Echocardiographic-Fluoroscopic Fusion Imaging Improves Interventionalists’ Learning Curve for Percutaneous Left Atrial Appendage Closure—Initial, Single-Center, Retrospective Observations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Intervention
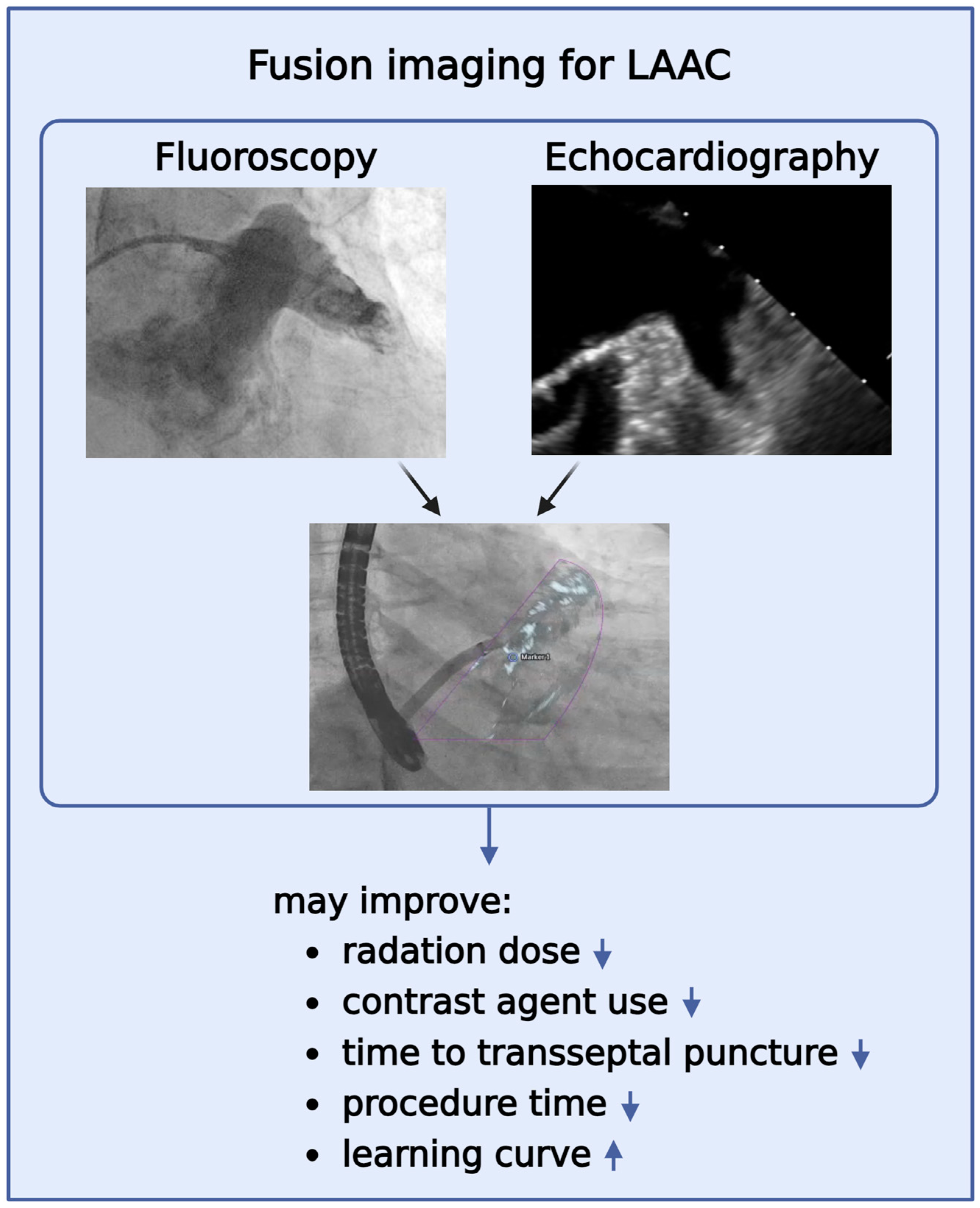
2.3. Real-Time Echocardiography-Fluoroscopy Fusion (FI) Imaging during LAAC
2.4. Learning Curve and Procedural Parameters
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Learning Curve Analyses
3.3. Learning Curve Analysis of Interventionalist 1
3.4. Learning Curve Analysis of Interventionalist 2
3.5. Analysis of FI’s Impact on Learning Curves
3.6. Procedural Complications
4. Discussion
- The left atrial appendage closure learning curve has a flat course without FI.
