Emotional Distress and Cardiovascular Health in Young Adults with Type 1 Diabetes
Abstract
1. Introduction
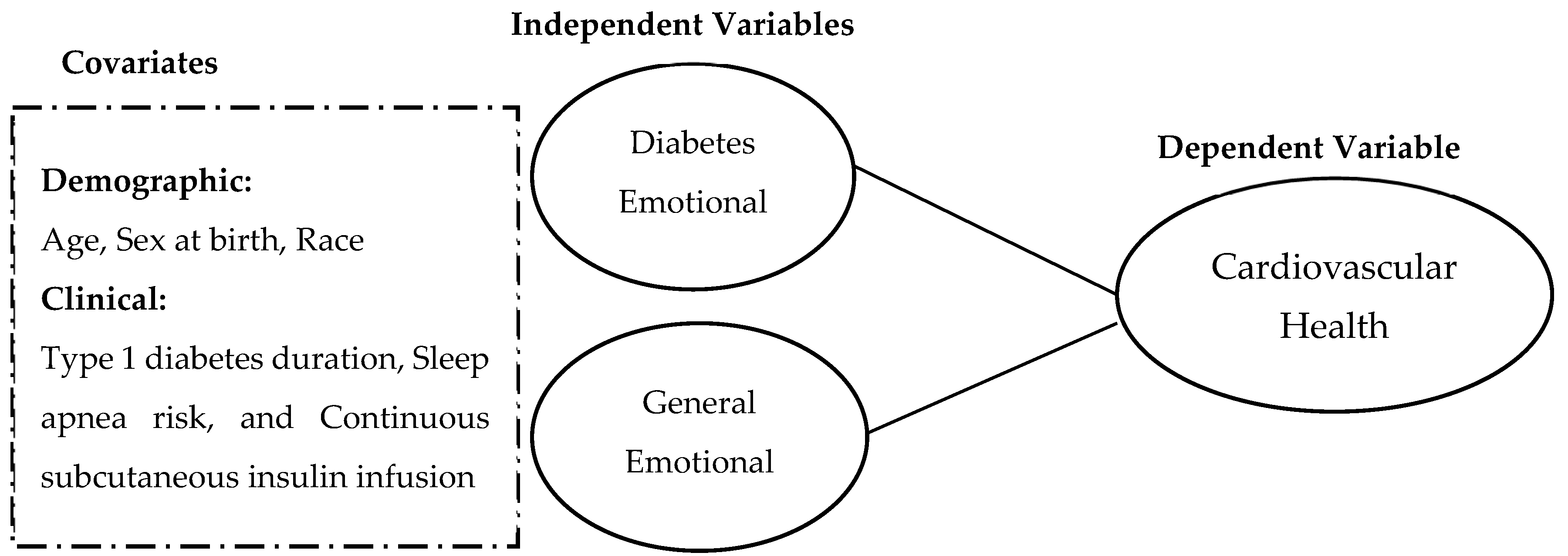
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Covariates
2.4. Emotional Distress Symptoms
2.5. Cardiovascular Health
2.6. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. General and Diabetes Emotional Distress
3.3. Associations Between Diabetes Emotional Distress and Cardiovascular Health
3.4. Associations Between General Emotional Distress and Cardiovascular Health
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Committee, A.D.A.P.P.; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Beverly, E.A.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Darville, A.; Das, S.R.; et al. Introduction and Methodology: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S1–S4. [Google Scholar] [CrossRef]
- Khunti, K.; Davies, M.; Majeed, A.; Thorsted, B.L.; Wolden, M.L.; Paul, S.K. Hypoglycemia and Risk of Cardiovascular Disease and All-Cause Mortality in Insulin-Treated People with Type 1 and Type 2 Diabetes: A Cohort Study. Diabetes Care 2015, 38, 316–322. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, M.; Buchan, I.; Capewell, S. Contributions of Treatment and Lifestyle to Declining CVD Mortality: Why Have CVD Mortality Rates Declined so Much since the 1960s? Heart 2013, 99, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Stouffer, G.A.; Kucharska-Newton, A.M.; Qamar, A.; Vaduganathan, M.; Pandey, A.; Porterfield, D.; Blankstein, R.; Rosamond, W.D.; Bhatt, D.L.; et al. Twenty Year Trends and Sex Differences in Young Adults Hospitalized With Acute Myocardial Infarction. Circulation 2019, 139, 1047–1056. [Google Scholar] [CrossRef]
- Soedamah-Muthu, S.S.; Chaturvedi, N.; Toeller, M.; Ferriss, B.; Reboldi, P.; Michel, G.; Manes, C.; Fuller, J.H. Risk Factors for Coronary Heart Disease in Type 1 Diabetic Patients in Europe. The EURODIAB Prospective Complications Study. Diabetes Care 2004, 27, 530–537. [Google Scholar] [CrossRef]
- Soedamah-Muthu, S.S.; Fuller, J.H.; Mulnier, H.E.; Raleigh, V.S.; Lawrenson, R.A.; Colhoun, H.M. High Risk of Cardiovascular Disease in Patients With Type 1 Diabetes in the U.K. Diabetes Care 2006, 29, 798–804. [Google Scholar] [CrossRef]
- Soedamah-Muthu, S.S.; Fuller, J.H.; Mulnier, H.E.; Raleigh, V.S.; Lawrenson, R.A.; Colhoun, H.M. All-Cause Mortality Rates in Patients with Type 1 Diabetes Mellitus Compared with a Non-Diabetic Population from the UK General Practice Research Database, 1992–1999. Diabetologia 2006, 49, 660–666. [Google Scholar] [CrossRef]
- Secrest, A.M.; Becker, D.J.; Kelsey, S.F.; LaPorte, R.E.; Orchard, T.J. Cause-Specific Mortality Trends in a Large Population-Based Cohort With Long-Standing Childhood-Onset Type 1 Diabetes. Diabetes 2010, 59, 3216–3222. [Google Scholar] [CrossRef]
