Angioplasty in Patients with Central Venous Occlusion Prior to Device Lead Implantation
Abstract
1. Introduction
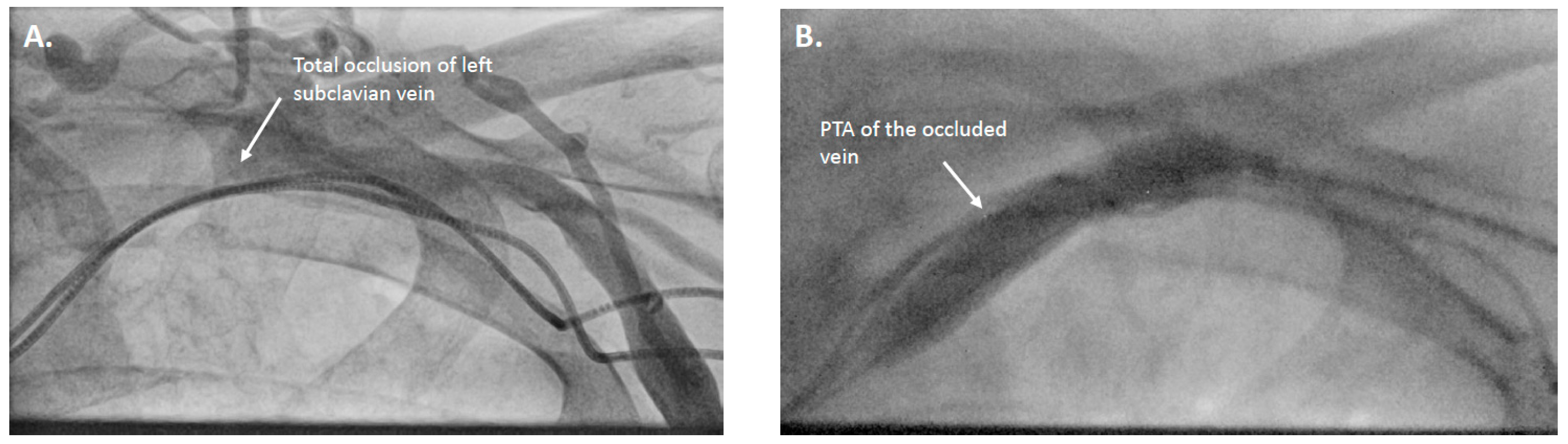
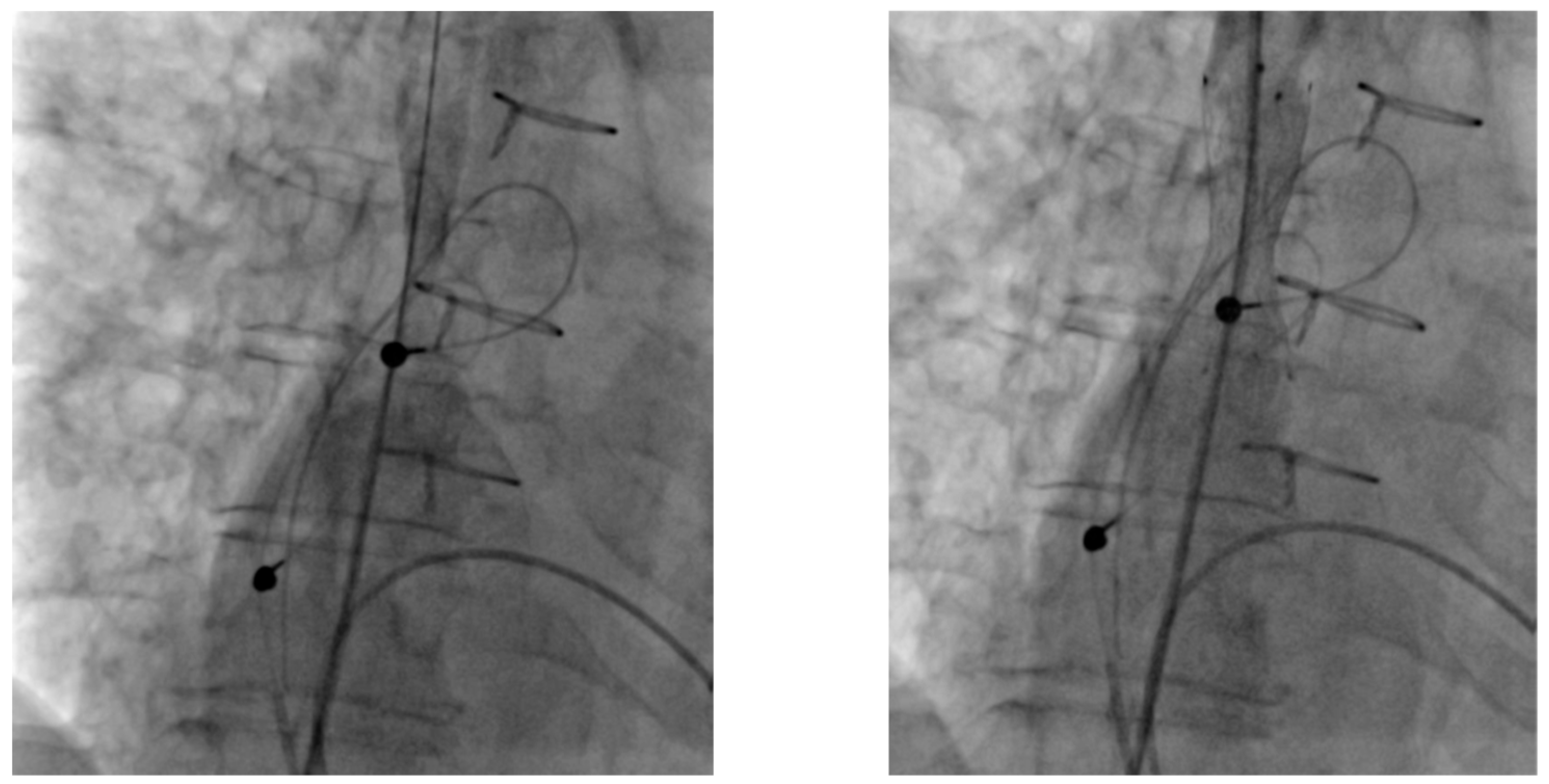
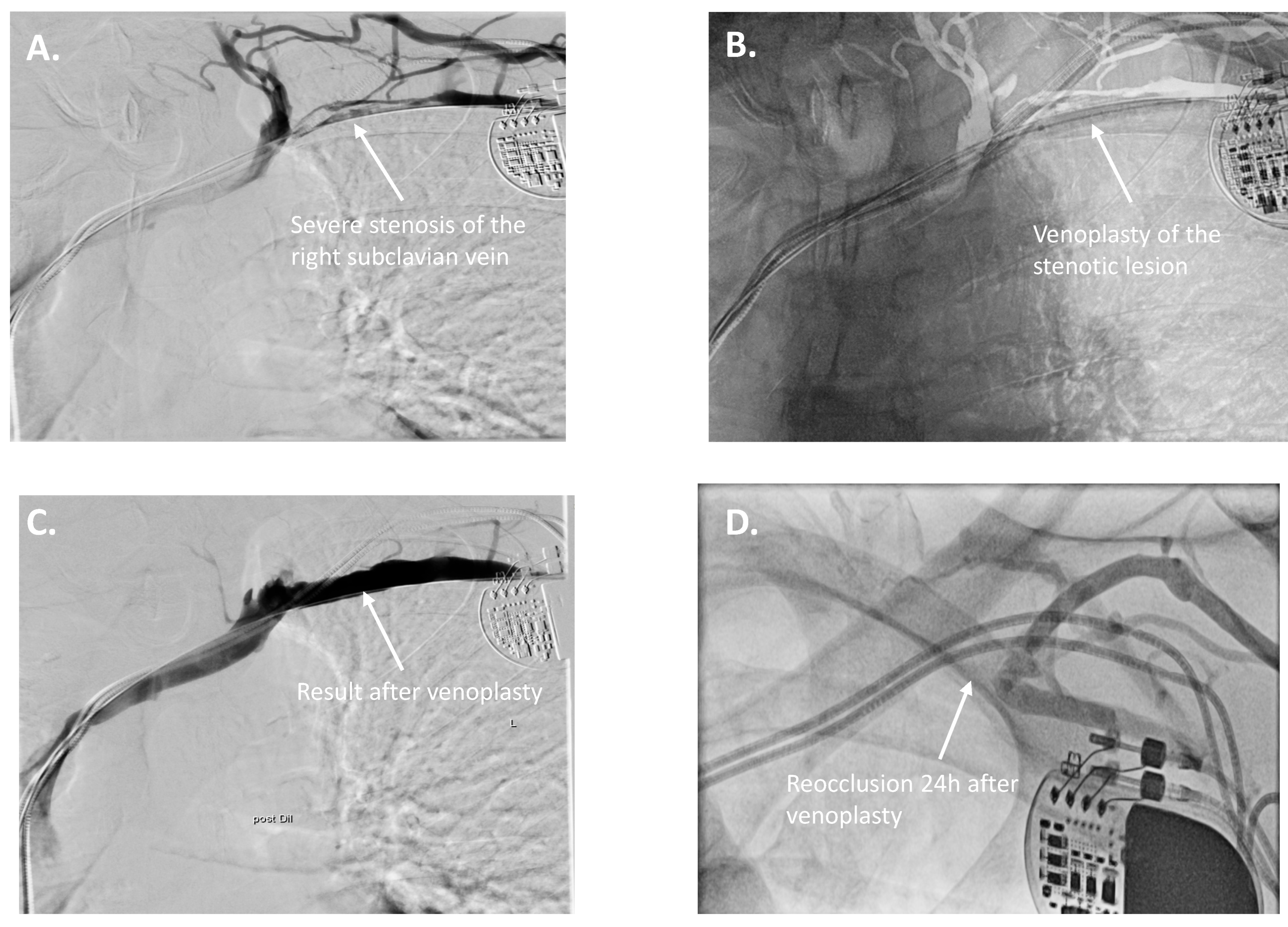
2. Cases
3. Therapeutic Interventions
4. Follow-Up and Outcomes
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glikson, M.; Nielsen, J.C.; Leclercq, C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabes, J.A.; Boriani, G.; Braunschweig, F.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Burri, H.; Starck, C.; Auricchio, A.; Biffi, M.; Burri, M.; D’avila, A.; Deharo, J.-C.; Glikson, M.; Israel, C.; Lau, C.-P.; et al. EHRA expert consensus statement and practical guide on optimal implantation technique for conventional pacemakers and implantable cardioverter-defibrillators: Endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart RhythmSociety (APHRS), and the Latin-American Heart RhythmSociety (LAHRS). Europace 2021, 23, 983–1008. [Google Scholar] [PubMed]
- Oginosawa, Y.; Abe, H.; Nakashima, Y. The incidence and risk factors for venous obstruction after implantation of transvenous pacing leads. PACE—Pacing Clin. Electrophysiol. 2002, 25, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Lickfett, L.; Bitzen, A.; Arepally, A.; Nasir, K.; Wolpert, C.; Jeong, K.M.; Krause, U.; Schimpf, R.; Lewalter, T.; Calkins, H.; et al. Incidence of venous obstruc-tion following insertion of an implantable cardioverter defibrillator. A study of systematic contrast venography on patients presenting for their first elective ICD generator replacement. Europace 2004, 6, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Morani, G.; Bolzan, B.; Valsecchi, S.; Morosato, M.; Ribichini, F.L. Chronic venous