The Impact of Triglyceride-Glucose (TyG) Index on Future Cardio and Cerebrovascular Events in Patients with Acute Coronary Syndrome, During 3 Years of Follow-Up
Abstract
1. Introduction
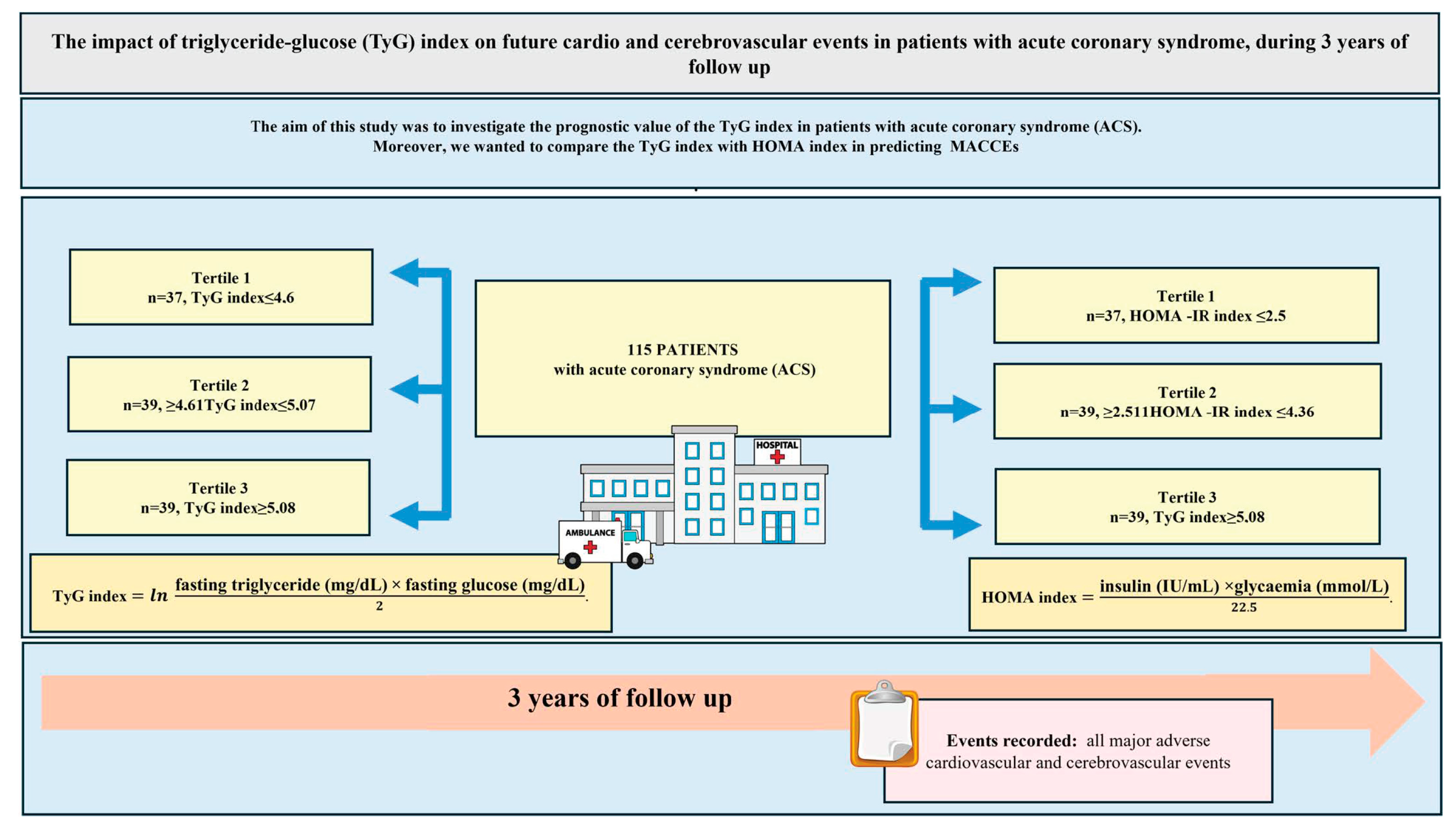
2. Methods
Statistical Analysis