- FI may improve the left atrial appendage closure learning curve.
- Even highly experienced interventionalists may benefit from FI guidance in their early phase of left atrial appendage closure training.
Limitations
5. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CAD | Coronary Artery Disease |
| CKD | Chronic Kidney Disease |
| COPD | Chronic Obstructive Pulmonary Disease |
| CV | Contrast Volume |
| DAP | Dose Area Product |
| EAPCI | European Association of Percutaneous Cardiovascular Interventions |
| FI | Fusion Imaging |
| FT | Fluoroscopy Time |
| IC | Interventional Cardiologist |
| IQR | Interquartile Range |
| LA | Left Atrium |
| LAA | Left Atrial Appendage |
| LAAC | Left Atrial Appendage Closure |
| MACE | Major Adverse Cardiac Events |
| NOAC | Novel oral anticoagulant |
| PT | Procedure Time |
| RAO | Right Anterior Oblique |
| SD | Standard Deviation |
| TEE | Transoesophageal Echocardiography |
| TSP | Transseptal Puncture |
| VKA | Vitamin K Antagonist |
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Doshi, S.K.; Kar, S.; Gibson, D.N.; Price, M.J.; Huber, K.; Horton, R.P.; Buchbinder, M.; Neuzil, P.; Gordon, N.T.; et al. 5-Year Outcomes after Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J. Am. Coll. Cardiol. 2017, 70, 2964–2975. [Google Scholar] [CrossRef] [PubMed]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. 4-Year Outcomes after Left Atrial Appendage Closure versus Nonwarfarin Oral Anticoagulation for Atrial Fibrillation. J. Am. Coll. Cardiol. 2022, 79, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, N.C.; Beigel, R.; Swaans, M.J.; Ho, S.Y.; Siegel, R.J. Percutaneous interventions for left atrial appendage exclusion: Options, assessment, and imaging using 2D and 3D echocardiography. JACC Cardiovasc. Imaging 2015, 8, 472–488. [Google Scholar] [CrossRef] [PubMed]
- Biaggi, P.; Sager, D.F.; Kulling, J.; Kuest, S.; Wyss, C.; Hurlimann, D.; Reho, I.; Buhler, I.; Noll, G.; Huber, M.; et al. Potential Value of Fusion Imaging and Automated Three-Dimensional Heart Segmentation during Transcatheter Aortic Valve Replacement. J. Am. Soc. Echocardiogr. 2020, 33, 516–517.E1. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R., Jr.; Korsholm, K.; Rodés-Cabau, J.; Saw, J.; Berti, S.; Alkhouli, M.A. Left atrial appendage occlusion. EuroIntervention 2023, 18, e1038–e1065. [Google Scholar] [CrossRef] [PubMed]