obstruction during cardiac device revision: Incidence, predictors, and efficacy of percutaneous techniques to overcome the stenosis. Heart Rhythm 2020, 17, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Worley, S.J.; Gohn, D.C.; Pulliam, R.W.; Raifsnider, M.A.; Ebersole, B.I.; Tuzi, J. Subclavian venoplasty by the implanting physicians in 373 patients over 11 years. Heart Rhythm 2011, 8, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.Y.; Gundewar, S.; Palma, E.C. Subclavian venoplasty may reduce implant times and implant failures in the era of increasing device upgrades. PACE—Pacing Clin. Electrophysiol. 2012, 35, 444–448. [Google Scholar] [CrossRef] [PubMed]
- McCotter, C.J.; Angle, J.F.; Prudente, L.A.; Mounsey, J.P.; Ferguson, J.D.; DiMarco, J.P.; Hummel, J.P.; Mangrum, J.M. Placement of transvenous pacemaker and ICD leads across total chronic occlusions. PACE—Pacing Clin. Electrophysiol. 2005, 28, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Lipšic, E.; Daniëls, F.; Groenveld, H.F.; Rienstra, M.; Maass, A.H. When and how to perform venoplasty for lead placement. Heart Rhythm 2024, 21, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.J.; Bode, W.D.; Frankel, D.S.; Garcia, F.; Supple, G.E.; Giri, J.S.; Kumareswaran, R.; Dixit, S.; Callans, D.J.; Marchlinski, F.E.; et al. Percutaneous balloon venoplasty for symptomatic lead-related venous stenosis. Heart Rhythm 2025, 22, 2082–2091. [Google Scholar] [CrossRef] [PubMed]
- Kishore, S.; Khaja, M.S.; Thornburg, B.; Sharma, A.M.; Knuttinen, M.G.; Shamoun, F.; Mantha, S.; Desai, K.R.; Sista, A.K.; Black, S.A.; et al. Antithrombotic therapy after venous interventions: AJR Expert Panel Narrative Review. Am. J. Roentgenol. 