Linking Myocardial Infarction and Frailty Status at Old Age in Europe: Moderation Effects of Country and Gender
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Variables
- Fatigue was measured using the following question: “In the last month, have you had little energy to do the things you wanted to do?” with a dichotomous answer of “yes” or “no”.
- Weakness was recorded from the highest of four consecutive dynamometer measurements of grip strength, making two in each hand, and applying the limits of gender and body mass index cutoffs set by Fried et al.
- Unintentional weight loss was recorded by reporting “a diminution in desire for food” in response to the question “How has your appetite been like?” or, in the case of an uncodable response to this question, by answering “less” to the question “So, have you been eating more or less than usual”?
- Physical activity was assessed in participants responding: “How often do you engage in activities that require a low or moderate level of energy, such as gardening, cleaning the car or going for a walk?” This criterion was fulfilled when the answer was “one to three times a month”, “hardly ever”, or “never”.
- Slowness was recorded as a positive answer to any of the following questions: “Because of a health problem, do you have difficulty walking 100 m?” and “Because of a health problem, do you have difficulty climbing one flight of stairs without resting?” The difficulties are expected to last more than 3 months.
2.3. Statistical Analyses
3. Results
3.1. Descriptive Statistics
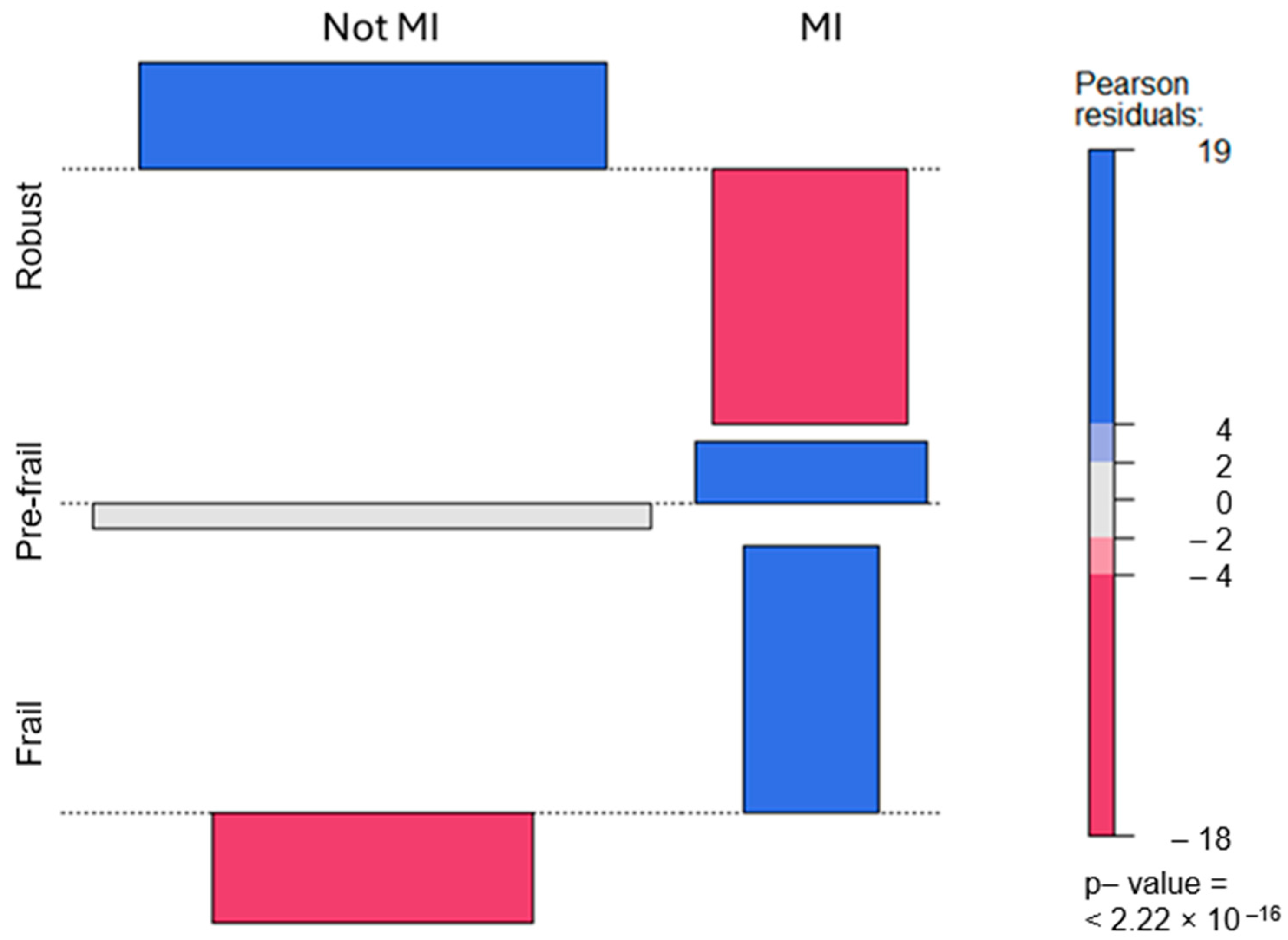
3.2. Relationship between Frailty and Myocardial Infarction
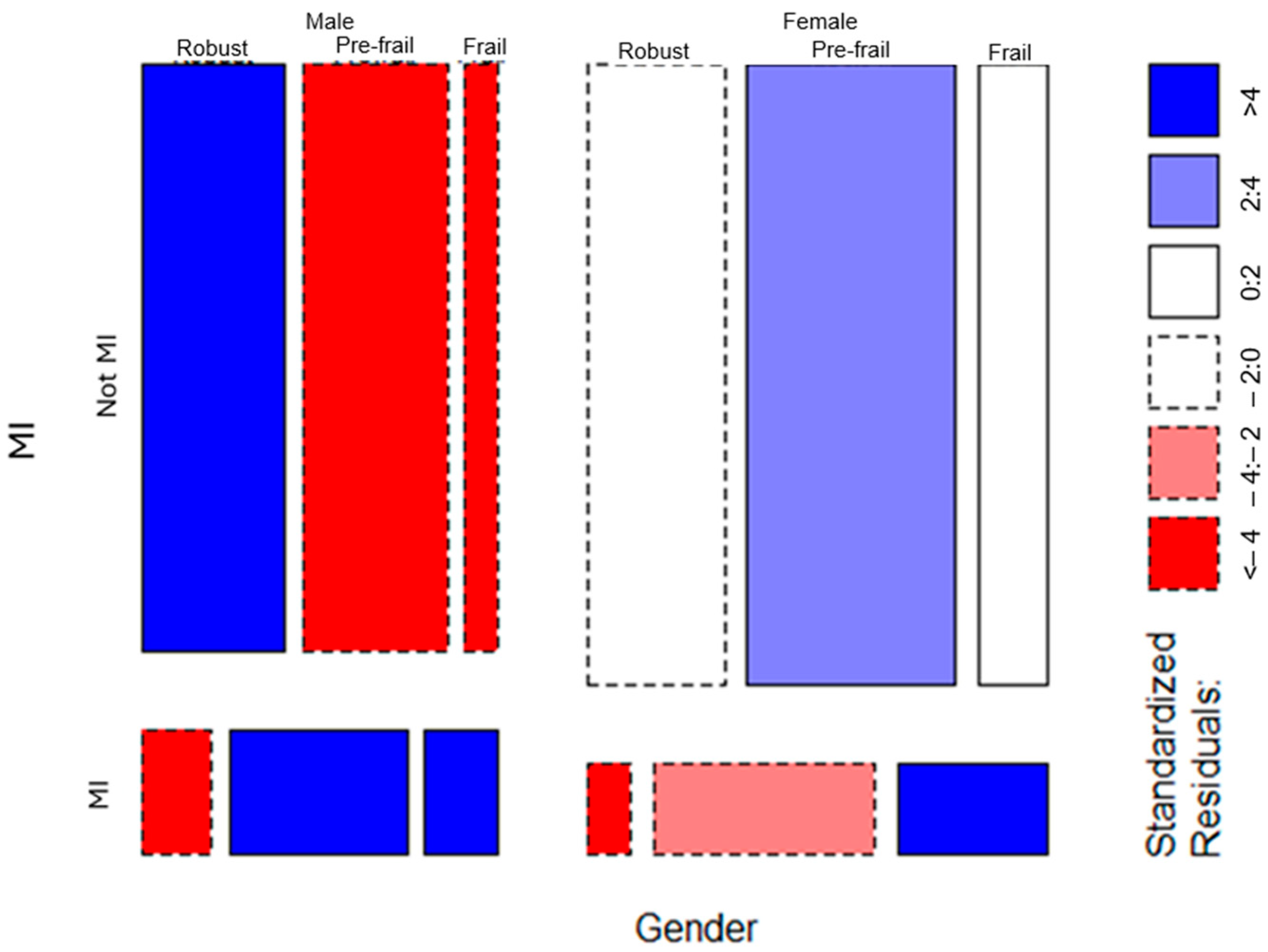
3.3. Moderation by Gender and Country
3.4. Multivariate Prediction of Myocardial Infarction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 25, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, B.R.; Cannata, F.; Stefanini, G.G.; Bolognese, L. Myocardial ischemia and coronary disease in heart failure. Heart Fail. Rev. 2020, 25, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.W.; Sidney, S.; Chandra, M.; Sorel, M.; Selby, J.V.; Go, A.S. Population trends in the incidence and outcomes of acute myocardial infarction. N. Engl. J. Med. 2010, 362, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Windecker, S. Fourth universal definition of myocardial infarction. Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The global prevalence of myocardial infarction: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef] [PubMed]
