Early Feasibility Study of a Hybrid Tissue-Engineered Mitral Valve in an Ovine Model
Abstract
1. Introduction
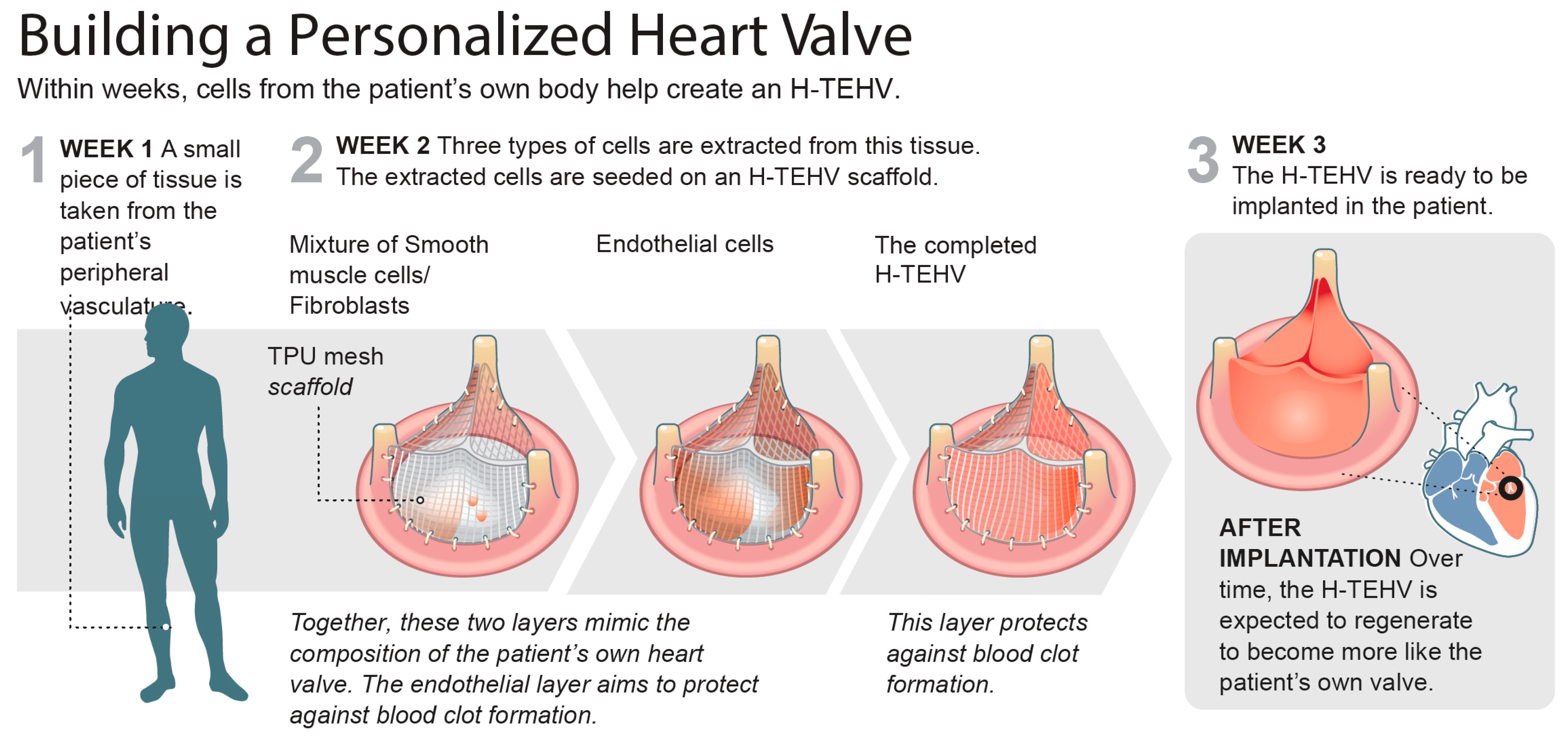
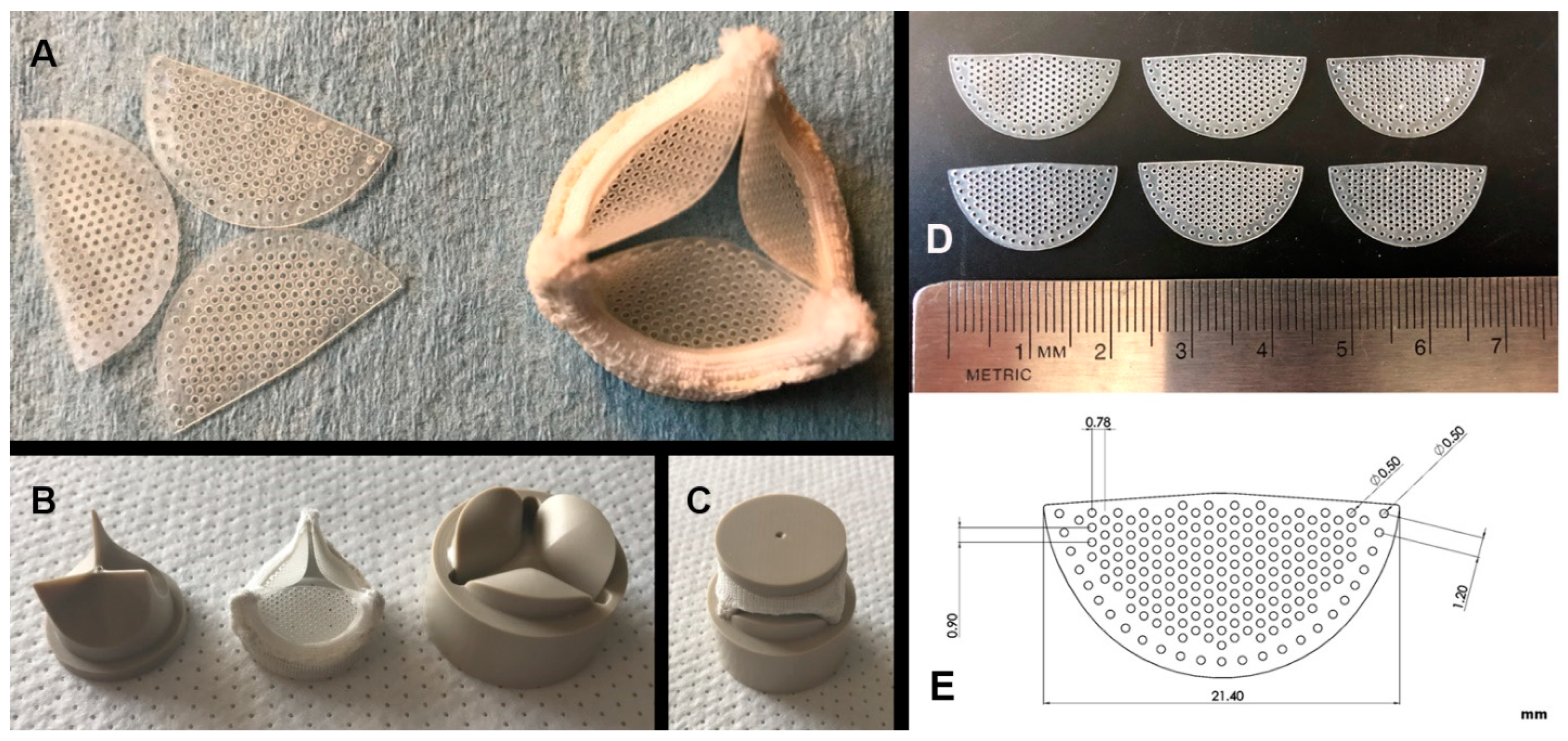
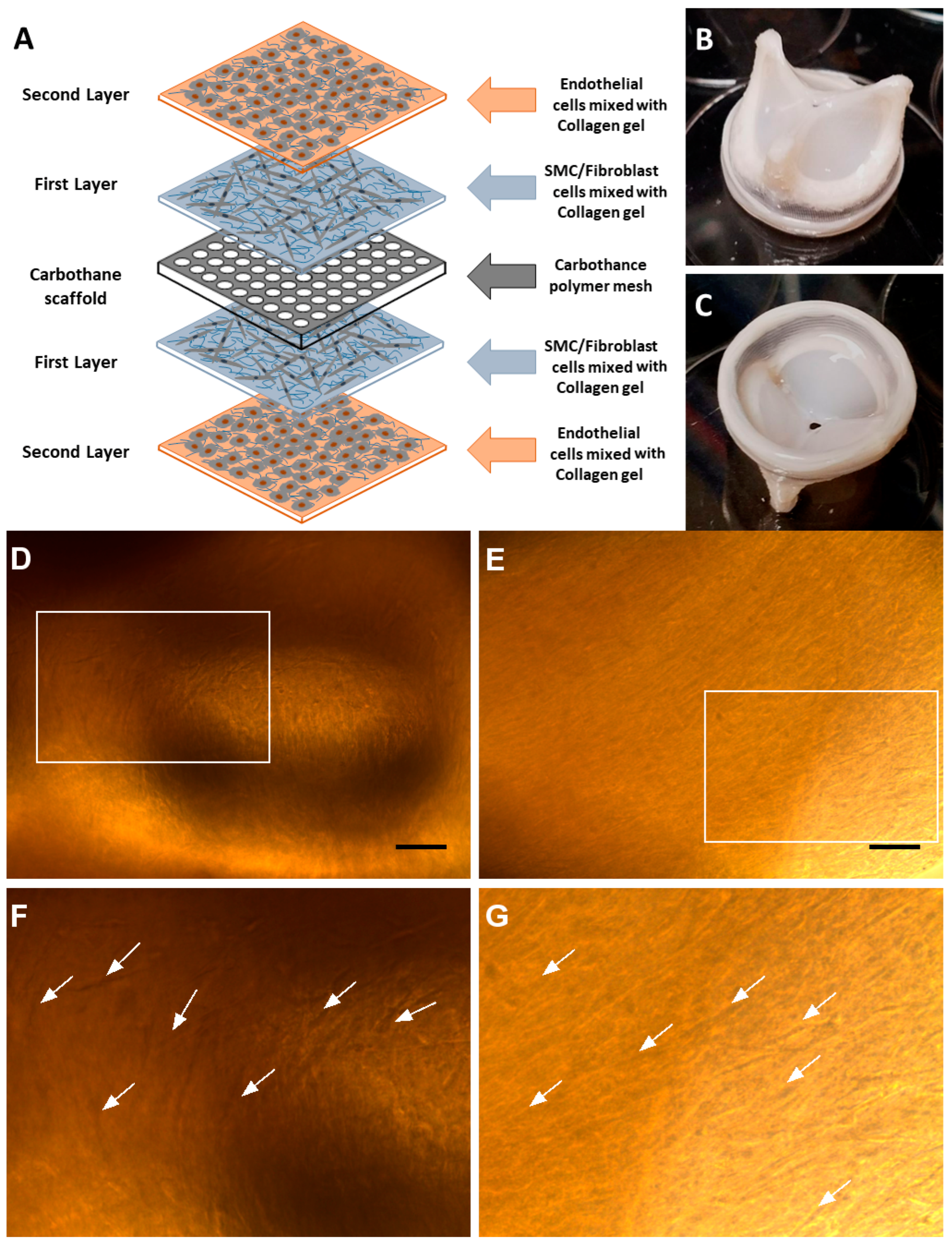