- Qamar, S.R.; Jalal, S.; Nicolaou, S.; Tsang, M.; Gilhofer, T.; Saw, J. Comparison of cardiac computed tomography angiography and transoesophageal echocardiography for device surveillance after left atrial appendage closure. EuroIntervention 2019, 15, 663–670. [Google Scholar] [CrossRef]
- Freixa, X.; Aminian, A.; Tzikas, A.; Saw, J.; Nielsen-Kudsk, J.E.; Ghanem, A.; Schmidt, B.; Hildick-Smith, D. Left atrial appendage occlusion with the Amplatzer Amulet: Update on device sizing. J. Interv. Card. Electrophysiol. 2020, 59, 71–78. [Google Scholar] [CrossRef]
- Simsek, B.; Kostantinis, S.; Karacsonyi, J.; Hakeem, A.; Prasad, A.; Prasad, A.; Bortnick, A.E.; Elbarouni, B.; Jneid, H.; Abbott, J.D.; et al. Educational Experience of Interventional Cardiology Fellows in the United States and Canada. JACC Cardiovasc. Interv. 2023, 16, 247–257. [Google Scholar] [CrossRef]
- Joshi, A.; Wragg, A. Simulator Training in Interventional Cardiology. Interv. Cardiol. Rev. 2016, 11, 70–73. [Google Scholar] [CrossRef]
- Ledwoch, J.; Krollmann, C.; Staubach, S.; Hug, M.; Strohm, H.; Mudra, H. Learning Curve Assessment for Percutaneous Left Atrial Appendage Closure with the WATCHMAN Occluder. J. Interv. Cardiol. 2016, 29, 393–399. [Google Scholar] [CrossRef]
- Jung, R.G.; Simard, T.; Killu, A.; Harris, A.A.; Hohmann, S.F.; Holmes, D.R.; Alkhouli, M. Learning Curve and Outcomes of Left Atrial Appendage Closure. JACC Cardiovasc. Interv. 2021, 14, 2750–2752. [Google Scholar] [CrossRef] [PubMed]
- Berti, S.; Pastormerlo, L.E.; Korsholm, K.; Saw, J.; Alkhouli, M.; Costa, M.P.; Odenstedt, J.; Packer, E.J.; Tondo, C.; Santoro, G.; et al. Intracardiac echocardiography for guidance of transcatheter left atrial appendage occlusion: An expert consensus document. Catheter. Cardiovasc. Interv. 2021, 98, 815–825. [Google Scholar] [CrossRef]
- Jungen, C.; Zeus, T.; Balzer, J.; Eickholt, C.; Petersen, M.; Kehmeier, E.; Veulemans, V.; Kelm, M.; Willems, S.; Meyer, C. Left Atrial Appendage Closure Guided by Integrated Echocardiography and Fluoroscopy Imaging Reduces Radiation Exposure. PLoS ONE 2015, 10, e0140386. [Google Scholar] [CrossRef] [PubMed]
- Balzer, J.; Zeus, T.; Hellhammer, K.; Veulemans, V.; Eschenhagen, S.; Kehmeier, E.; Meyer, C.; Rassaf, T.; Kelm, M. Initial clinical experience using the EchoNavigator®-system during structural heart disease interventions. World J. Cardiol. 2015, 7, 562–570. [Google Scholar] [CrossRef]
