Intravascular Imaging-Guided Versus Angiography-Guided Percutaneous Coronary Intervention in Patients with Non-ST-Segment Elevation Myocardial Infarction in the United States: Results from Big Data Analysis
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Population
2.3. Study Outcomes and Definitions
2.4. Statistical Analysis
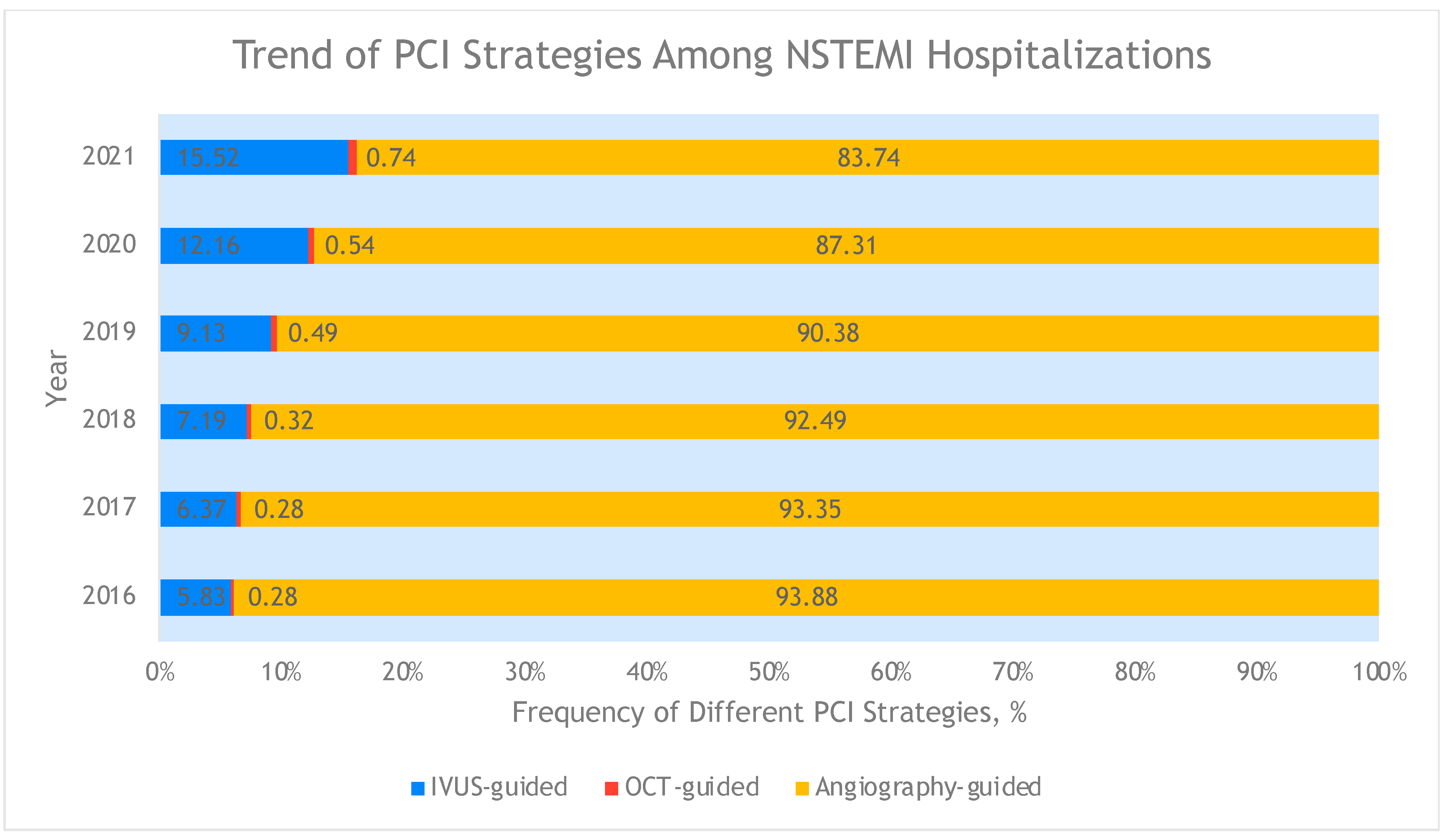
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rao, S.V.; O’Donoghue, M.L.; Ruel, M.; Rab, T.; Tamis-Holland, J.E.; Alexander, J.H.; Baber, U.; Baker, H.; Cohen, M.G.; Cruz-Ruiz, M.; et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025. [Google Scholar] [CrossRef]
- Groenland, F.T.W.; Neleman, T.; Kakar, H.; Scoccia, A.; Ziedses des Plantes, A.C.; Clephas, P.R.D.; Chatterjee, S.; Zhu, M.; den Dekker, W.K.; Diletti, R.; et al. Intravascular ultrasound-guided vs. coronary angiography-guided percutaneous coronary intervention in patients with acute myocardial infarction: A systematic review and meta-analysis. Int. J. Cardiol. 2022, 353, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Ang, S.P.; Qadeer, Y.K.; Wang, Z.; Alam, M.; Jneid, H.; Sharma, S. National Trends, Mortality and Outcomes in Intravascular Imaging-Guided vs. Angiography-Guided Percutaneous Coronary Intervention in the United States. Crit. Pathw. Cardiol. 2024, 23, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.P.; Chia, J.E.; Iglesias, J.; Usman, M.H.; Krittanawong, C. Coronary Intervention Outcomes in Patients with Liver Cirrhosis. Curr. Cardiol. Rep. 2025, 27, 2. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Ang, S.P.; Maitra, N.S.; Wang, Z.; Alam, M.; Jneid, H.; Sharma, S. Intravascular Imaging-Guided vs. Angiography-Guided Percutaneous Coronary Intervention in Patients With ST-Segment Elevation Myocardial Infarction in the United States. Catheter. Cardiovasc. Interv. 2025, 105, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.O.; Kinnaird, T.; Wijeysundera, H.C.; Johnson, T.W.; Zaman, S.; Rashid, M.; Moledina, S.; Ludman, P.; Mamas, M.A. Impact of Intracoronary Imaging-Guided Percutaneous Coronary Intervention on Procedural Outcomes Among Complex Patient Groups. J. Am. Heart Assoc. 2022, 11, e026500. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.J.; Lim, S.; Choo, E.H.; Hwang, B.H.; Kim, C.J.; Park, M.W.; Lee, J.M.; Park, C.S.; Kim, H.Y.; Yoo, K.D.; et al. Impact of Intravascular Ultrasound on Long-Term Clinical Outcomes in Patients with Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2021, 14, 2431–2443. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bae, S.; Johnson, T.W.; Son, N.H.; Sim, D.S.; Hong, Y.J.; Kim, S.W.; Cho, D.K.; Kim, J.S.; Kim, B.K.; et al. Role of Intravascular Ultrasound-Guided Percutaneous Coronary Intervention in Optimizing Outcomes in Acute Myocardial Infarction. J. Am. Heart Assoc. 