2022, 219, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Chen, C.; Li, Y.; Lv, Q.; Li, X.; Guo, D.; Shi, Z.; Fu, W.; Zhang, W.W.; POATIVES Collaborative Study Group; et al. Principles of optimal antithrombotic therapy for iliac venous stenting (POATIVES): A national expert-based Delphi consensus study. J. Vasc. Surg. Venous Lymphat. Disord. 2024, 12, 101739. [Google Scholar] [CrossRef]
- Rozmus, G.; Daubert, J.P.; Huang, D.T.; Rosero, S.; Hall, B.; Francis, C. Venous thrombosis and stenosis after implantation of pacemakers and defibrillators. J. Interv. Card. Electrophysiol. 2005, 13, 9–19. [Google Scholar] [CrossRef] [PubMed]
| Total n = 11 | |
|---|---|
| Age | 75.4 ± 10.6 |
| Female sex | 3 (27%) |
| Creatinine, mg/dL | 1.6 ± 1.1 |
| Hb, mg/dL | 11.8 ± 2.2 |
| LVEF, % | 34.6 ± 11.6 |
| Hypertension | 5 (45%) |
| Diabetes | 3 (27%) |
| CAD | 10 (91%) |
| Prior MI | 3 (27%) |
| Smoker | 4 (36%) |
| PAVK | 2 (18%) |
| PE | 0 (0%) |
| On anticoagulation | 8 (73%) |
| Patient | Planned Procedure | Indication | Venous Lesion | Intervention | Outcome |
|---|---|---|---|---|---|
| 1 | ICD → CRT-D upgrade | HFrEF, LSB, QRS 190 ms | Left subclavian vein occlusion | Attempted recanalization via right femoral vein | Recanalization unsuccessful—CRT-D not implanted |
| 2 | 2-chamber pacemaker → CRT-P upgrade | HFrEF, LSB, QRS 190 ms | Left subclavian and brachiocephalic vein stenosis | Successful PTA via left subclavian vein | CRT-P successfully implanted |
| 3 | 2-chamber pacemaker → CRT upgrade | HFmrEF, increased ventricular pacing | Left subclavian vein occlusion | Recanalization and PTA via axillary/brachial vein | Reocclusion next day |
| 4 | New DDD pacemaker post Candida endocarditis | Endocarditis | Superior vena cava (SVC) stenosis after atrioplasty | SVC stenting via right femoral vein, lead extraction and reimplantation | Device revision successful |