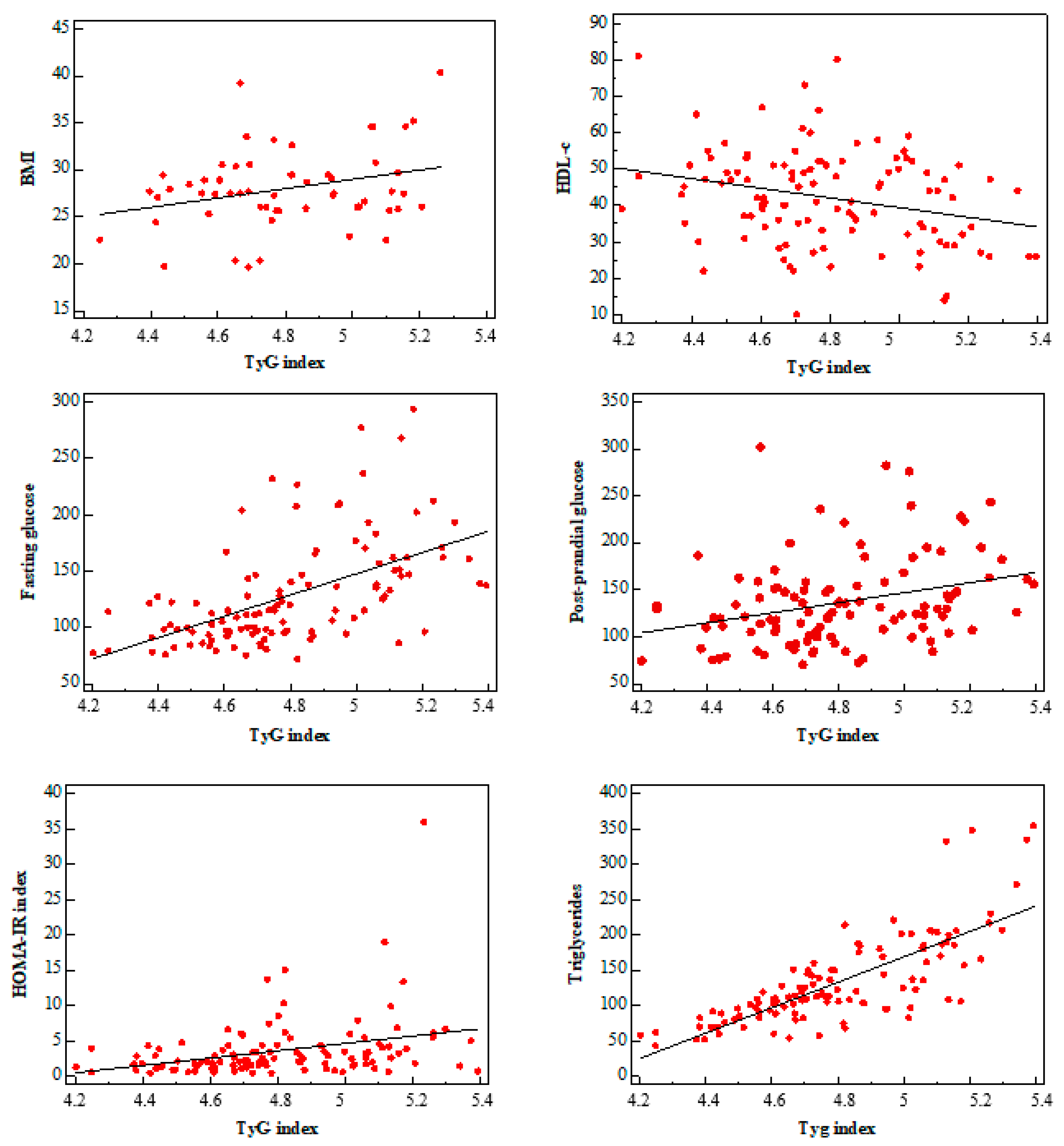
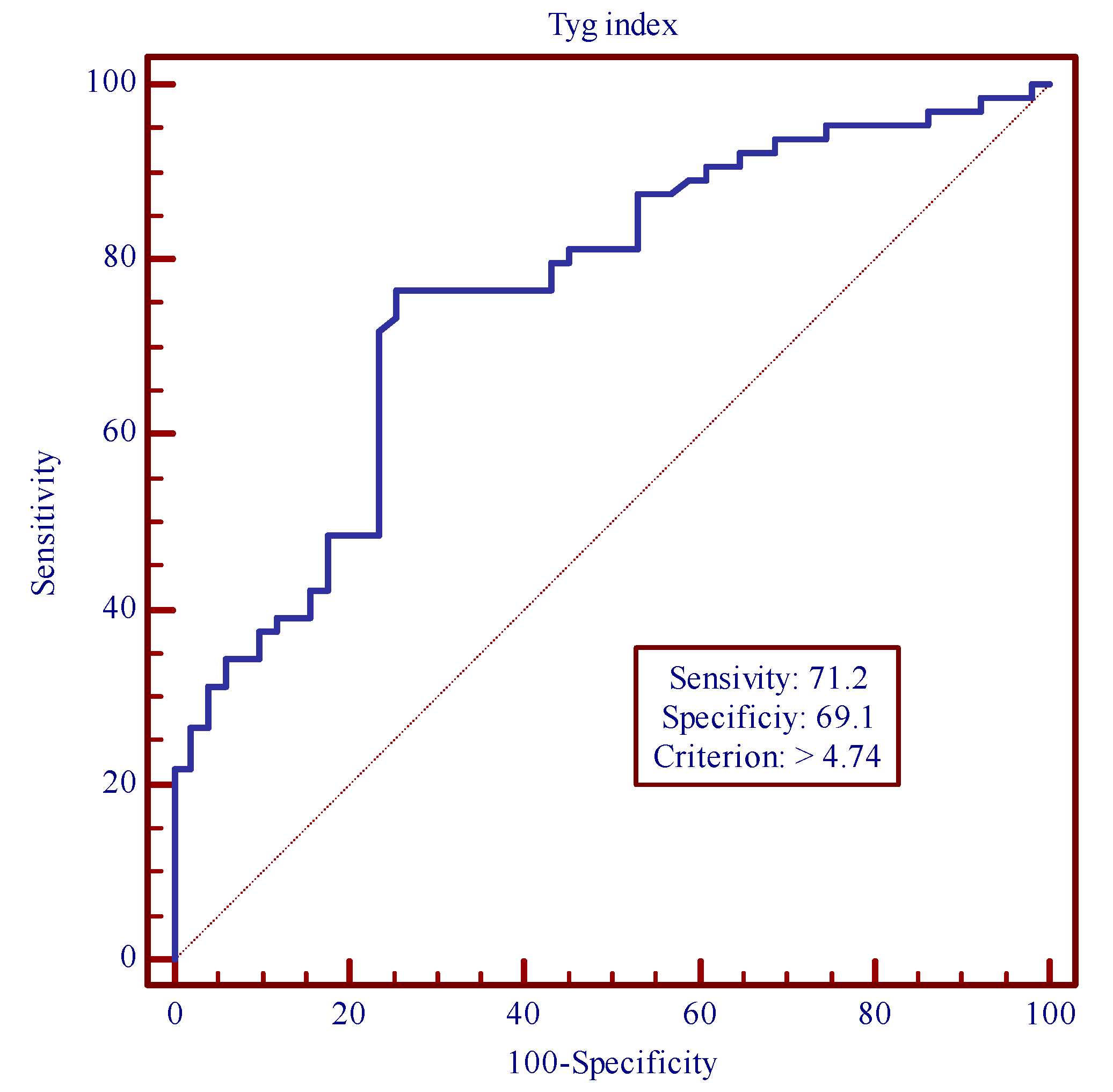
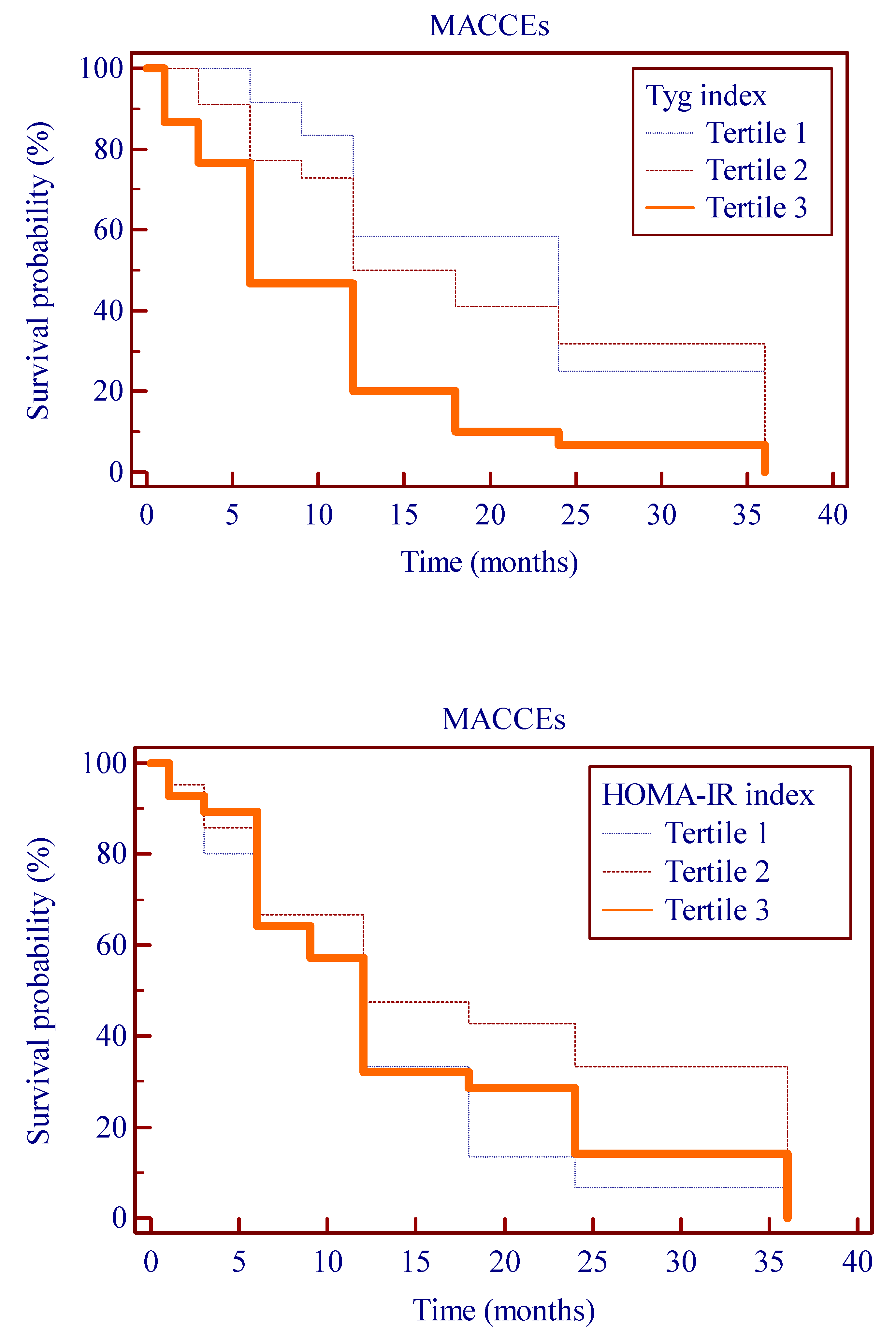
3. Results
Follow-Up
4. Discussion
- (1)
- Patients with a higher TyG index had a significantly increased risk of developing cardiovascular events.