- Zorinas, A.; Zakarkaitė, D.; Janušauskas, V.; Austys, D.; Puodžiukaitė, L.; Zuozienė, G.; Samalavičius, R.S.; Jovaišienė, I.; Davidavičius, G.; Ručinskas, K.; et al. Technical Recommendations for Real-Time Echocardiography and Fluoroscopy Imaging Fusion in Catheter-Based Mitral Valve Paravalvular Leak and Other Procedures. J. Clin. Med. 2022, 11, 1328. [Google Scholar] [CrossRef]
- Afzal, S.; Piayda, K.; Hellhammer, K.; Veulemans, V.; Wolff, G.; Heidari, H.; Stüwe, D.; Kanschik, D.; Polzin, A.; Kelm, M.; et al. Real-time echocardiography-fluoroscopy fusion imaging for left atrial appendage closure: Prime time for fusion imaging? Acta Cardiol. 2021, 76, 1004–1012. [Google Scholar] [CrossRef]
- Van Belle, E.; Teles, R.C.; Pyxaras, S.A.; Kalpak, O.; Johnson, T.W.; Barbash, I.M.; De Luca, G.; Kostov, J.; Parma, R.; Vincent, F.; et al. EAPCI Core Curriculum for Percutaneous Cardiovascular Interventions (2020): Committee for Education and Training European Association of Percutaneous Cardiovascular Interventions (EAPCI). A branch of the European Society of Cardiology. EuroIntervention 2021, 17, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. EuroIntervention 2020, 15, 1133–1180. [Google Scholar] [CrossRef]
- Jone, P.N.; Haak, A.; Petri, N.; Ross, M.; Morgan, G.; Wiktor, D.M.; Gill, E.; Quaife, R.A.; Messenger, J.C.; Salcedo, E.E.; et al. Echocardiography-Fluoroscopy Fusion Imaging for Guidance of Congenital and Structural Heart Disease Interventions. JACC Cardiovasc. Imaging 2019, 12, 1279–1282. [Google Scholar] [CrossRef]
- Tzikas, A.; Holmes, D.R., Jr.; Gafoor, S.; Ruiz, C.E.; Blomstrom-Lundqvist, C.; Diener, H.C.; Cappato, R.; Kar, S.; Lee, R.J.; Byrne, R.A.; et al. Percutaneous left atrial appendage occlusion: The Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. Europace 2017, 19, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Rodeghiero, F.; Tosetto, A.; Abshire, T.; Arnold, D.M.; Coller, B.; James, P.; Neunert, C.; Lillicrap, D. ISTH/SSC bleeding assessment tool: A standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J. Thromb. Haemost. 2010, 8, 2063–2065. [Google Scholar] [CrossRef]
- Aeckersberg, G.; Gkremoutis, A.; Schmitz-Rixen, T.; Kaiser, E. The relevance of low-fidelity virtual reality simulators compared with other learning methods in basic endovascular skills training. J. Vasc. Surg. 2019, 69, 227–235. [Google Scholar] [CrossRef]