2022, 11, e023481. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ge, Z.; Kan, J.; Anjum, M.; Xie, P.; Chen, X.; Khan, H.S.; Guo, X.; Saghir, T.; Chen, J.; et al. Intravascular ultrasound-guided vs. angiography-guided percutaneous coronary intervention in acute coronary syndromes (IVUS-ACS): A two-stage, multicentre, randomised trial. Lancet 2024, 403, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, J.; Reddy, R.K.; Jamil, Y.; Malik, A.; Chamie, D.; Howard, J.P.; Nanna, M.G.; Mintz, G.S.; Maehara, A.; Ali, Z.A.; et al. Intravascular Imaging-Guided vs. Angiography-Guided Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis of Randomized Trials. J. Am. Heart Assoc. 2024, 13, e031111. [Google Scholar] [CrossRef] [PubMed]
- Armillotta, M.; Bergamaschi, L.; Paolisso, P.; Belmonte, M.; Angeli, F.; Sansonetti, A.; Stefanizzi, A.; Bertolini, D.; Bodega, F.; Amicone, S.; et al. Prognostic Relevance of Type 4a Myocardial Infarction and Periprocedural Myocardial Injury in Patients with Non-ST-Segment-Elevation Myocardial Infarction. Circulation 2025, 151, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Mentias, A.; Sarrazin, M.V.; Saad, M.; Panaich, S.; Kapadia, S.; Horwitz, P.A.; Girotra, S. Long-Term Outcomes of Coronary Stenting with and Without Use of Intravascular Ultrasound. JACC Cardiovasc. Interv. 2020, 13, 1880–1890. [Google Scholar] [CrossRef] [PubMed]

| Variables | Angiography-Guided (n = 984,940) | ICI-Guided (n = 106,060) | Total (n = 1,091,000) | p |
|---|---|---|---|---|
| Age | 66.23 ± 12.46 | 66.39 ± 12.50 | 66.24 ± 12.46 | 0.102 |
| Female | 343,170 (34.84) | 36,345 (34.27) | 379,515 (34.79) | 0.1065 |
| Race | <0.001 | |||
| White | 741,560 (75.29) | 79,100 (74.58) | 820,660 (75.22) | |
| Black | 103,045 (10.46) | 10,310 (9.72) | 113,355 (10.39) | |
| Hispanic | 80,410 (8.16) | 8850 (8.34) | 89,260 (8.18) | |
| Asian or Pacific Islander | 25,375 (2.58) | 3205 (3.02) | 28,580 (2.62) | |
| Native American | 5685 (0.58) | 930 (0.88) | 6615 (0.61) | |
| Other | 28,865 (2.93) | 3665 (3.46) | 32,530 (2.98) | |
| Hospital Bed Size | <0.001 | |||
| Small | 162,335 (16.48) | 14,905 (14.05) | 177,240 (16.25) | |
| Medium | 299,675 (30.43) | 26,990 (25.45) | 326,665 (29.94) | |
| Large | 522,930 (53.09) | 64,165 (60.50) | 587,095 (53.81) | |
| Hospital Teaching Status | <0.001 | |||
| Rural | 59,665 (6.06) | 5435 (5.12) | 65,100 (5.97) | |
| Urban Non-teaching | 202,555 (20.57) | 18,620 (17.56) | 221,175 (20.27) | |
| Urban Teaching | 722,720 (73.38) | 82,005 (77.32) | 804,725 (73.76) | |