| 5 | Re-implantation of LV lead | LV lead dislocation | Venous stenosis | Successful PTA of subclavian and brachiocephalic veins via left subclavian vein | LV lead revised and repositioned |
| 6 | 2-chamber pacemaker → CRT-D upgrade | LSB, HFrEF | Chronic brachiocephalic and subclavian vein occlusion | Successful recanalization and PTA | CRT-D upgrade successful |
| 7 | 2-chamber pacemaker → CRT-D upgrade | LVEF deterioration, increased ventricular pacing | Right subclavian vein stenosis | Right subclavian PTA via axillary/brachial vein | CRT-D upgrade successful |
| 8 | Dual-chamber pacemaker → CRT-P upgrade | LVEF deterioration, increased ventricular pacing | Left subclavian vein occlusion | Recanalization and PTA via lateral left subclavian vein | CRT-P upgrade successful |
| 9 | 2-chamber pacemaker → CRT-D upgrade | LVEF deterioration, increased ventricular pacing | Left subclavian vein occlusion | Recanalization and PTA via left subclavian vein | Recanalization successful; CRT-D upgrade not possible due to coronary sinus anatomy—upgraded to LBB stimulation system |
| 10 | Dual-chamber pacemaker → CRT-P upgrade | LSB, HFrEF | Left subclavian vein occlusion | Recanalization and PTA via left subclavian vein | CRT-P upgrade successful |
| 11 | ICD implantation | HFrEF | Right subclavian vein occlusion, left shunt arm | PTA of right brachiocephalic vein | CRT upgrade successful |
| Patient | Follow-Up Duration | Follow-Up Outcome |
|---|---|---|
| 1 | 7 months | unremarkable findings |
| 2 | 15 months | unremarkable findings |
| 3 | 39 months | unremarkable findings |
| 4 | No follow-up at our center | |