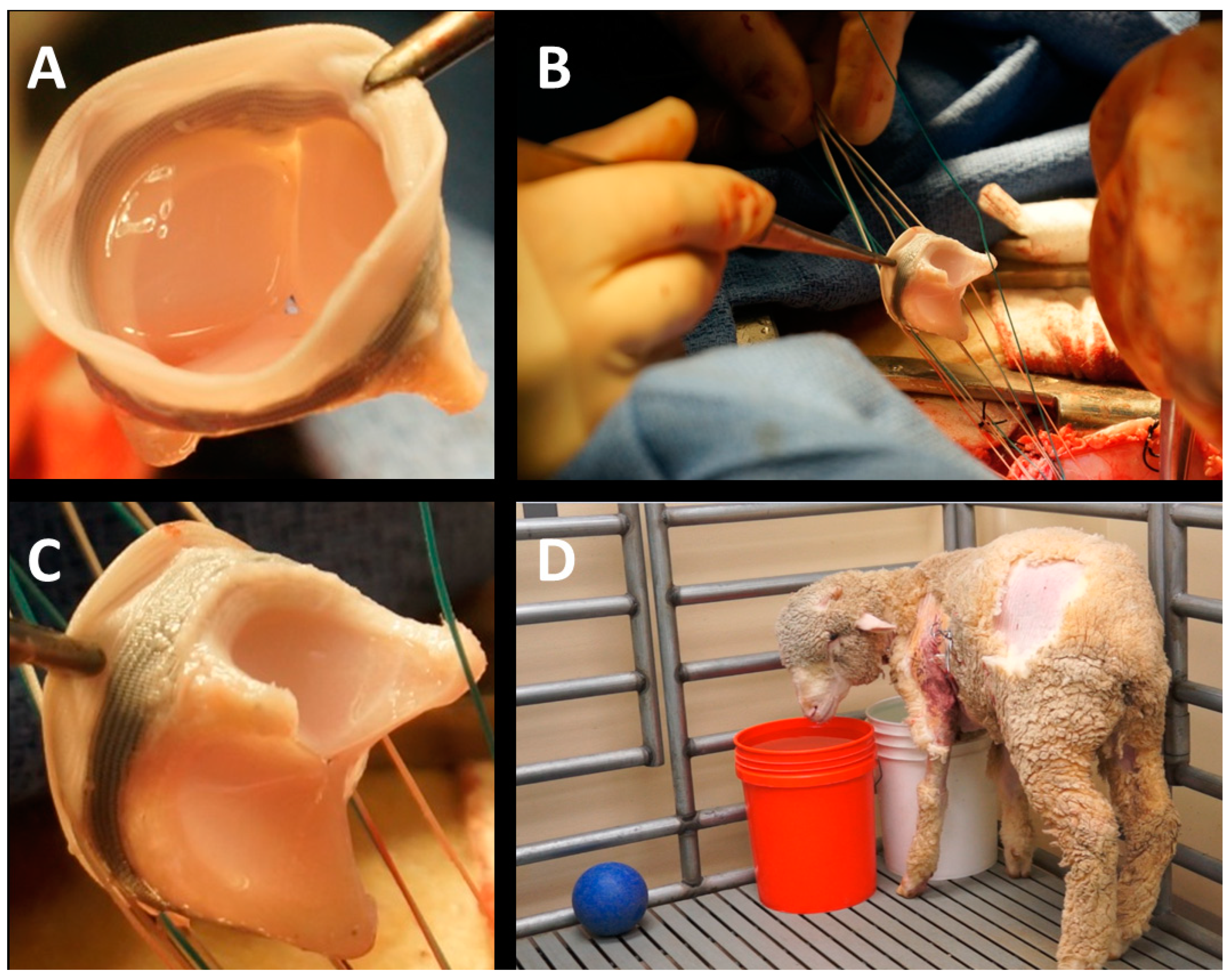
2. Material and Methods
- (1)
- Endothelial cell growth medium (EGM-2, Lonza CC-3162) enriched with FBS (10.00 mL), hydrocortisone (0.20 mL), hFGF-B (2.00 mL), VEGF (0.50 mL), R3-IGF-1 (0.50 mL), ascorbic acid (0.50 mL), hEGF (0.50 mL), GA-1000 (0.50 mL), and heparin (0.50 mL);
- (2)
- Fibroblast growth medium (FGM-2, Lonza CC-3132) enriched with insulin (0.50 mL), hFGF-B (0.50 mL), GA-1000 (0.50 mL), and FBS (10.00 mL);
- (3)
- Smooth muscle cell growth medium (SmGM-2, Lonza CC-3182) enriched with insulin (0.50 mL), hFGF-B (1.00 mL), GA-1000 (0.50 mL), FBS (25.00 mL), and hEGF (0.50 mL).