- Kalra, A.; Bhatt, D.L.; Kleiman, N.S. A 24-Month Interventional Cardiology Fellowship: Learning Motor Skills through Blocked Repetition. JACC Cardiovasc. Interv. 2017, 10, 210–211. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, N.S.; Welt, F.G.P.; Truesdell, A.G.; Sherwood, M.; Kadavath, S.; Shah, P.B.; Klein, L.W.; Hogan, S.; Kavinsky, C.; Rab, T. Should Interventional Cardiologists Super-Subspecialize?: Moving from Patient Selection to Operator Selection. JACC Cardiovasc. Interv. 2021, 14, 97–100. [Google Scholar] [CrossRef]
- Salemi, A.; Sedrakyan, A.; Mao, J.; Elmously, A.; Wijeysundera, H.; Tam, D.Y.; Di Franco, A.; Redwood, S.; Girardi, L.N.; Fremes, S.E.; et al. Individual Operator Experience and Outcomes in Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chhatriwalla, A.K.; Vemulapalli, S.; Szerlip, M.; Kodali, S.; Hahn, R.T.; Saxon, J.T.; Mack, M.J.; Ailawadi, G.; Rymer, J.; Manandhar, P.; et al. Operator Experience and Outcomes of Transcatheter Mitral Valve Repair in the United States. J. Am. Coll. Cardiol. 2019, 74, 2955–2965. [Google Scholar] [CrossRef]
- Bertsche, D.; Pfisterer, M.; Dahme, T.; Schneider, L.M.; Metze, P.; Vernikouskaya, I.; Rasche, V. MRI-based training model for left atrial appendage closure. Int. J. Comput. Assist. Radiol. Surg. 2023, 18, 2111–2116. [Google Scholar] [CrossRef]
- Sawant, A.C.; Seibolt, L.; Sridhara, S.; Rodriguez, J.; Distler, E.; Murarka, S.; Lazkani, M.; Kumar, A.; Kanwar, N.; Prakash, M.P.H.; et al. Operator Experience and Outcomes after Transcatheter Left Atrial Appendage Occlusion with the Watchman Device. Cardiovasc. Revasc. Med. 2020, 21, 467–472. [Google Scholar] [CrossRef]
- Wang, D.D.; Eng, M.; Kupsky, D.; Myers, E.; Forbes, M.; Rahman, M.; Zaidan, M.; Parikh, S.; Wyman, J.; Pantelic, M.; et al. Application of 3-Dimensional Computed Tomographic Image Guidance to WATCHMAN Implantation and Impact on Early Operator Learning Curve: Single-Center Experience. JACC Cardiovasc. Interv. 2016, 9, 2329–2340. [Google Scholar] [CrossRef]
- Afzal, S.; Veulemans, V.; Balzer, J.; Rassaf, T.; Hellhammer, K.; Polzin, A.; Kelm, M.; Zeus, T. Safety and efficacy of transseptal puncture guided by real-time fusion of echocardiography and fluoroscopy. Neth. Heart J. 2017, 25, 131–136. [Google Scholar] [CrossRef]
- Ebelt, H.; Domagala, T.; Offhaus, A.; Wiora, M.; Schwenzky, A.; Hoyme, M.; Anacker, J.; Röhl, P. Fusion Imaging of X-ray and Transesophageal Echocardiography Improves the Procedure of Left Atrial Appendage Closure. Cardiovasc. Drugs Ther. 2020, 34, 781–787. [Google Scholar] [CrossRef]