| Admission | 0.7649 | |||
| Elective | 46,925 (4.76) | 5115 (4.82) | 52,040 (4.77) | |
| Primary payment coverage | <0.001 | |||
| Medicare | 557,375 (56.59) | 60,810 (57.34) | 618,185 (56.66) | |
| Medicaid | 87,605 (8.89) | 9485 (8.94) | 97,090 (8.90) | |
| Private insurance | 264,490 (26.85) | 28,425 (26.80) | 292,915 (26.85) | |
| Self-pay | 42,670 (4.33) | 3925 (3.70) | 46,595 (4.27) | |
| No charge | 4185 (0.42) | 265 (0.25) | 4450 (0.41) | |
| Other | 28,615 (2.91) | 3150 (2.97) | 31,765 (2.91) | |
| Median household income, USD | <0.001 | |||
| 1–28,999 | 303,835 (30.85) | 28,860 (27.21) | 332,695 (30.49) | |
| 29,000–35,999 | 273,740 (27.79) | 27,025 (25.48) | 300,765 (27.57) | |
| 36,000–46,999 | 232,390 (23.59) | 26,530 (25.01) | 258,920 (23.73) | |
| 47,000+ | 174,975 (17.77) | 23,645 (22.29) | 198,620 (18.21) | |
| Hospital Region | <0.001 | |||
| Northeast | 157,880 (16.03) | 17,810 (16.79) | 175,690 (16.10) | |
| Midwest | 234,935 (23.85) | 24,980 (23.55) | 259,915 (23.82) | |
| South | 422,420 (42.89) | 36,435 (34.35) | 458,855 (42.06) | |
| West | 169,705 (17.23) | 26,835 (25.30) | 196,540 (18.01) | |
| Comorbidities | ||||
| Congestive heart failure | 377,540 (38.33) | 45,240 (42.66) | 422,780 (38.75) | <0.001 |
| Atrial fibrillation | 145,165 (14.74) | 15,580 (14.69) | 160,745 (14.73) | 0.8539 |
| Valvular heart diseases | 130,020 (13.20) | 16,215 (15.29) | 146,235 (13.40) | <0.001 |
| Peripheral vascular disease | 120,515 (12.24) | 13,720 (12.94) | 134,235 (12.30) | 0.0036 |
| Hypertension | 833,140 (84.59) | 89,550 (84.43) | 922,690 (84.57) | 0.5922 |
| Chronic lung disease | 210,935 (21.42) | 22,675 (21.38) | 233,610 (21.41) | 0.9047 |
| Diabetes | 436,820 (44.35) | 46,690 (44.02) | 483,510 (44.32) | 0.3861 |
| Hyperlipidemia | 730,170 (74.13) | 80,410 (75.82) | 810,580 (74.30) | <0.001 |
| CKD | 237,855 (24.15) | 27,920 (26.32) | 265,775 (24.36) | <0.001 |
| Liver disease | 30,840 (3.13) | 3705 (3.49) | 34,545 (3.17) | 0.004 |
| Anemia | 33,020 (3.35) | 4315 (4.07) | 37,335 (3.42) | <0.001 |
| Cancer | 24,315 (2.47) | 2845 (2.68) | 27,160 (2.49) | 0.0525 |
| Obesity | 227,690 (23.12) | 25,685 (24.22) | 253,375 (23.22) | 0.0012 |
| Alcohol use | 29,320 (2.98) | 2895 (2.73) | 32,215 (2.95) | 0.0507 |
| Smoking | 240,780 (24.45) | 24,120 (22.74) | 264,900 (24.28) | <0.001 |
| Prior MI | 179,280 (18.20) | 20,330 (19.17) | 199,610 (18.30) | 0.0013 |
| Prior PCI | 14,395 (1.46) | 1150 (1.08) | 15,545 (1.42) | <0.001 |
| Prior CABG | 112,060 (11.38) | 8795 (8.29) | 120,855 (11.08) | <0.001 |
| Elixhauser comorbidity score ≥ 4 | 486,435 (49.38) | 55,945 (52.78) | 542,380 (49.71) | <0.001 |
| Angiographic characteristics | ||||