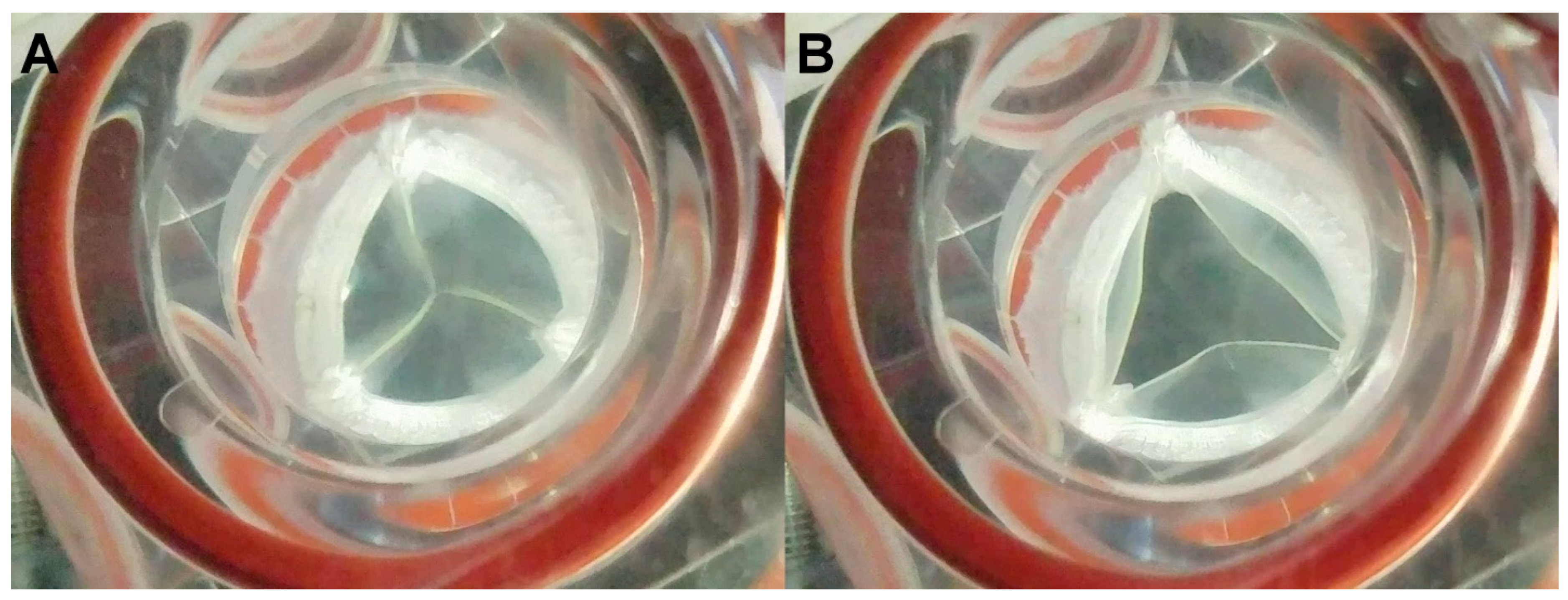
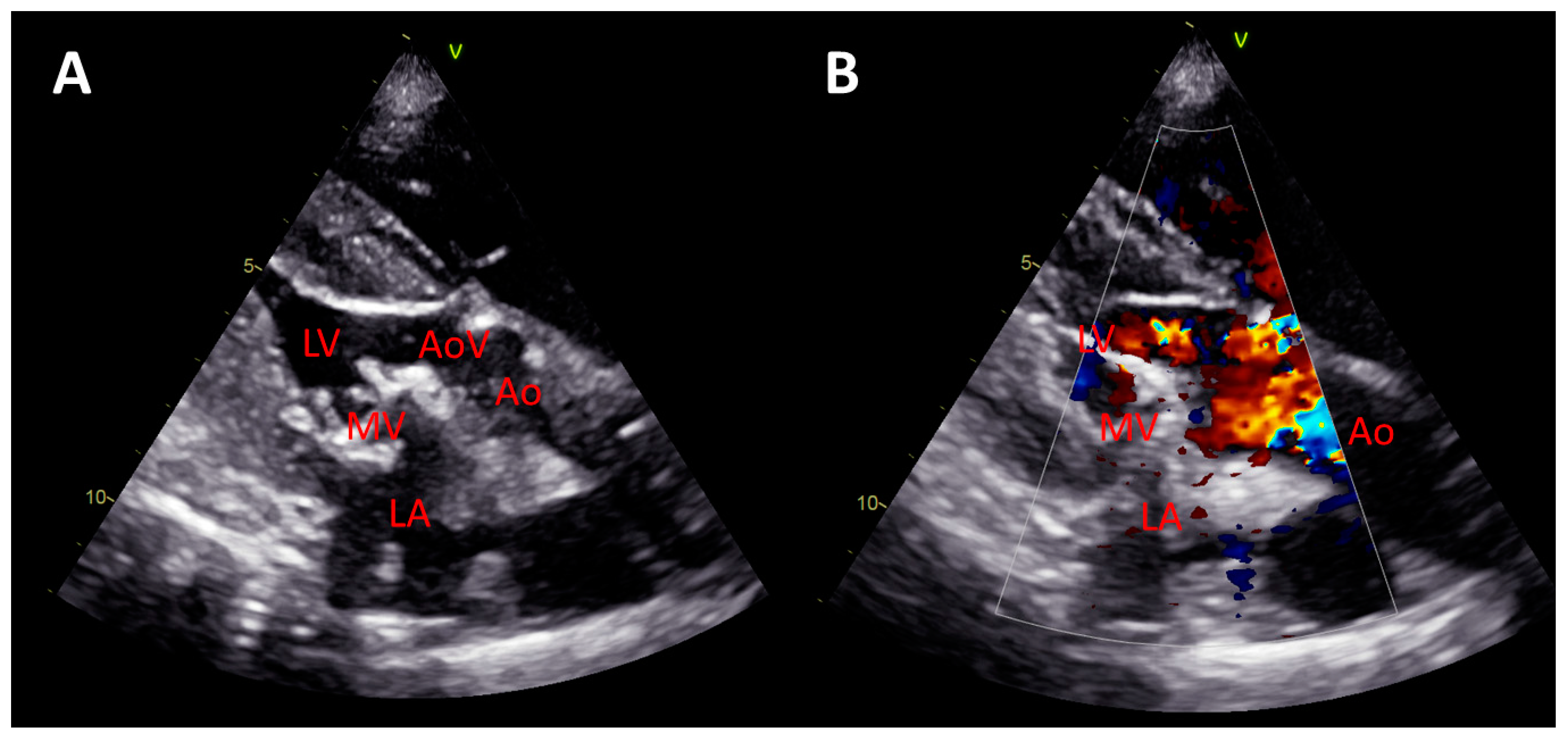
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldstone, A.B.; Chiu, P.; Baiocchi, M.; Lingala, B.; Patrick, W.L.; Fischbein, M.P.; Woo, Y.J. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N. Engl. J. Med. 2017, 377, 1847–1857. [Google Scholar] [CrossRef]
- Jose, J.; Sulimov, D.S.; El-Mawardy, M.; Sato, T.; Allali, A.; Holy, E.W.; Becker, B.; Landt, M.; Kebernik, J.; Schwarz, B.; et al. Clinical Bioprosthetic Heart Valve Thrombosis After Transcatheter Aortic Valve Replacement: Incidence, Characteristics, and Treatment Outcomes. JACC 2017, 10, 686–697. [Google Scholar] [CrossRef]
- Stephens, E.H.; de Jonge, N.; McNeill, M.P.; Durst, C.A.; Grande-Allen, K.J. Age-Related Changes in Material Behavior of Porcine Mitral and Aortic Valves and Correlation to Matrix Composition. Tissue Eng. Part A 2009, 16, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.H.; Mueller, R.; Iversen, S. Early calcific degeneration of a CoreValve transcatheter aortic bioprosthesis. Eur. Heart J. 2012, 33, 586. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.; Lutter, G.; Cremer, J. Durability of bioprosthetic cardiac valves. Dtsch. Arztebl. Int. 2008, 105, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Stock, U.A.; Nagashima, M.; Khalil, P.N.; Nollert, G.D.; Herden, T.; Sperling, J.S.; Moran, A.; Lien, J.; Martin, D.P.; Schoen, F.J.; et al. Tissue-engineered valved conduits in the pulmonary circulation. J. Thorac. Cardiovasc. Surg. 2000, 119 Pt 1, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Sodian, R.; Hoerstrup, S.P.; Sperling, J.S.; Daebritz, S.; Martin, D.P.; Moran, A.M.; Kim, B.S.; Schoen, F.J.; Vacanti, J.P.; Mayer, J.E., Jr. Early in vivo experience with tissue-engineered trileaflet heart valves. Circulation 2000, 102 (Suppl. S3), III22–III29. [Google Scholar] [CrossRef] [PubMed]
- Driessen-Mol, A.; Emmert, M.Y.; Dijkman, P.E.; Frese, L.; Sanders, B.; Weber, B.; Cesarovic, N.; Sidler, M.; Leenders, J.; Jenni, R.; et al. Transcatheter implantation of homologous “off-the-shelf” tissue-engineered heart valves with self-repair capacity: Long-term functionality and rapid in vivo remodeling in sheep. J. Am. Coll. Cardiol. 2014, 63, 1320–1329. [Google Scholar] [CrossRef]
