1. Introduction
Insights regarding cardiac work and energetic metrics from experimental and clinical studies were developed from the seminal work by Suga and colleagues quantifying the left ventricular pressure–volume (PV) construct [
1,
2,
3,
4,
5,
6,
7]. The PV construct allows for comparing mechanical efficiency (ME) and myocardial efficiency (MyoEff) [
5,
6]. Mechanical efficiency is derived from stroke work (SW) divided by the pressure–volume area (PVA) and has been shown to be altered in various physiologic and disease states [
8,
9,
10]. Mechanical efficiency (SW/PVA) is an engineering perspective and is intended to be a complementary metric to the PV indices of contractility.
The ME and MyoEff metrics have been studied in chronically instrumented canine models and patient studies [
9,
11,
12], and these metrics are not interchangeable [
8,
9]. The MyoEff metric can be derived using either the SW or the PVA variables along with myocardial oxygen consumption (MVO
2). The SW/MVO
2 metric (MyoEff–SW) yields a lower value than the PVA/MVO
2 (MyoEff–PVA) metric but represents the metabolic efficiency related to completed work [
9,
13]. Several studies have used the MyoEff–SW metric and quantified how disease states impact this measurement [
8,
9,
10].
Assessing MVO
2 has evolved from invasive measures to the development of the pressure-work index (PWI) by Rooke and Feigl [
14] and noninvasive measures using positron emission tomography (PET) imaging [
15]. Combining PET with transthoracic echocardiography offers a practical tool to measure the SW, MVO
2, and MyoEff–SW metrics. However, the ability for the wide use of PET for serial evaluation is low. The PWI or estimated MVO
2 (eMVO
2) were highly correlated with the PET measures of MVO
2 [
15]. Combining echocardiography measurements of SW and left ventricular mass with arterial pressures to estimate MVO
2 offers a noninvasive method for the serial evaluation of MyoEff. Other conventional, indirect measures of MyoEff, such as the rate pressure product and mechano-energetic efficiency, offer insights but do not provide a true ratio [
16]. A comparison of indirect measures has been studied, and while the PVA tool was determined to be the best metric, its invasive nature reduces its clinical utility.
Over the past three decades, numerous publications have revisited the importance of aortic compliance (C
A) in cardiovascular health [
17,
18,
19]. Aortic compliance refers to the ability of the aorta to expand and contract in response to changes in blood volume. These findings have consistently shown that a loss in C
A is associated with an elevation of central aortic blood pressure, decreases in cardiac function, and an impact on SW [
20]. The impact of simulated VA on eMVO
2 and MyoEff remains to be evaluated.
Due to the previously described methods using the PVA construct and the inability to conduct invasive serial studies, we used a computational model (CM) to investigate how vascular aging impacted the metrics quantifying cardiac function and ventricular vascular coupling (VVC). The CM generated PV loop parameters along with eMVO2 in a simulated aging environment as CA was reduced from “normal to stiff”. We coupled this with the impact of an elevated heart rate, providing insights into the two paradigms: MyoEff–PVA and MyoEff–SW.