- Dégano, I.R.; Salomaa, V.; Veronesi, G.; Ferriéres, J.; Kirchberger, I.; Laks, T.; Havulinna, A.S.; Ruidavets, J.-B.; Ferrario, M.M.; Meisinger, C.; et al. Twenty-five-year trends in myocardial infarction attack and mortality rates, and case-fatality, in six European populations. Heart 2015, 101, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Qian, S.; Huang, Y.; Zhang, Y.; Peng, Z.; Li, Q.; Shu, B.; Zhu, L.; Wang, M. Drug Discovery for Coronary Artery Disease. Adv. Exp. Med. Biol. 2020, 1177, 297–339. [Google Scholar] [CrossRef] [PubMed]
- López, J.A.; Bellido, C.M.; Simón, P.H.; Padial, L.R. Cardiopatía isquémica: Concepto, clasificación, epidemiología, factores de riesgo, pronóstico y prevención. Medicine 2017, 12, 2145–2152. [Google Scholar] [CrossRef]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European society of cardiology: Cardiovascular disease statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global epidemiology of ischemic heart disease: Results from the global burden of disease study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R. Cardiovascular disease and frailty: What are the mechanistic links? Clin. Chem. 2019, 65, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Buth, K.J.; Martin, B.J.; Yip, A.M.; Hirsch, G.M. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation 2010, 121, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Cereda, E.; Stubbs, B.; Solmi, M.; Luchini, C.; Manzato, E.; Sergi, G.; Manu, P.; Harris, T.; Fontana, L.; et al. Risk of cardiovascular disease morbidity and mortality in frail and pre-frail older adults: Results from a meta-analysis and exploratory meta-regression analysis. Ageing Res. Rev. 2017, 35, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, T.; Raja, M.; Radha, D.; Gaur, T.A.; Geetha, J.; Sakthivadivel, V. Risk factors and inflammatory markers in acute coronary syndrome-ST elevation myocardial infarction (STEMI). Horm. Mol. Biol. Clin. Investig. 2023, 44, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Wleklik, M.; Denfeld, Q.; Lisiak, M.; Czapla, M.; Kałuzna-Oleksy, M.; Uchmanowicz, I. Frailty Syndrome in Older Adults with Cardiovascular Diseases–What Do We Know and What Requires Further Research? Int. J. Environ. Res. Public Health 2022, 19, 2234. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Arik, F.; Smith, L.; Jackson, S.E.; Isik, A.T. Inflammation, Frailty and Cardiovascular Disease. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2020; Volume 1216, pp. 55–64. [Google Scholar] [CrossRef]
- Udell, J.A.; Lu, D.; Bagai, A.; Dodson, J.A.; Desai, N.R.; Fonarow, G.C.; Goyal, A.; Garratt, K.N.; Lucas, J.; Weintraub, W.S.; et al. Preexisting frailty and outcomes in older patients with acute myocardial infarction. Am. Heart J. 2022, 249, 34–44. [Google Scholar] [CrossRef]