- Laing, S.P.; Swerdlow, A.J.; Slater, S.D.; Botha, J.L.; Burden, A.C.; Waugh, N.R.; Smith, A.W.M.; Hill, R.D.; Bingley, P.J.; Patterson, C.C.; et al. The British Diabetic Association Cohort Study, II: Cause-Specific Mortality in Patients with Insulin-Treated Diabetes Mellitus. Diabet. Med. 1999, 16, 466–471. [Google Scholar] [CrossRef]
- Skrivarhaug, T.; Bangstad, H.J.; Stene, L.C.; Sandvik, L.; Hanssen, K.F.; Joner, G. Long-Term Mortality in a Nationwide Cohort of Childhood-Onset Type 1 Diabetic Patients in Norway. Diabetologia 2006, 49, 298–305. [Google Scholar] [CrossRef]
- Fang, N.; Jiang, M.; Fan, Y. Ideal Cardiovascular Health Metrics and Risk of Cardiovascular Disease or Mortality: A Meta-Analysis. Int. J. Cardiol. 2016, 214, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.M.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory from the American Heart Association. Circulation 2022, 146, E18–E43. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.M.; Foster, N.C.; Beck, R.W.; Bergenstal, R.M.; DuBose, S.N.; DiMeglio, L.A.; Maahs, D.M.; Tamborlane, W.V. Current State of Type 1 Diabetes Treatment in the U.S.: Updated Data From the T1D Exchange Clinic Registry. Diabetes Care 2015, 38, 971–978. [Google Scholar] [CrossRef]
- Foster, N.C.; Beck, R.W.; Miller, K.M.; Clements, M.A.; Rickels, M.R.; Dimeglio, L.A.; Maahs, D.M.; Tamborlane, W.V.; Bergenstal, R.; Smith, E.; et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol. Ther. 2019, 21, 66–72. [Google Scholar] [CrossRef]
- Malik, F.S.; Sauder, K.A.; Isom, S.; Reboussin, B.A.; Dabelea, D.; Lawrence, J.M.; Roberts, A.; Mayer-Davis, E.J.; Marcovina, S.; Dolan, L.; et al. Trends in Glycemic Control Among Youth and Young Adults With Diabetes: The SEARCH for Diabetes in Youth Study. Diabetes Care 2022, 45, 285–294. [Google Scholar] [CrossRef]
- Batelaan, N.M.; Seldenrijk, A.; Bot, M.; Van Balkom, A.J.L.M.; Penninx, B.W.J.H. Anxiety and New Onset of Cardiovascular Disease: Critical Review and Meta-Analysis. Br. J. Psychiatry 2016, 208, 223–231. [Google Scholar] [CrossRef]
- Edmondson, D.; von Känel, R. Post-Traumatic Stress Disorder and Cardiovascular Disease. Lancet Psychiatry 2017, 4, 320–329. [Google Scholar] [CrossRef]
- Richardson, S.; Shaffer, J.A.; Falzon, L.; Krupka, D.; Davidson, K.W.; Edmondson, D. Meta-Analysis of Perceived Stress and Its Association with Incident Coronary Heart Disease. Am. J. Cardiol. 2012, 110, 1711–1716. [Google Scholar] [CrossRef]
- Hare, D.L. Depression and Cardiovascular Disease. Curr. Opin. Lipidol. 2021, 32, 167–174. [Google Scholar] [CrossRef]
- Amsberg, S.; Anderbro, T.; Wredling, R.; Lisspers, J.; Lins, P.-E.; Adamson, U.; Johansson, U.-B. A Cognitive Behavior Therapy-Based Intervention among Poorly Controlled Adult Type 1 Diabetes Patients—A Randomized Controlled Trial. Patient Educ. Couns. 2009, 77, 72–80. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.; Sun, J. Effects of Cognitive Behavioral Therapy–Based Intervention on Improving Glycaemic, Psychological, and Physiological Outcomes in Adult Patients With Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Front. Psychiatry 2020, 11, 711. [Google Scholar] [CrossRef] [PubMed]