- Gottlieb, D.; Kunal, T.; Emani, S.; Aikawa, E.; Brown, D.W.; Powell, A.J.; Nedder, A.; Engelmayr, G.C., Jr.; Melero-Martin, J.M.; Sacks, M.S.; et al. In vivo monitoring of function of autologous engineered pulmonary valve. J. Thorac. Cardiovasc. Surg. 2010, 139, 723–731. [Google Scholar] [CrossRef]
- Flanagan, T.C.; Sachweh, J.S.; Frese, J.; Schnoring, H.; Gronloh, N.; Koch, S.; Tolba, R.H.; Schmitz-Rode, T.; Jockenhoevel, S. In vivo remodeling and structural characterization of fibrin-based tissue-engineered heart valves in the adult sheep model. Tissue Eng. Part. A 2009, 15, 2965–2976. [Google Scholar] [CrossRef]
- Hoerstrup, S.P.; Sodian, R.; Daebritz, S.; Wang, J.; Bacha, E.A.; Martin, D.P.; Moran, A.M.; Guleserian, K.J.; Sperling, J.S.; Kaushal, S.; et al. Functional living trileaflet heart valves grown in vitro. Circulation 2000, 102 (Suppl. S3), III44–III49. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, F.W.; Perry, T.E.; Yu, Y.; Sherwood, M.C.; Rabkin, E.; Masuda, Y.; Garcia, G.A.; McLellan, D.L.; Engelmayr, G.C., Jr.; Sacks, M.S.; et al. From stem cells to viable autologous semilunar heart valve. Circulation 2005, 111, 2783–2791. [Google Scholar] [CrossRef]
- Kluin, J.; Talacua, H.; Smits, A.I.; Emmert, M.Y.; Brugmans, M.C.; Fioretta, E.S.; Dijkman, P.E.; Sontjens, S.H.; Duijvelshoff, R.; Dekker, S.; et al. In situ heart valve tissue engineering using a bioresorbable elastomeric implant—From material design to 12 months follow-up in sheep. Biomaterials 2017, 125, 101–117. [Google Scholar] [CrossRef]
- Jockenhoevel, S.; Chalabi, K.; Sachweh, J.S.; Groesdonk, H.V.; Demircan, L.; Grossmann, M.; Zund, G.; Messmer, B.J. Tissue engineering: Complete autologous valve conduit—A new moulding technique. Thorac. Cardiovasc. Surg. 2001, 49, 287–290. [Google Scholar] [CrossRef]
- Alavi, S.H.; Kheradvar, A. Metal mesh scaffold for tissue engineering of membranes. Tissue Eng. Part C Methods 2012, 18, 293–301. [Google Scholar] [CrossRef]
- Alavi, S.H.; Kheradvar, A. A hybrid tissue-engineered heart valve. Ann. Thorac. Surg. 2015, 99, 2183–2187. [Google Scholar] [CrossRef]