| CTO | 45,565 (4.63) | 5060 (4.77) | 50,625 (4.64) | 0.3855 |
| FFR | 40,060 (4.07) | 7945 (7.49) | 48,005 (4.40) | <0.001 |
| DES | 882,740 (89.62) | 98,180 (92.57) | 980,920 (89.91) | <0.001 |
| BMS | 53,080 (5.39) | 3585 (3.38) | 56,665 (5.19) | <0.001 |
| In-hospital management | ||||
| Use of MCS | 35,320 (3.59) | 8340 (7.86) | 43,660 (4.00) | <0.001 |
| LVAD | 19,095 (1.94) | 5620 (5.30) | 24,715 (2.27) | <0.001 |
| ECMO | 770 (0.08) | 125 (0.12) | 895 (0.08) | 0.0546 |
| IABP | 17,755 (1.80) | 3305 (3.12) | 21,060 (1.93) | <0.001 |
| Variables | aOR (95% CI) | p |
|---|---|---|
| Age | ||
| Age < 65 | Ref | |
| Age ≥ 65 | 0.97 (0.92–1.01) | 0.157 |
| Sex | ||
| Male | Ref | |
| Female | 0.97 (0.94–1.00) | 0.096 |
| Race | ||
| White | Ref | |
| Black | 1.01 (0.94–1.09) | 0.705 |
| Hispanic | 0.97 (0.90–1.05) | 0.442 |
| Asian or Pacific Islander | 0.89 (0.80–0.99) | 0.027 |
| Native American | 1.44 (1.19–1.75) | <0.001 |
| Other | 1.11 (1.00–1.25) | 0.06 |
| Hospital Bed Size | ||
| Small | Ref | |
| Medium | 0.99 (0.88–1.10) | 0.815 |
| Large | 1.33 (1.20–1.48) | <0.001 |
| Hospital Teaching Status | ||
| Rural | Ref | |
| Urban Non-teaching | 0.98 (0.81–1.17) | 0.795 |
| Urban Teaching | 1.21 (1.02–1.44) | 0.034 |
| Hospital Region | ||
| Northeast | Ref | |
| Midwest | 0.99 (0.87–1.13) | 0.882 |
| South | 0.86 (0.76–0.98) | 0.02 |
| West | 1.42 (1.25–1.61) | <0.001 |
| Primary payment coverage | ||
| Medicare | Ref | |
| Medicaid | 0.96 (0.90–1.02) | 0.202 |
| Private insurance | 0.99 (0.94–1.04) | 0.773 |
| Self-pay | 0.97 (0.88–1.06) | 0.479 |
| No charge | 0.69 (0.51–0.93) | 0.017 |
| Other | 1.03 (0.94–1.13) | 0.552 |
| Median household income, USD | ||
| 1–28,999 | Ref | |
| 29,000–35,999 | 1.01 (0.96–1.06) | 0.789 |
| 36,000–46,999 | 1.11 (1.04–1.17) | 0.001 |
| 47,000+ | 1.27 (1.17–1.36) | <0.001 |
| Congestive heart failure | 1.11 (1.07–1.16) | <0.001 |
| Valvular heart disease | 1.10 (1.05–1.15) | <0.001 |
| Peripheral vascular diseases | 1.01 (0.96–1.06) | 0.703 |
| Atrial fibrillation | 0.93 (0.89–0.97) | 0.001 |
| Hypertension | 0.96 (0.91–1.00) | 0.052 |
| Anemia | 1.14 (1.06–1.23) | 0.001 |
| Cancer | 1.06 (0.97–1.15) | 0.223 |
| Diabetes | 0.94 (0.91–0.97) | <0.001 |
| Hyperlipidemia | 1.12 (1.07–1.16) | <0.001 |
| Smoking | 0.98 (0.95–1.02) | 0.366 |
| CKD | 1.05 (1.01–1.09) | 0.02 |
| Chronic liver disease | 0.96 (0.89–1.04) | 0.376 |
| Obesity | 1.06 (1.02–1.10) | 0.002 |
| Alcohol use | 0.91 (0.83–1.00) | 0.044 |
| Prior PCI | 0.76 (0.66–0.88) | <0.001 |
| Prior MI | 1.06 (1.02–1.10) | 0.004 |
| Prior CABG | 0.71 (0.67–0.75) | <0.001 |
| FFR | 1.85 (1.73–1.98) | <0.001 |
| CTO | 1.02 (0.95–1.10) | 0.55 |
| BMS | 0.77 (0.69–0.86) | <0.001 |