2. Methods
2.1. Computational Model Concept
A lumped parameter CM was implemented using MATLAB (2020b release, MathWorks, 1 Apple Hill Drive, Natick, MA, USA). This model includes 240 mathematical equations based on a comprehensive representation of the human cardiopulmonary system [
20,
21,
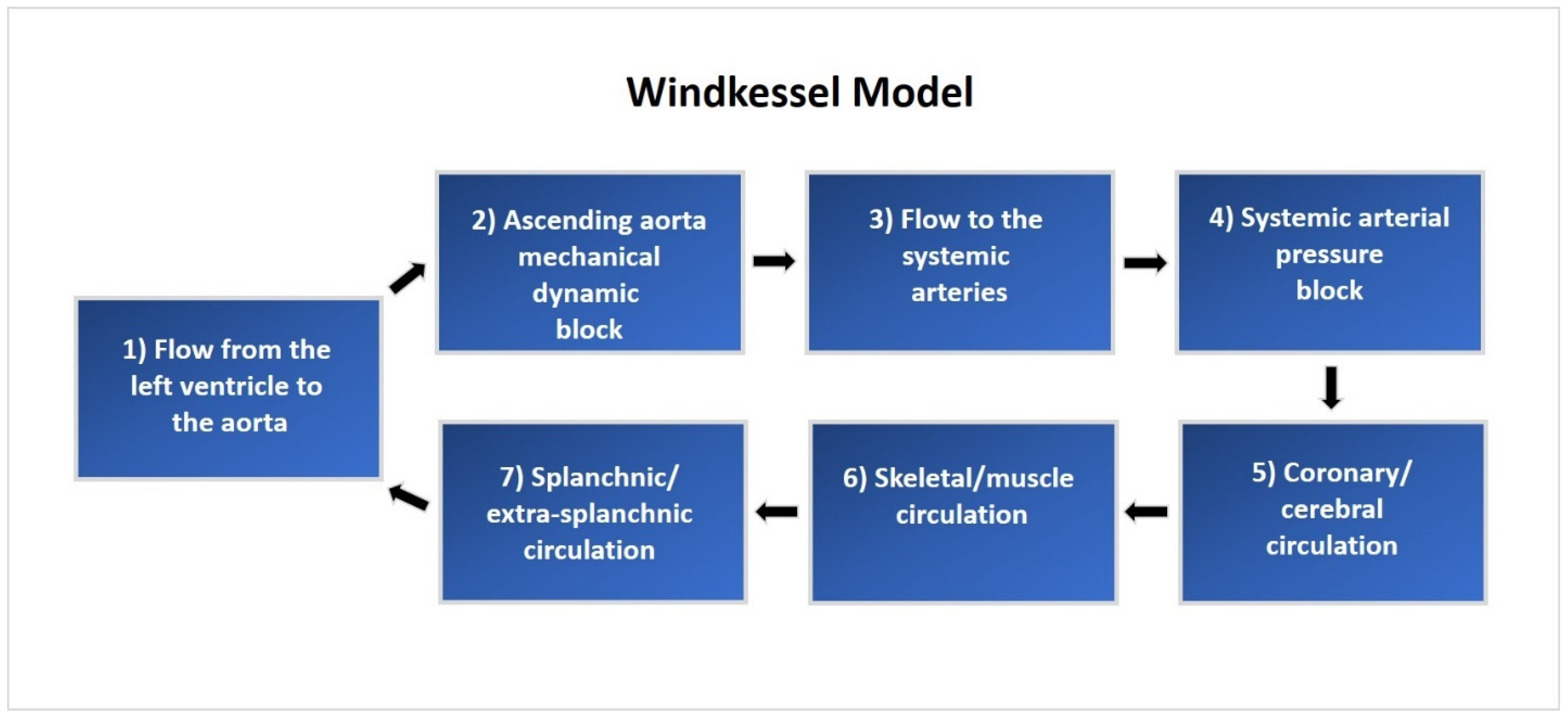
22]. The CM is composed of a five-compartment systemic vascular system, pulmonary circulation, lung mechanics, and blood gas exchange, as well as the left and right atria and ventricles. Hemodynamic function is regulated by autonomic neurological control and local autoregulation based on blood gas concentrations. These integrated mathematical elements work together to create an accurate model of the human cardiopulmonary system. A system overview of cardiovascular circulation through the proximal and distal Windkessel model used in this study is illustrated in
Figure 1.
2.2. Baseline Model Verification
The integrity of the CM was assessed by setting the C
A and vascular peripheral resistance parameters within the model to their published values and evaluating the simulated cardiovascular response based on published PV study data [
8,
9]. Nominal C
A and vascular peripheral resistance (R
T) were changed to 0.7 mL/mmHg and 1.28 mmHg·mL
−1·s
−1, respectively, based on prior studies of normal cardiovascular physiology [
23,
24]. We directly compared the cardiovascular waveforms generated by the model with key measurements, including the left ventricular pressure, left ventricular volume, aortic pressure, and the PV loop response (
Figure 2).