- Nelles, D.; Schrickel, J.W.; Nickenig, G.; Sedaghat, A. Percutaneous left atrial appendage closure using the TrueFusion™ fusion-imaging technology. Clin. Res. Cardiol. 2020, 109, 646–648. [Google Scholar] [CrossRef]
- Blusztein, D.I.; Gogia, S.; Hahn, R.T.; Sommer, R.J.; Ng, V.; Forman, J.; Lebehn, M.; Ranard, L.; Vahl, T.P. Zero-Contrast Left Atrial Appendage Occlusion Using a Hybrid Echocardiography-Fluoroscopy Technique without Iodinated Contrast. Am. J. Cardiol. 2023, 198, 53–55. [Google Scholar] [CrossRef]
- Chen, T.; Liu, G.; Mu, Y.; Xu, W.H.; Guo, Y.T.; Guo, J.; Chen, Y.D. Application of cardiac computed tomographic imaging and fluoroscopy fusion for guiding left atrial appendage occlusion. Int. J. Cardiol. 2021, 331, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.K.; Horvilleur, J.; Cormier, B.; Cazalas, M.; Fernandez, L.; Patane, M.; Neylon, A.; Spaziano, M.; Sawaya, F.J.; Arai, T.; et al. Novel integrated 3D multidetector computed tomography and fluoroscopy fusion for left atrial appendage occlusion procedures. Catheter. Cardiovasc. Interv. 2018, 91, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Mo, B.F.; Wan, Y.; Alimu, A.; Sun, J.; Zhang, P.P.; Yu, Y.; Chen, M.; Li, W.; Wang, Z.Q.; Wang, Q.S.; et al. Image fusion of integrating fluoroscopy into 3D computed tomography in guidance of left atrial appendage closure. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Motiwala, A.; O’Neill, B.; Patil, P. Novel use of fused cardiac computed tomography and transesophageal echocardiography for left atrial appendage closure. Catheter. Cardiovasc. Interv. 2021, 97, E719–E723. [Google Scholar] [CrossRef] [PubMed]
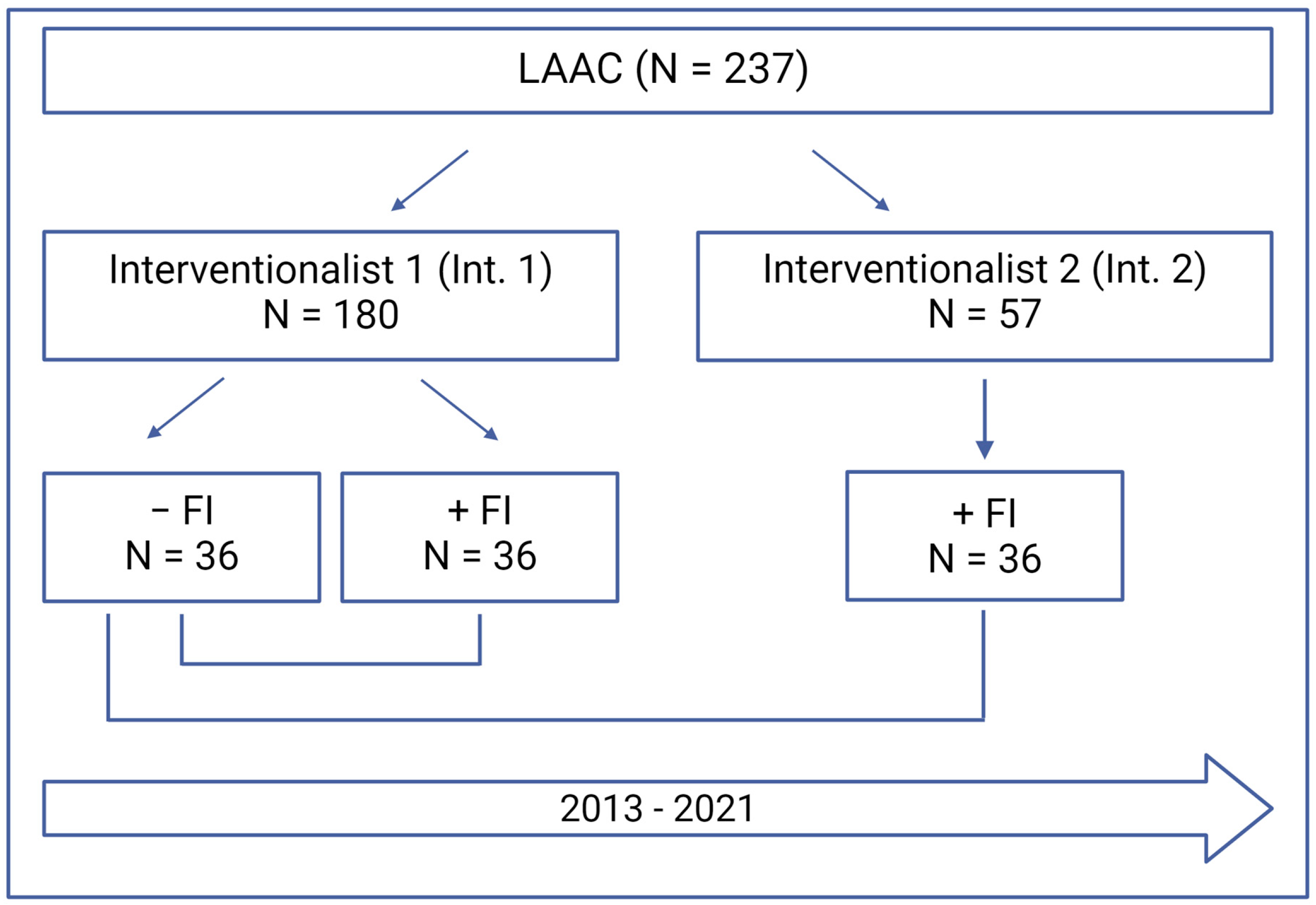


| Variable | Interventionalist 1 −FI, N = 36 | Interventionalist 1 +FI, N = 36 | Interventionalist 2 +FI, N = 36 | p-Value |
|---|---|---|---|---|
| Age (Y), M ± SD | 75 ± 9 | 78 ± 6 | 75 ± 9 | 0.222 |
| Height (cm), M ± SD | 170.6 ± 8.3 | 169.8 ± 8.7 | 171 ± 9.6 | 0.919 |
| Weight (kg), M ± SD | 80.1 ± 14.2 | 78.6 ± 14.1 | 80.7 ± 21.9 | 0.816 |
| BMI (m/kg2), M ± SD | 27.4 ± 2.2 | 27.3 ± 4.4 | 27.5 ± 7.1 | 0.764 |
| CHA2DS2-VASc-Score | 4 ± 1 | 4 ± 2 | 4 ± 1 | 0.919 |
| HAS-BLED-Score | 3 ± 1 | 4 ± 1 | 2 ± 1 | <0.001 |
| Heart failure, N (%) | 20 (55.6%) | 17 (47.2%) | 22 (61.1%) | 0.492 |
| CKD, N (%) | 19 (52.8%) | 17 (47.2%) | 13 (36.1%) | 0.351 |
| CAD, N (%) | 29 (80.6%) | 22 (61.1%) | 20 (55.6%) | 0.064 |
| Heart surgery, N (%) | 11 (30.6%) | 7 (19.4%) | 6 (16.7%) | 0.325 |