| DES | 1.24 (1.16–1.33) | <0.001 |
| Use of MCS | 2.11 (1.97–2.25) | <0.001 |
| Elixhauser score ≥ 4 | 1.05 (1.00–1.10) | 0.037 |
| In-Hospital Outcomes | Angiography-Guided (n = 984,940) | ICI-Guided (n = 106,060) | Total (n = 1,091,000) | p | aOR (95% CI) | p | PSM OR (95% CI) | p |
|---|---|---|---|---|---|---|---|---|
| Mortality | 19,620 (1.99) | 2245 (2.12) | 21,865 (2.00) | 0.222 | 0.75 (0.67–0.83) | <0.001 | 0.85 (0.74–0.96) | 0.012 |
| AKI | 171,005 (17.36) | 20,040 (18.89) | 191,045 (17.51) | <0.001 | 0.97 (0.93–1.01) | 0.193 | 1.00 (0.95–1.06) | 0.874 |
| Cardiogenic shock | 33,485 (3.40) | 4890 (4.61) | 38,375 (3.52) | <0.001 | 0.84 (0.77–0.92) | <0.001 | 0.91 (0.83–0.99) | 0.04 |
| Cardiac Arrest | 18,120 (1.84) | 2010 (1.90) | 20,130 (1.85) | 0.5694 | 0.88 (0.79–0.98) | 0.018 | 0.90 (0.79–1.04) | 0.157 |
| Length of stay | 3.98 ± 4.93 | 4.47 ± 5.18 | 4.03 ± 4.96 | <0.001 | - | - | ||
| Cost of hospitalization | 25,942 ± 20,647 | 33,177 ± 25,533 | 26,642 ± 21,277 | <0.001 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krittanawong, C.; Ang, S.P.; Maitra, N.S.; Wang, Z.; Alam, M.; Jneid, H.; Sharma, S. Intravascular Imaging-Guided Versus Angiography-Guided Percutaneous Coronary Intervention in Patients with Non-ST-Segment Elevation Myocardial Infarction in the United States: Results from Big Data Analysis. J. Cardiovasc. Dev. Dis. 2025, 12, 161. https://doi.org/10.3390/jcdd12040161
Krittanawong C, Ang SP, Maitra NS, Wang Z, Alam M, Jneid H, Sharma S. Intravascular Imaging-Guided Versus Angiography-Guided Percutaneous Coronary Intervention in Patients with Non-ST-Segment Elevation Myocardial Infarction in the United States: Results from Big Data Analysis. Journal of Cardiovascular Development and Disease. 2025; 12(4):161. https://doi.org/10.3390/jcdd12040161
Chicago/Turabian StyleKrittanawong, Chayakrit, Song Peng Ang, Neil Sagar Maitra, Zhen Wang, Mahboob Alam, Hani Jneid, and Samin Sharma. 2025. "Intravascular Imaging-Guided Versus Angiography-Guided Percutaneous Coronary Intervention in Patients with Non-ST-Segment Elevation Myocardial Infarction in the United States: Results from Big Data Analysis" Journal of Cardiovascular Development and Disease 12, no. 4: 161. https://doi.org/10.3390/jcdd12040161
APA StyleKrittanawong, C., Ang, S. P., Maitra, N. S., Wang, Z., Alam, M., Jneid, H., & Sharma, S. (2025). Intravascular Imaging-Guided Versus Angiography-Guided Percutaneous Coronary Intervention in Patients with Non-ST-Segment Elevation Myocardial Infarction in the United States: Results from Big Data Analysis. Journal of Cardiovascular Development and Disease, 12(4), 161. https://doi.org/10.3390/jcdd12040161