Model verification was based on the agreement of simulated PV data versus established normal physiological cardiovascular responses. This process involved fine-tuning the ancillary system parameters of the CM to match the original model’s waveforms and standard human physiological cardiac PV responses. This alignment with typical human physiology assures the model’s accuracy. Under normal compliance and resistance conditions, the model produced PV waveforms that resulted in a systolic pressure of approximately 120 mmHg, a diastolic pressure of 80 mmHg, and a pulse pressure of 40 mmHg.
2.3. Experiment Setup
After verifying the model’s capability to recreate normal physiologic cardiovascular responses, it was adapted to fit the experimental setup and procedure of the canine experiment described by Kelly et al. [
25]. The model was modified to recreate the scenario used in invasive PVL studies, which includes right atrial pacing, autonomic nervous system blockade, and a decrease in preload through vena cava occlusion (VCO). The nominal vascular compliance and peripheral resistances were adjusted to represent normal and stiff conditions, mimicking human cardiac and arterial function and physiology. The developed model illustrates venous blood flow to the right atrium through the superior and inferior vena cava, representing the total summation of flow converging through the veins in the thoracic cavity. This flow convergence is modeled by pooling the total venous blood flow from the five vascular compartments.
2.4. Vena Caval Occlusion
Occlusion of the venous return was simulated using a time-based increase in the hemodynamic resistance of the thoracic cavity veins after allowing the system to reach a steady state three minutes before the VCO. The occlusion occurred proximal to the tricuspid valve and downstream of the five systemic vein sub-compartments. This in silico approach simulates venous return occlusion through a balloon-expandable catheter delivery system, which mimics a reduction in the venous blood return.
2.5. Simulated Vascular Aging
Validation of the CM involved the assessment of its response and capability to simulate the effects of progressive vascular aging on cardiac function and efficiency. Vascular aging of the aorta and systemic vascular resistance described in the Kelly et al. study [
25] was simulated within the CM by the direct modification of the proximal C
A and peripheral vascular resistance elements. Due to the variance in vascular compliance and resistance between a human arterial system and a canine subject, and additional verification between the human CM cardiovascular response and the canine pressure–volume study results was performed to determine parity. A comparable response was found between the native arterial parameters outlined in the Kelly et al. study [
25] (C
A of 1.65 mL/mmHg and R
T of 3.04 mmHg·mL
−1·s
−1) and the nominal CM arterial parameters (C
A of 0.7 mL/mmHg and R
T of 1.28 mmHg·mL
−1·s
−1). The stiff Tygon tubing parameters (C
A of 0.19 mL/mmHg and R
T of 3.66 mmHg·mL
−1·s
−1) created a similar cardiac function and efficiency response when set in the CM. The progression of vascular aging was simulated by proportional scaling of the arterial compliances and resistances of the five compartments and aorta starting from nominal settings and linearly scaling compliance to stiff Tygon conditions. The creation of the variables including the stroke volume, aortic flow, and left ventricular pressures were derived from the Windkessel properties of the model. Further details on this process have been published in our prior study [
20].