| PCI, N (%) | 20 (55.6%) | 14 (38.9%) | 11 (30.6%) | 0.091 |
| Hypercholesterolemia, N (%) | 30 (83.3%) | 28 (77.8%) | 26 (72.2%) | 0.526 |
| Arterial hypertension, N (%) | 32 (88.9%) | 33 (91.7%) | 34 (94.4%) | 0.695 |
| Diabetes mellitus, N (%) | 10 (27.8%) | 12 (33.3%) | 12 (33.3%) | 0.842 |
| COPD, N (%) | 2 (5.6%) | 8 (22.2%) | 4 (11.1%) | 0.100 |
| Variable | Interventionalist 1 −FI, N = 36 | Interventionalist 1 +FI, N = 36 | p-Value |
|---|---|---|---|
| Fluoroscopy time (min) | 18.6 ± 8.3 | 12.5 ± 4.5 | <0.001 |
| Dose area product (cGy·cm2) | 5034.1 ± 4304 | 4368.6 ± 2087.3 | 0.039 |
| Procedure time (min) | 68.5 ± 26.8 | 59 ± 7.5 | 0.004 |
| Contrast volume (mL) | 145 ± 100 | 63.5 ± 30 | <0.001 |
| Variable | Interventionalist 1 −FI, N = 36 | Interventionalist 2 +FI, N = 36 | p-Value |
|---|---|---|---|
| Fluoroscopy time (min) | 18.6 ± 8.3 | 13.7 ± 7 | 0.038 |
| Dose area product (cGy·cm2) | 5034.1 ± 4304 | 2787.2 ± 2284.4 | <0.001 |
| Procedure time (min) | 68.5 ± 26.8 | 56.5 ± 7 | <0.001 |
| Contrast volume (mL) | 163.8 ± 110.5 | 55 ± 22.5 | <0.001 |
| Variable | Interventionalist 1 −FI, N = 36 | Interventionalist 1 +FI, N = 36 | p-Value |
|---|---|---|---|
| Pericardial effusion | 0 | 0 | - |
| Bleeding | 4 (11%) | 1 (3%) | 0.164 |
| Vascular complications | 0 | 1 (3%) | 0.314 |
| Stroke | 0 | 0 | - |
| Dislocation | 0 | 0 | - |
| Arrhythmia | 3 (8%) | 0 | 0.077 |
| Variable | Interventionalist 1 −FI, N = 36 | Interventionalist 2 +FI, N = 36 | p-Value |
|---|---|---|---|
| Pericardial effusion | 0 | 0 | - |
| Bleeding | 4 (11%) | 2 (5.5%) | 0.394 |
| Vascular complications | 0 | 2 (5.5%) | 0.151 |
| Stroke | 0 | 0 | - |
| Dislocation | 0 | 0 | - |
| Arrhythmia | 3 (8%) | 0 | 0.077 |
| Authors | Study Design | N (+FI/−FI) | Methods | Results |
|---|---|---|---|---|
| Afzal et al. [17] | Observational study | 155 (34/121) | Echocardiography + fluoroscopy | FI reduced the total procedure time, the time to successful transseptal, and periprocedural amount of contrast agent. |
| Ebelt et al. [32] | Observational study | 75 (25/50) | Echocardiography + fluoroscopy | FI significantly reduced procedure time and the amount of contrast medium |
| Nelles et al. [33] | Case report | 1 (1/0) | Echocardiography + fluoroscopy | FI is safe and feasible |
| Blusztein et al. [34] | Observational study | 31 (31/0) | Echocardiography + fluoroscopy | FI using for zero-contrast LAAC is safe and feasible |