- Snoek, F.J.; van der Ven, N.C.W.; Lubach, C.H.C.; Chatrou, M.; Adèr, H.J.; Heine, R.J.; Jacobson, A.M. Effects of Cognitive Behavioural Group Training (CBGT) in Adult Patients with Poorly Controlled Insulin-Dependent (Type 1) Diabetes: A Pilot Study. Patient Educ. Couns. 2001, 45, 143–148. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Whittemore, R.; Gholson, G.; Grey, M. Diabetes Distress, Depressive Symptoms, and Cardiovascular Health in Adults With Type 1 Diabetes. Nurs. Res. 2019, 68, 445–452. [Google Scholar] [CrossRef]
- Pilkonis, P.A.; Choi, S.W.; Reise, S.P.; Stover, A.M.; Riley, W.T.; Cella, D. Item Banks for Measuring Emotional Distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): Depression, Anxiety, and Anger. Assessment 2011, 18, 263–283. [Google Scholar] [CrossRef]
- Griggs, S.; Grey, M.; Ash, G.I.; Li, C.R.; Crawford, S.L.; Hickman, R.L. Objective Sleep-Wake Characteristics Are Associated With Diabetes Symptoms in Young Adults With Type 1 Diabetes. Sci. Diabetes Self-Manag. Care 2022, 48, 149–156. [Google Scholar] [CrossRef]
- Polonsky, W.H.; Fisher, L.; Earles, J.; Dudl, R.J.; Lees, J.; Mullan, J.; Jackson, R.A. Assessing Psychosocial Distress in Diabetes: Development of the Diabetes Distress Scale. Diabetes Care 2005, 28, 626–631. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef]
- Schmitt, A.; Kulzer, B.; Ehrmann, D.; Haak, T.; Hermanns, N. A Self-Report Measure of Diabetes Self-Management for Type 1 and Type 2 Diabetes: The Diabetes Self-Management Questionnaire-Revised (DSMQ-R)—Clinimetric Evidence From Five Studies. Front. Clin. Diabetes Healthc. 2021, 2, 823046. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)–A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Masharani, U.; Strycker, L.A.; Fisher, L. The Ubiquity of Diabetes Distress among Adults with Type 1 Diabetes in an Urban, Academic Practice: A Template for Intervention. Diabet. Med. 2022, 39, e14832. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, W.H.; Anderson, B.J.; Lohrer, P.A.; Welch, G.; Jacobson, A.M.; Aponte, J.E.; Schwartz, C.E. Assessment of Diabetes-Related Distress. Diabetes Care 1995, 18, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Cho, Y.; Seo, D.H.; Ahn, S.H.; Hong, S.; Suh, Y.J.; Chon, S.; Woo, J.T.; Baik, S.H.; Lee, K.W.; et al. Impact of Diabetes Distress on Glycemic Control and Diabetic Complications in Type 2 Diabetes Mellitus. Sci. Rep. 2024, 14, 5568. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; McQueen, A.; Chen, T.C.; Wang, J.C. Regulation of Glucose Homeostasis by Glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar] [CrossRef]