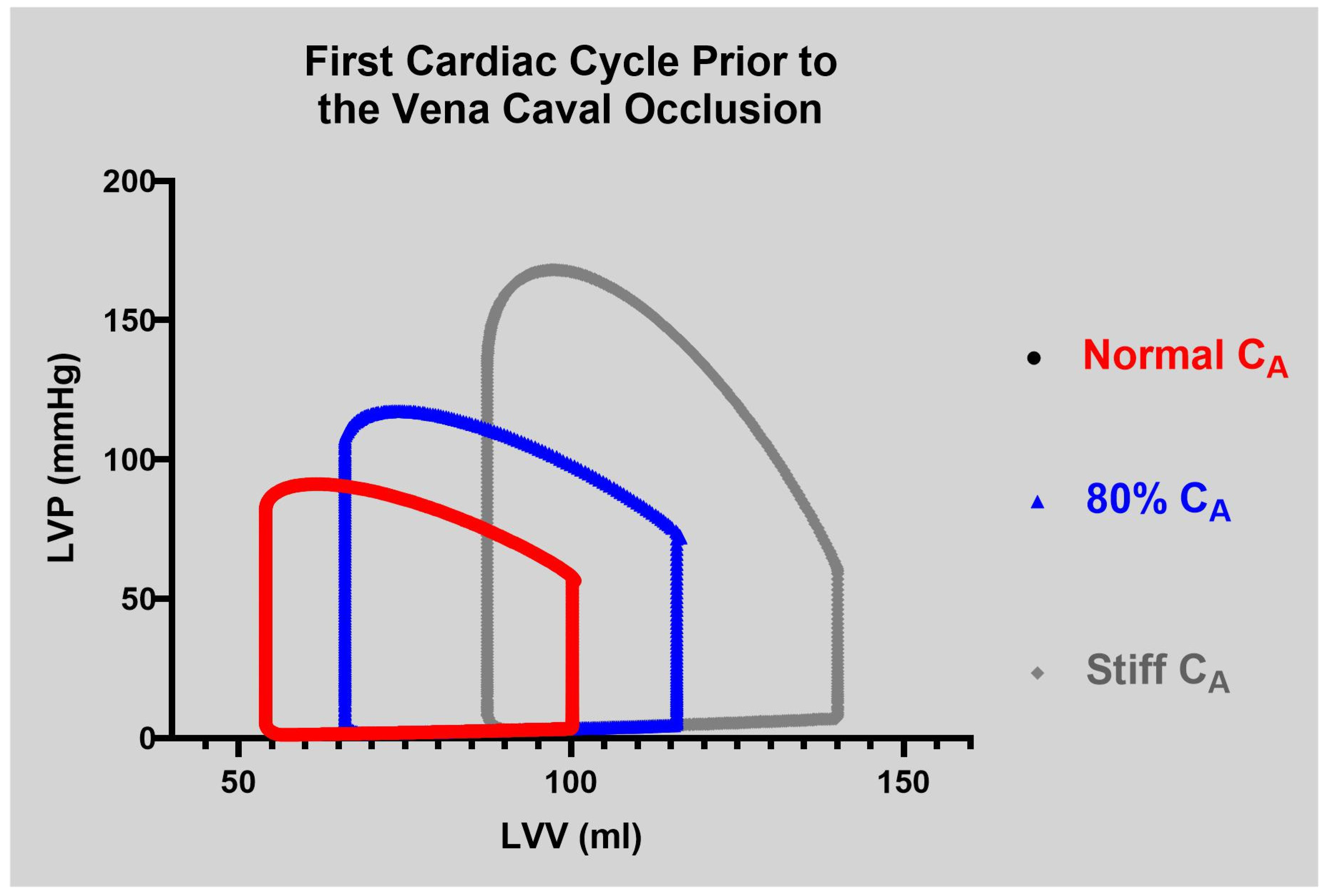
The CM was evaluated during two phases. In Phase I, right atrial pacing was simulated at 80 bpm across the five levels of compliance during each VCO simulation. In Phase II, the normal and the stiff CA settings were evaluated at 60, 100, and 140 bpm. Beat-to-beat data were collected by simulating the VCO under all levels of compliance. The results were verified through an examination of end-diastolic volume (EDV), end-systolic volume (ESV), end-systolic pressure (Pes), end-diastolic pressure (EDP), PP, dP/dtmax, and PVA.
2.6. Evaluation of Cardiac Function
Similar to clinical and pre-clinical studies, LV volume was calculated for each beat during the simulated VCO. The PVA was described by Suga [
1] and the parameters were calculated for each beat during the VCO. The ESPVR relation (Pes and Ves) was fit using a linear least-square algorithm to the following equation [
2,
3]:
where Ees is the slope of the relation and Vo is its volume-axis intercept [
3].
