025). Non-Traditional Cardiovascular Risk Factors: Tailored Assessment and Clinical Implications. Journal of Cardiovascular Development and Disease, 12(5), 171. https://doi.org/10.3390/jcdd12050171