- Kelley, C.; Vander Molen, J.; Choi, J.; Bhai, S.; Martin, K.; Cochran, C.; Puthanveetil, P. Impact of Glucocorticoids on Cardiovascular System—The Yin Yang Effect. J. Pers. Med. 2022, 12, 1829. [Google Scholar] [CrossRef]
- Kraus-Friedmann, N. Hormonal Regulation of Hepatic Gluconeogenesis. Physiol. Rev. 1984, 64, 170–259. [Google Scholar] [CrossRef]
- Exton, J.H. Regulation of Gluconeogenesis by Glucocorticoids. Monogr. Endocrinol. 1979, 12, 535–546. [Google Scholar] [CrossRef]
- Ruzzin, J.; Wagman, A.S.; Jensen, J. Glucocorticoid-Induced Insulin Resistance in Skeletal Muscles: Defects in Insulin Signalling and the Effects of a Selective Glycogen Synthase Kinase-3 Inhibitor. Diabetologia 2005, 48, 2119–2130. [Google Scholar] [CrossRef]
- Charmandari, E.; Tsigos, C.; Chrousos, G. Endocrinology of the Stress Response. Annu. Rev. Physiol. 2005, 67, 259–284. [Google Scholar] [CrossRef]
- Griggs, S.; Conley, S.; Batten, J.; Grey, M. A Systematic Review and Meta-Analysis of Behavioral Sleep Interventions for Adolescents and Emerging Adults. Sleep. Med. Rev. 2020, 54, 101356. [Google Scholar] [CrossRef] [PubMed]
- Lauderdale, D.S.; Knutson, K.L.; Yan, L.L.; Liu, K.; Rathouz, P.J. Self-Reported and Measured Sleep Duration: How Similar Are They? Epidemiology 2008, 19, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Grulovic, N.; Rojnic Kuzman, M.; Baretic, M. Prevalence and Predictors of Diabetes-Related Distress in Adults with Type 1 Diabetes. Sci. Rep. 2022, 12, 15758. [Google Scholar] [CrossRef]
- Cowie, J.; Alfano, C.A.; Patriquin, M.A.; Reynolds, K.C.; Talavera, D.; Clementi, M.A. Addressing Sleep in Children with Anxiety Disorders. Sleep. Med. Clin. 2014, 9, 137–148. [Google Scholar] [CrossRef]
- Li, D.B.; Xu, T.; Xie, D.Q.; Wang, M.Y.; Sun, S.P.; Wang, M.; Zhang, S.S.; Yang, X.R.; Zhang, Z.N.; Wang, S.; et al. Efficacy of Mobile-Based Cognitive Behavioral Therapy on Lowering Low-Density Lipoprotein Cholesterol Levels in Patients With Atherosclerotic Cardiovascular Disease: Multicenter, Prospective Randomized Controlled Trial. J. Med. Internet Res. 2023, 25, e44939. [Google Scholar] [CrossRef]
- Dong, N.; Wang, X.; Yang, L. The Short- and Long-Term Effects of Cognitive Behavioral Therapy on the Glycemic Control of Diabetic Patients: A Systematic Review and Meta-Analysis. Biopsychosoc. Med. 2023, 17, 18. [Google Scholar] [CrossRef]
- Jenkinson, E.; Knoop, I.; Hudson, J.L.; Moss-Morris, R.; Hackett, R.A. The Effectiveness of Cognitive Behavioural Therapy and Third-wave Cognitive Behavioural Interventions on Diabetes-related Distress: A Systematic Review and Meta-analysis. Diabet. Med. 2022, 39, e14948. [Google Scholar] [CrossRef]
| Characteristic | Mean | SD |
|---|---|---|
| Age in years | 21.5 | 2.5 |
| T1D Duration in years | 10.3 | 5.6 |
| N | (%) | |
| Education (% college student) | 90 | 54.9 |
| Sex at birth (female) | 104 | 63 |