- Alavi, S.H.; Liu, W.F.; Kheradvar, A. Inflammatory Response Assessment of a Hybrid Tissue-Engineered Heart Valve Leaflet. Ann. Biomed. Eng. 2013, 41, 316–326. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.; Hahn, C.; King, M.W.; Guidoin, R. Assessing the resistance to calcification of polyurethane membranes used in the manufacture of ventricles for a totally implantable artificial heart. J. Biomed. Mater. Res. 1999, 48, 648–659. [Google Scholar] [CrossRef]
- Kheradvar, A.; Zareian, R.; Kawauchi, S.; Goodwin, R.L.; Rugonyi, S. Animal models for heart valve research and development. Drug Discov. Today Dis. Models 2017, 24, 55–62. [Google Scholar] [CrossRef]
- Weber, S.C.; Gratopp, A.; Akanbi, S.; Rheinlaender, C.; Sallmon, H.; Barikbin, P.; Koehne, P.S. Isolation and Culture of Fibroblasts, Vascular Smooth Muscle, and Endothelial Cells From the Fetal Rat Ductus Arteriosus. Pediatr. Res. 2011, 70, 236–241. [Google Scholar] [CrossRef]
- Alavi, S.H.; Kheradvar, A. Apparatus and Process for Growing a Heart Valve in Three-Dimensions; USPTO, 10,016,461; The Regents of the University of California: Oakland, CA, USA, 2018. [Google Scholar]
- Daebritz, S.H.; Sachweh, J.S.; Hermanns, B.; Fausten, B.; Franke, A.; Groetzner, J.; Klosterhalfen, B.; Messmer, B.J. Introduction of a flexible polymeric heart valve prosthesis with special design for mitral position. Circulation 2003, 108 (Suppl. S1), II134–II139. [Google Scholar] [CrossRef]
- Daebritz, S.H.; Fausten, B.; Hermanns, B.; Schroeder, J.; Groetzner, J.; Autschbach, R.; Messmer, B.J.; Sachweh, J.S. Introduction of a flexible polymeric heart valve prosthesis with special design for aortic position. Eur. J. Cardio-Thorac. Surg. 2004, 25, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Rotman, O.M.; Kovarovic, B.; Chiu, W.-C.; Bianchi, M.; Marom, G.; Slepian, M.J.; Bluestein, D. Novel Polymeric Valve for Transcatheter Aortic Valve Replacement Applications: In Vitro Hemodynamic Study. Ann. Biomed. Eng. 2019, 47, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Simon-Walker, R.; Cavicchia, J.; Prawel, D.A.; Dasi, L.P.; James, S.P.; Popat, K.C. Hemocompatibility of hyaluronan enhanced linear low density polyethylene for blood contacting applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1964–1975. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.; Vaesken, A.; Amri, A.; Dasi, L.P.; Heim, F. Heart Valves from Polyester Fibers vs. Biological Tissue: Comparative Study In Vitro. Ann. Biomed. Eng. 2017, 45, 476–486. [Google Scholar] [CrossRef]