- Yu, Q.; Guo, D.; Peng, J.; Wu, Q.; Yao, Y.; Ding, M.; Wang, J. Prevalence and adverse outcomes of frailty in older patients with acute myocardial infarction after percutaneous coronary interventions: A systematic review and meta-analysis. Clin. Cardiol. 2023, 46, 5–12. [Google Scholar] [CrossRef]
- Zhang, S.; Meng, H.; Chen, Q.; Wang, X.; Zou, J.; Hao, Q.; Yang, M.; Wu, J. Is frailty a prognostic factor for adverse outcomes in older patients with acute coronary syndrome? Aging. Clin. Exp. Res. 2020, 32, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Majid, Z.; Welch, C.; Davies, J.; Jackson, T. Global frailty: The role of ethnicity, migration and socioeconomic factors. Maturitas 2020, 139, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fathima, S.N. An Update on Myocardial Infarction. In Current Research and Trends in Medical Science and Technology; Scripown Publications: New Delhi, India, 2021; Volume 1, p. 216. [Google Scholar]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Rohlfs, I.; García, M.M.; Gavaldà, L.; Medrano, M.J.; Juvinyà, D.; Baltasar, A.; Saurina, C.; Faixedasa, M.T.; Muñoz, D. Género y cardiopatía isquémica. Gac. Sanit. 2004, 18, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ahrenfeldt, L.J.; Möller, S.; Thinggaard, M.; Christensen, K.; Lindahl-Jacobsen, R. Sex Differences in Comorbidity and Frailty in Europe. Int. J. Public Health 2019, 64, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.H.; Peel, N.M.; Samanta, M.; Theou, O.; Howlett, S.E.; Hubbard, R.E. Sex differences in frailty: A systematic review and meta-analysis. Exp. Gerontol. 2017, 89, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.M.; Strange, J.E.; Falkentoft, A.C.; El-Chouli, M.; Ravn, P.B.; Ruwald, A.C.; Fosbøl, E.; Køber, L.; Gislason, G.; Sehested, T.S.G.; et al. Frailty, Treatments, and Outcomes in Older Patients with Myocardial Infarction: A Nationwide Registry-Based Study. J. Am. Heart Assoc. 2023, 12, e030561. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.M.; Gale, C.P.; Lip, G.; Martin-Sanchez, F.J.; McIntyre, H.F.; Mueller, C.; Price, S.; Sanchis, J.; Vidan, M.T.; Wilkinson, C.; et al. Editor’s Choice—Frailty and the management of patients with acute cardiovascular disease: A position paper from the Acute Cardiovascular Care Association. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 176–193. [Google Scholar] [CrossRef]