- (2)
- The association between TyG index and MACCEs is independent of traditional cardiovascular risk factors and other well-known potential con founders. It follows that the TyG index could be an additional marker for cardiovascular risk assessment.
- (3)
- Both TyG index and HOMA-IR had a relationship with MACCEs, but high values of TyG index are superior to HOMA-IR in predicting MACCEs.
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Luo, E.; Wang, D.; Yan, G.; Qiao, Y.; Liu, B.; Hou, J.; Tang, C. High triglyceride–glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc. Diabetol. 2019, 18, 150. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 150. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef]
- Tahapary, D.L.; Pratisthita, L.B.; Fitri, N.A.; Marcella, C.; Wafa, S.; Kurniawan, F.; Rizka, A.; Tarigan, T.J.E.; Harbuwono, D.S.; Purnamasari, D.; et al. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102581. [Google Scholar] [CrossRef]
- Caccamo, G.; Bonura, F.; Bonura, F.; Vitale, G.; Novo, G.; Evola, S.; Evola, G.; Grisanti, M.R.; Novo, S. Insulin resistance and acute coronary syndrome. Atherosclerosis 2010, 211, 672–675. [Google Scholar] [CrossRef]
- Marcovina, S.; Bowsher, R.R.; Miller, W.G.; Staten, M.; Myers, G.; Caudill, S.P.; Campbell, S.E.; Steffes, M.W. Standardization of Insulin Immunoassays: Report of the American Diabetes Association Workgroup. Clin. Chem. 2007, 53, 711–716. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, T.-Y.; Cheng, Y.-J.; Ma, Y.; Xu, Y.-K.; Yang, J.-Q.; Zhou, Y.-J. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: Results from an observational cohort study in China. Cardiovasc. Diabetol. 2020, 19, 108. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The Product of Fasting Glucose and Triglycerides As Surrogate for Identifying Insulin Resistance in Apparently Healthy Subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Khan, S.H.; Sobia, F.; Niazi, N.K.; Manzoor, S.M.; Fazal, N.; Ahmad, F. Metabolic clustering of risk factors: Evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol. Metab. Syndr. 2018, 10, 74. [Google Scholar] [CrossRef]
- Mazidi, M.; Kengne, A.-P.; Katsiki, N.; Mikhailidis, D.P.; Banach, M. Lipid accumulation product and triglycerides/glucose index are useful predictors of insulin resistance. J. Diabetes Its Complicat. 2018, 32, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zuo, Y.; Chen, S.; Liu, Q.; Tao, B.; Wu, S.; Wang, A. Triglyceride–glucose index is associated with the risk of myocardial infarction: An 11-year prospective study in the Kailuan cohort. Cardiovasc. Diabetol. 2021, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, Q.; Wu, Y.; Cao, L. Cumulative triglyceride-glucose index is a risk for CVD: A prospective cohort study. Cardiovasc. Diabetol. 2022, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Tian, X.; Zuo, Y.; Chen, S.; Meng, X.; Wu, S.; Wang, Y. Change in triglyceride-glucose index predicts the risk of cardiovascular disease in the general population: A prospective cohort study. Cardiovasc. Diabetol. 2021, 20, 113. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2024, 13, 55–161. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Rev. Españ. Cardiol. 2022, 75, 523. [Google Scholar] [CrossRef]