The slope and volume axis of the SW–EDV relation were determined using a linear least squares algorithm and were fit to the following equation [
26]:
where Mw is the slope of the relation and Vw is its volume axis.
The slope and volume axis of the dP/dt
max–EDV relation were determined in the same beats as used for the ESPVR and pre-load recruitable stroke work (PRSW) relations using a linear least squares algorithm and were fit to the following equation [
27]:
In the slope of the dP/dt
max–EDV relation, dE/dt
max represents the maximum rate of change of LV elastance [
28]. In a similar manner, the relation between PVA and EDV was fit to the following equation [
29]:
where MPVA is the slope of the relation and VPVA its volume intercept.
The calculation of eMVO
2 is determined with the equation below:
where:
SBP = systolic blood pressure (peak systolic pressure, mmHg);
DBP = diastolic blood pressure (mmHg);
HR = heart rate (beats/min);
SV = stroke volume (mL);
BW = body weight (kg) (set to 75 kg);
K1 = 4.08 × 10−4;
K2 = 3.25 × 10−4.
Myocardial efficiency was calculated using two approaches. First, the SW was divided by eMVO
2, as described by Rooke and Feigl [
14]. We chose a left ventricular mass of 200 g in the eMVO
2 equation, representing a person of 80 kg to produce the steady state MyoEff–SW. In unpublished data, we measured the LV mass in eight subjects with a range of left ventricular mass (87.3 to 217.4 g) and a range of eMVO
2 (6.1 to 12.5 mL/min/200 g). A comparison of MyoEff–SW versus MyoEff–PVA at a steady state and during the VCO allowed a direct comparison of these metrics. The CM provided five levels of static C
A as described above. In both Phase I and II, the parameters were calculated during the first beat of the VCO (steady state,
Figure 3) and the transient VCO process.
Phase I and II results were verified and validated through an examination of EDV, ESV, ESP, end-diastolic pressure (EDP), dP/dt
max, SW, PVA, ESPVR, and eMVO
2 beat-to-beat data. Validation of the human CM results was conducted through a comparison of the hemodynamic relationships seen in the works of Kelly et al. [
25] and Moulton and Secomb [
30], and physiological knowledge of the effects of stiffening vascular beds on the heart. Statistical analysis of the data was conducted using GraphPad Prism software Version 10.4 (225 Franklin St, Boston, MA, USA).
6. Limitations
The current model evaluates how VA alters cardiac function, including metrics of VVC and myocardial energetics in the normal heart. Quantifying changes in complex ventricular and vascular physiology would require employing both finite element and lumped parameter approaches. The CM attempted to replicate pre-clinical animal studies and found agreement in several aspects. However, pre-clinical studies to mimic the impact of VA coupled with elevated heart rates have not been conducted, for obvious reasons. The model did not include parameters to alter the impact of wave reflection or the timing or magnitude on VVC, as that would have necessitated a finite element component. The ventricular component was based on the time-varying elastance construct for the normal heart [
42].
The PV loop construct offers valuable insights into cardiac function in both pre-clinical and clinical studies. With the addition of the MVO
2 measurement, the construct was expanded to include an evaluation of MyoEff, utilizing the PVA-MVO
2 metric. Several publications have compared SW–MVO
2 with PVA–MVO
2 and have demonstrated the that the addition of PE leads to an overestimation of MyoEff [
8,
9,
10]. Moreover, the PVA-MVO
2 metric has limitations. It relies on invasive measurements, making it unsuitable for serial monitoring. Additionally, it necessitates altering the afterload to simulate a VCO, which poses further challenges in the clinical setting. Furthermore, the PVA–MVO
2MyoEff metric is overestimated due to the inclusion of PE in its calculation. By contrast, the MyoEff–SW does not require invasive assessment or load manipulation and provides a clear physiologic measure of MyoEff.