- Börsch-Supan, A.; Brandt, M.; Hunkler, C.; Kneip, T.; Korbmacher, J.; Malter, F.; Schaan, B.; Stuck, S.; Zuber, S.; SHARE Central Coordination Team. Data Resource Profile: The Survey of Health, Ageing and Retirementin Europe (SHARE). Int. J. Epidemiol. 2013, 42, 992–1001. [Google Scholar] [CrossRef]
- Börsch-Supan, A. Survey of Health Ageing and Retirement in Europe (SHARE) Wave 6 Release, Version: 6.1.1; SHARE-ERIC: Munich, Germany, 2018. [Google Scholar]
- Malter, F.; Börsch-Supan, A. SHARE Wave 6: Panel Innovations and Collecting Dried Blood Spots; Munich Center for the Economics of Aging (MEA): Munich, Germany, 2017. [Google Scholar]
- Santos-Eggimann, B.; Cuenoud, P.; Spagnoli, J.; Junod, J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2009, 64, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ortuno, R.; Fouweather, T.; Jagger, C. Cross-national disparities in sex differences in life expectancy with and without frailty. Age Ageing 2014, 43, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ortuño, R. The frailty instrument for primary care of the Survey of Health, Ageing and Retirement in Europe (SHARE-FI) predicts mortality beyond age, comorbidities, disability, self-rated health, education and depression. Eur. Geriatr. Med. 2011, 2, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ortuño, R.; Walsh, C.D.; Lawlor, B.A.; Kenny, R.A. A frailty instrument for primary care: Findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). BMC Geriatr. 2010, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Macklai, N.S.; Spagnoli, J.; Junod, J.; Santos-Eggimann, B. Prospective association of the SHARE-operationalized frailty phenotype with adverse health outcomes: Evidence from 60+ community-dwelling Europeans living in 11 countries. BMC Geriatr. 2013, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Lakomy, M. Prevalence of activities in later life across European regions. Cent. Eur. J. Public Policy 2020, 14, 14–27. [Google Scholar] [CrossRef]
- Formanek, T.; Kagstrom, A.; Winkler, P.; Cermakova, P. Differences in cognitive performance and cognitive decline across European regions: A population-based prospective cohort study. Eur. Psychiatry 2019, 58, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics; Harper Collins: New York, NY, USA, 2012. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 16 February 2024).
- Warnes, G.R.; Bolker, B.; Lumley, T.; Johnson, R.C. gmodels: Various R Programming Tools for Model Fitting R Package, Version 2.18.1.1; 2022. Available online: https://CRAN.R-project.org/package=gmodels (accessed on 16 February 2024).
- Meyer, D.; Zeileis, A.; Hornik, K. vcd: Visualizing Categorical Data R Package, Version 4.1.3. 2022, pp. 10–14. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=d5c07c051d6a8fc15e5667648e1d796a0a276e78 (accessed on 8 May 2024).