| Gender identity | ||
| Woman or female | 97 | 59.5 |
| Man or male | 44 | 27.0 |
| Trans-male | 1 | 0.6 |
| Genderqueer | 2 | 1.2 |
| Non-binary | 4 | 2.5 |
| Self-reported race and ethnicity | ||
| Non-Hispanic White | 124 | 77 |
| Non-Hispanic Black | 11 | 6.8 |
| Non-Hispanic Asian | 8 | 5.0 |
| Hispanic White | 9 | 5.6 |
| Hispanic Black | 3 | 1.9 |
| Bi/Multi race/ethnicity | 6 | 3.7 |
| Characteristic | Mean | SD |
|---|---|---|
| Emotional Distress | ||
| Diabetes Emotional Distress Scale | 36.3 | 15.7 |
| General Emotional Distress (PROMIS t score) | 52.5 | 9.6 |
| Cardiovascular Health | ||
| Total Cardiovascular Health Score | 65.5 | 16.3 |
| N | % | |
| Ideal Cardiovascular Health | 33 | 20.6 |
| Intermediate Cardiovascular Health | 105 | 65.6 |
| Poor Cardiovascular Health | 22 | 13.8 |
| Independent Variables | B | SE | β | p Value | R2 |
|---|---|---|---|---|---|
| Diabetes Emotional Distress | −0.293 | 0.099 | −0.301 | 0.004 | 0.210 |
| Age | 1.799 | 0.754 | 0.229 | 0.019 | |
| Sex at birth | 2.287 | 3.452 | 0.068 | 0.509 | |
| Race | 5.242 | 4.226 | 0.121 | 0.218 | |
| Sleep apnea risk | −9.005 | 4.901 | −0.187 | 0.069 | |
| T1D Duration | −0.288 | 0.298 | −0.099 | 0.336 | |
| Continuous subcutaneous insulin infusion | −2.412 | 3.567 | −0.069 | 0.501 |
| Independent Variables | B | SE | β | p Value | R2 |
|---|---|---|---|---|---|
| General Emotional Distress | −0.543 | 0.152 | −0.338 | <0.001 | 0.239 |
| Age | 1.382 | 0.746 | 0.176 | 0.067 | |
| Sex at birth | 0.876 | 3.235 | 0.026 | 0.787 | |
| Race | 5.037 | 4.148 | 0.117 | 0.228 | |
| Sleep apnea risk | −9.505 | 4.731 | −0.197 | 0.047 | |
| T1D Duration | −0.331 | 0.293 | −0.114 | 0.262 | |
| Continuous subcutaneous insulin infusion | −1.280 | 3.496 | −0.037 | 0.715 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armentrout, B.L.; Ahmed, B.H.; Waraphok, S.; Huynh, J.; Griggs, S. Emotional Distress and Cardiovascular Health in Young Adults with Type 1 Diabetes. J. Cardiovasc. Dev. Dis. 2024, 11, 391. https://doi.org/10.3390/jcdd11120391
Armentrout BL, Ahmed BH, Waraphok S, Huynh J, Griggs S. Emotional Distress and Cardiovascular Health in Young Adults with Type 1 Diabetes. Journal of Cardiovascular Development and Disease. 2024; 11(12):391. https://doi.org/10.3390/jcdd11120391
Chicago/Turabian StyleArmentrout, Bethany L., Bootan H. Ahmed, Sineenat Waraphok, Johnathan Huynh, and Stephanie Griggs. 2024. "Emotional Distress and Cardiovascular Health in Young Adults with Type 1 Diabetes" Journal of Cardiovascular Development and Disease 11, no. 12: 391. https://doi.org/10.3390/jcdd11120391
APA StyleArmentrout, B. L., Ahmed, B. H., Waraphok, S., Huynh, J., & Griggs, S. (2024). Emotional Distress and Cardiovascular Health in Young Adults with Type 1 Diabetes. Journal of Cardiovascular Development and Disease, 11(12), 391. https://doi.org/10.3390/jcdd11120391