- Prawel, D.A.; Dean, H.; Forleo, M.; Lewis, N.; Gangwish, J.; Popat, K.C.; Dasi, L.P.; James, S.P. Hemocompatibility and Hemodynamics of Novel Hyaluronan–Polyethylene Materials for Flexible Heart Valve Leaflets. Cardiovasc. Eng. Technol. 2014, 5, 70–81. [Google Scholar] [CrossRef]
- Bezuidenhout, D.; Williams, D.F.; Zilla, P. Polymeric heart valves for surgical implantation, catheter-based technologies and heart assist devices. Biomaterials 2015, 36, 6–25. [Google Scholar] [CrossRef]
- Singh, S.K.; Kachel, M.; Castillero, E.; Xue, Y.; Kalfa, D.; Ferrari, G.; George, I. Polymeric prosthetic heart valves: A review of current technologies and future directions. Front. Cardiovasc. Med. 2023, 10, 1137827. [Google Scholar] [CrossRef]
- Kulinsky, L.; Kheradvar, A. Imprinter for Conformal Coating of three-Dimensional Surfaces; USPTO, 10,245,614; The Regents of the University of California: Oakland, CA, USA, 2019. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zareian, R.; Zuke, S.D.; Morisawa, D.; Geertsema, R.S.; Majid, M.; Wynne, C.; Milliken, J.C.; Kheradvar, A. Early Feasibility Study of a Hybrid Tissue-Engineered Mitral Valve in an Ovine Model. J. Cardiovasc. Dev. Dis. 2024, 11, 69. https://doi.org/10.3390/jcdd11020069
Zareian R, Zuke SD, Morisawa D, Geertsema RS, Majid M, Wynne C, Milliken JC, Kheradvar A. Early Feasibility Study of a Hybrid Tissue-Engineered Mitral Valve in an Ovine Model. Journal of Cardiovascular Development and Disease. 2024; 11(2):69. https://doi.org/10.3390/jcdd11020069
Chicago/Turabian StyleZareian, Ramin, Samuel D. Zuke, Daisuke Morisawa, Roger S. Geertsema, Mariwan Majid, Clinton Wynne, Jeffrey C. Milliken, and Arash Kheradvar. 2024. "Early Feasibility Study of a Hybrid Tissue-Engineered Mitral Valve in an Ovine Model" Journal of Cardiovascular Development and Disease 11, no. 2: 69. https://doi.org/10.3390/jcdd11020069
APA StyleZareian, R., Zuke, S. D., Morisawa, D., Geertsema, R. S., Majid, M., Wynne, C., Milliken, J. C., & Kheradvar, A. (2024). Early Feasibility Study of a Hybrid Tissue-Engineered Mitral Valve in an Ovine Model. Journal of Cardiovascular Development and Disease, 11(2), 69. https://doi.org/10.3390/jcdd11020069