- Signorell, A.; Aho, K.; Alfons, A.; Anderegg, N.; Aragon, T.; Arachchige, C.; Arppe, A.; Baddeley, A.; Barton, K.; Bolker, B.; et al. DescTools: Tools for Descriptive Statistics R Package, Version 0.99.47. 2022. Available online: https://cran.r-project.org/package=DescTools (accessed on 8 May 2024).
- Patel, A.; Goodman, S.G.; Yan, A.T.; Alexander, K.P.; Wong, C.L.; Cheema, A.N.; Udell, J.A.; Kaul, P.; D’Souza, M.; Hyun, K.; et al. Frailty and Outcomes After Myocardial Infarction: Insights from the CONCORDANCE Registry. J. Am. Heart Assoc. 2018, 7, e009859. [Google Scholar] [CrossRef] [PubMed]
- Putthapiban, P.; Vutthikraivit, W.; Rattanawong, P.; Sukhumthammarat, W.; Kanjanahattakij, N.; Kewcharoen, J.; Amanullah, A. Association of frailty with all-cause mortality and bleeding among elderly patients with acute myocardial infarction: A systematic review and meta-analysis. J. Geriatr. Cardiol. 2020, 17, 270–278. [Google Scholar]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef]
- Garcia, M.; Mulvagh, S.L.; Merz, C.N.; Buring, J.E.; Manson, J.E. Cardiovascular Disease in Women: Clinical Perspectives. Circ. Res. 2016, 118, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.E.; Rockwood, K. Frailty in older women. Maturitas 2011, 69, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.C.; Wozniak, A.; Scott, C.; Chatterjee, A.; Titterton, G.N.; Corrigan, A.E.; Kuri, A.; Shah, V.; Soh, I.; Kaski, J.C. Between-Sex Differences in Risk Factors for Cardiovascular Disease among Patients with Myocardial Infarction—A Systematic Review. J. Clin. Med. 2023, 12, 5163. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.S.; Islam, S.; Rosengren, A.; Franzosi, M.G.; Steyn, K.; Yusufali, A.H.; Keltai, M.; Diaz, R.; Rangarajan, S.; Yusuf, S. Risk factors for myocardial infarction in women and men: Insights from the INTERHEART study. Eur. Heart J. 2008, 29, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V. Sex and gender differences in health: Science & Society Series on Sex and Science. EMBO Rep. 2012, 13, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Sagris, M.; Antonopoulos, A.S.; Theofilis, P.; Oikonomou, E.; Siasos, G.; Tsalamandris, S.; Antoniades, C.; Brilakis, E.S.; Kaski, J.C.; Tousoulis, D. Risk factors profile of young and older patients with myocardial infarction. Cardiovasc. Res. 2022, 118, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Cai, J.; Pamuk, E.R.; Williamson, D.F.; Thun, M.J.; Wood, J.L. The effect of age on the association between body-mass index and mortality. N. Engl. J. Med. 1998, 338, 1–7. [Google Scholar] [CrossRef]