| 5 | 1 months | diaphragmatic stimulation, reprogramming; no further abnormalities |
| 6 | No follow-up yet | |
| 7 | No follow-up yet | |
| 8 | 4 months | RV lead with complete exit block 2 months after implantation |
| 9 | No follow-up at our center | |
| 10 | 4 months | LV lead impedance drop due to dislocation 2 months after implantation. Re-occlusion on the subclavian vein. |
| 11 | 34 months | unremarkable findings |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frydas, A.; Baehr, F.-L.; Dreger, H.; Boldt, L.-H.; Parwani, A.S.; Hindricks, G.; Gebauer, B.; Hilgendorf, I.; Blaschke, F. Angioplasty in Patients with Central Venous Occlusion Prior to Device Lead Implantation. J. Cardiovasc. Dev. Dis. 2025, 12, 457. https://doi.org/10.3390/jcdd12120457
Frydas A, Baehr F-L, Dreger H, Boldt L-H, Parwani AS, Hindricks G, Gebauer B, Hilgendorf I, Blaschke F. Angioplasty in Patients with Central Venous Occlusion Prior to Device Lead Implantation. Journal of Cardiovascular Development and Disease. 2025; 12(12):457. https://doi.org/10.3390/jcdd12120457
Chicago/Turabian StyleFrydas, Athanasios, Felix-Lucas Baehr, Henryk Dreger, Leif-Hendrik Boldt, Abdul Shokor Parwani, Gerhard Hindricks, Bernhard Gebauer, Ingo Hilgendorf, and Florian Blaschke. 2025. "Angioplasty in Patients with Central Venous Occlusion Prior to Device Lead Implantation" Journal of Cardiovascular Development and Disease 12, no. 12: 457. https://doi.org/10.3390/jcdd12120457
APA StyleFrydas, A., Baehr, F.-L., Dreger, H., Boldt, L.-H., Parwani, A. S., Hindricks, G., Gebauer, B., Hilgendorf, I., & Blaschke, F. (2025). Angioplasty in Patients with Central Venous Occlusion Prior to Device Lead Implantation. Journal of Cardiovascular Development and Disease, 12(12), 457. https://doi.org/10.3390/jcdd12120457