| Chen et al. [35] | Observational study | 82 (41/41) | Computed tomography + fluoroscopy | FI is feasible, safe, and applicable; it reduces the radiation exposure, procedure duration, and volume of contrast media |
| Roy et al. [36] | Observational study | 57 (16/41) | Computed tomography + fluoroscopy | FI reduced contrast volume, procedure time, and fluoroscopy time |
| Mo et al. [37] | Observational study | 117 (39/78) | Computed tomography + fluoroscopy | FI enabled a lower average number of recapture times and the number of devices per patient with a higher one-time successful deployment rate |
| Peters et al. [38] | Case series | 3 (3/0) | Computed tomography + echocardiography | FI improved the detection of LAA anatomy and delivery catheter orientation within the LAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanschik, D.; Heidari, H.; Klein, K.; Polzin, A.; Veulemans, V.; Leick, J.; Kelm, M.; Jung, C.; Zeus, T.; Afzal, S. Echocardiographic-Fluoroscopic Fusion Imaging Improves Interventionalists’ Learning Curve for Percutaneous Left Atrial Appendage Closure—Initial, Single-Center, Retrospective Observations. J. Cardiovasc. Dev. Dis. 2024, 11, 82. https://doi.org/10.3390/jcdd11030082
Kanschik D, Heidari H, Klein K, Polzin A, Veulemans V, Leick J, Kelm M, Jung C, Zeus T, Afzal S. Echocardiographic-Fluoroscopic Fusion Imaging Improves Interventionalists’ Learning Curve for Percutaneous Left Atrial Appendage Closure—Initial, Single-Center, Retrospective Observations. Journal of Cardiovascular Development and Disease. 2024; 11(3):82. https://doi.org/10.3390/jcdd11030082
Chicago/Turabian StyleKanschik, Dominika, Houtan Heidari, Kathrin Klein, Amin Polzin, Verena Veulemans, Jürgen Leick, Malte Kelm, Christian Jung, Tobias Zeus, and Shazia Afzal. 2024. "Echocardiographic-Fluoroscopic Fusion Imaging Improves Interventionalists’ Learning Curve for Percutaneous Left Atrial Appendage Closure—Initial, Single-Center, Retrospective Observations" Journal of Cardiovascular Development and Disease 11, no. 3: 82. https://doi.org/10.3390/jcdd11030082
APA StyleKanschik, D., Heidari, H., Klein, K., Polzin, A., Veulemans, V., Leick, J., Kelm, M., Jung, C., Zeus, T., & Afzal, S. (2024). Echocardiographic-Fluoroscopic Fusion Imaging Improves Interventionalists’ Learning Curve for Percutaneous Left Atrial Appendage Closure—Initial, Single-Center, Retrospective Observations. Journal of Cardiovascular Development and Disease, 11(3), 82. https://doi.org/10.3390/jcdd11030082