- Bender, R.; Jöckel, K.H.; Trautner, C.; Spraul, M.; Berger, M. Effect of age on excess mortality in obesity. JAMA 1999, 281, 1498–1504. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Liu, L.; et al. Obesity and the risk of myocardial infarction in 27 000 participants from 52 countries: A case-control study. Lancet 2005, 366, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Roever, L.; Resende, E.S.; Diniz, A.L.; Penha-Silva, N.; Veloso, F.C.; Casella-Filho, A.; Dourado, P.M.; Chagas, A.C. Ectopic adiposopathy and association with cardiovascular disease risk factors: The Uberlandia Heart Study. Int. J. Cardiol. 2015, 190, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Xanthopoulos, A.; Parissis, J.; Butler, J.; Farmakis, D. Pathogenesis of chronic heart failure: Cardiovascular aging, risk factors, comorbidities, and disease modifiers. Heart Fail. Rev. 2022, 27, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wang, J.; Tian, J.; Tang, Y.D. Coronary Artery Disease: From Mechanism to Clinical Practice. Adv. Exp. Med. Biol. 2020, 1177, 1–36. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean ± SD o n (%) |
|---|---|
| Age | 71.96 ± 8.27 |
| Gender | |
| Male | 9766 (43.7) |
| Female | 12,590 (56.3) |
| Country | |
| Sweden | 3397 (15.2) |
| Spain | 4497 (20.1) |
| France | 2861 (12.8) |
| Greece | 3558 (15.9) |
| Czech Republic | 3896 (17.4) |
| Estonia | 4147 (18.5) |
| Marital status | |
| Married, living together with spouse | 14,316 (64.7) |
| Registered partnership | 231 (1.0) |
| Married, living separated from spouse | 240 (1.1) |
| Never married | 1036 (4.7) |
| Divorced | 1735 (7.8) |
| Widowed | 4581 (20.7) |
| Years of education | 10.48 ± 4.32 |
| Body mass index | 27.31 ± 4.57 |
| Number of comorbid conditions | 2.06 ± 1.65 |
| Myocardial infarction | 3307 (14.8) |
| High blood pressure or hypertension | 10705 (47.9) |
| High blood cholesterol | 5912 (26.4) |
| Diabetes or high blood sugar | 3777 (16.9) |
| Frailty | |
| Robust | 7744 (34.6) |
| Pre-frail | 10,985 (49.1) |
| Frail | 3627 (16.2) |
| Country | n (%) |
|---|---|
| Sweden | 394 (11.6%) |
| Spain | 543 (12.2%) |
| France | 446 (15.6%) |
| Greece | 506 (14.3%) |
| Czech Republic | 555 (14.3%) |
| Estonia | 865 (20.9%) |
| Not MI | MI | ||
|---|---|---|---|
| Count | 7166 | 538 | |
| Robust | Row percent | 93.017% | 6.983% |
| Standardized residual | 7.461 | −17.881 | |
| Count | 9174 | 1799 | |
| Pre-frail | Row percent | 83.605% | 16.395% |
| Standardized residual | −1.778 | 4.261 | |
| Count | 2655 | 970 | |
| Frail | Row percent | 73.241% | 26.759% |
| Standardized residual | −7.783 | 18.654 |
| Effects Included | Deviance | AIC | BIC | |
|---|---|---|---|---|
| Main | 1582.6 | 1880 | 1894 | |
| Country moderates | Main + two-way interactions | 12.4 | 344 | 385 |
| Main + two-way + three-way interactions | 0 | 352 | 409 | |
| Main | 1369.4 | 1486 | 1488 | |
| Gender moderates | Main + two-way interactions | 13 | 139 | 144 |
| Main + two-way + three-way interactions | 0 | 130 | 136 |
| 95% CI | ||||
|---|---|---|---|---|
| Predictor | p | OR | Lower | Upper |
| Constant | <0.001 | 0.01 | 0.00 | 0.01 |
| Age | <0.001 | 1.02 | 1.01 | 1.03 |
| Female vs. male | <0.001 | 0.51 | 0.46 | 0.56 |
| Body mass index | 0.927 | 0.99 | 0.98 | 1.01 |
| Physically inactive vs. active | <0.001 | 0.74 | 0.65 | 0.85 |
| Number of chronic diseases | <0.001 | 1.79 | 1.73 | 1.85 |
| Staying in hospital vs. not | <0.001 | 0.65 | 0.59 | 0.73 |
| Stroke vs. not | <0.001 | 0.28 | 0.23 | 0.34 |
| Blood cholesterol vs. not | 0.034 | 0.89 | 0.81 | 0.99 |
| Blood pressure vs. not | 0.002 | 0.84 | 0.76 | 0.93 |
| Coronary disease vs. not | <0.001 | 9.37 | 8.38 | 10.47 |
| Frailty | ||||
| Pre-frail vs. robust | <0.001 | 1.39 | 1.23 | 1.57 |
| Frail vs. robust | <0.001 | 1.56 | 1.34 | 1.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sentandreu-Mañó, T.; Torres, Z.; Luján-Arribas, C.; Tomás, J.M.; González-Cervantes, J.J.; Marques-Sule, E. Linking Myocardial Infarction and Frailty Status at Old Age in Europe: Moderation Effects of Country and Gender. J. Cardiovasc. Dev. Dis. 2024, 11, 176. https://doi.org/10.3390/jcdd11060176
Sentandreu-Mañó T, Torres Z, Luján-Arribas C, Tomás JM, González-Cervantes JJ, Marques-Sule E. Linking Myocardial Infarction and Frailty Status at Old Age in Europe: Moderation Effects of Country and Gender. Journal of Cardiovascular Development and Disease. 2024; 11(6):176. https://doi.org/10.3390/jcdd11060176
Chicago/Turabian StyleSentandreu-Mañó, Trinidad, Zaira Torres, Cecilia Luján-Arribas, José M. Tomás, José Javier González-Cervantes, and Elena Marques-Sule. 2024. "Linking Myocardial Infarction and Frailty Status at Old Age in Europe: Moderation Effects of Country and Gender" Journal of Cardiovascular Development and Disease 11, no. 6: 176. https://doi.org/10.3390/jcdd11060176
APA StyleSentandreu-Mañó, T., Torres, Z., Luján-Arribas, C., Tomás, J. M., González-Cervantes, J. J., & Marques-Sule, E. (2024). Linking Myocardial Infarction and Frailty Status at Old Age in Europe: Moderation Effects of Country and Gender. Journal of Cardiovascular Development and Disease, 11(6), 176. https://doi.org/10.3390/jcdd11060176