- Di Lisi, D.; Ciampi, Q.; Madaudo, C.; Manno, G.; Macaione, F.; Novo, S.; Novo, G. Contractile Reserve in Heart Failure with Preserved Ejection Fraction. J. Cardiovasc. Dev. Dis. 2022, 9, 248. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, H.; Ma, Y.; Han, X.; Cao, Z.; Wu, Y. Triglyceride-glucose index associated with the risk of cardiovascular disease: The Kailuan study. Endocrine 2021, 75, 392–399. [Google Scholar] [CrossRef]
- Wei, R.; Chen, S.; Huang, X.; Zhai, Z.; Wang, Q.; Sun, J.; Mo, J.; Huang, J.; Xu, Y.; Lu, W. The triglyceride glucose index as a sensitive predictor for the risk of MACCEs in patients with diabetic foot ulcers: An ambispective longitudinal cohort study. Int. Wound J. 2024, 21, e14874. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cong, H.-l.; Zhang, J.-X.; Hu, Y.-C.; Wei, A.; Zhang, Y.-Y.; Yang, H.; Ren, L.-B.; Qi, W.; Li, W.-Y.; et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc. Diabetol. 2020, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Vasques, A.C.J.; Novaes, F.S.; de Oliveira, M.d.S.; Matos Souza, J.R.; Yamanaka, A.; Pareja, J.C.; Tambascia, M.A.; Saad, M.J.A.; Geloneze, B. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 2011, 93, e98–e100. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kwon, H.-S.; Park, Y.-M.; Ha, H.-S.; Jeong, S.H.; Yang, H.K.; Lee, J.-H.; Yim, H.-W.; Kang, M.-I.; Lee, W.-C.; et al. Predicting the Development of Diabetes Using the Product of Triglycerides and Glucose: The Chungju Metabolic Disease Cohort (CMC) Study. PLoS ONE 2014, 9, e90430. [Google Scholar] [CrossRef]
- Irace, C.; Carallo, C.; Scavelli, F.B.; De Franceschi, M.S.; Esposito, T.; Tripolino, C.; Gnasso, A. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int. J. Clin. Pract. 2013, 67, 665–672. [Google Scholar] [CrossRef]
- Cho, Y.K.; Lee, J.; Kim, H.S.; Kim, E.H.; Lee, M.J.; Yang, D.H.; Kang, J.-W.; Jung, C.H.; Park, J.-Y.; Kim, H.-K.; et al. Triglyceride Glucose-Waist Circumference Better Predicts Coronary Calcium Progression Compared with Other Indices of Insulin Resistance: A Longitudinal Observational Study. J. Clin. Med. 2020, 10, 92. [Google Scholar] [CrossRef]
- Sánchez-Íñigo, L.; Navarro-González, D.; Fernández-Montero, A.; Pastrana-Delgado, J.; Martínez, J.A. The TyG index may predict the development of cardiovascular events. Eur. J. Clin. Investig. 2016, 46, 189–197. [Google Scholar] [CrossRef]
- Cho, Y.-R.; Ann, S.H.; Won, K.-B.; Park, G.-M.; Kim, Y.-G.; Yang, D.H.; Kang, J.-W.; Lim, T.-H.; Kim, H.-K.; Choe, J.; et al. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci. Rep. 2019, 9, 6129. [Google Scholar] [CrossRef]
- Tao, L.-C.; Xu, J.-N.; Wang, T.-T.; Hua, F.; Li, J.-J. Triglyceride-glucose index as a marker in cardiovascular diseases: Landscape and limitations. Cardiovasc. Diabetol. 2022, 21, 68. [Google Scholar] [CrossRef]
| Tertile 1 (n = 37) | Tertile 2 (n = 39) | Tertile 3 (n = 39) | p Value < 0.05 | |
|---|---|---|---|---|
| Mean age (n/SD) | 67.94 ± 14.16 | 64.97 ± 11.01 | 64.35 ± 11.23 | 0.396 |
| Male n (%) | 23 (62.12%) | 29 (74.35%) | 29 (74.35%) | 0.408 |
| Current smokers | 12(32.43.20%) | 16 (41.02%) | 11 (28.20%) | 0.476 |
| Ex smokers | 11 (29.72%) | 18 (46.15%) | 16 (41.02%) | 0.326 |
| Hypertension | 28(75.67%) | 30 (46.15%) | 29 (74.35%) | 0.890 |
| Diabetes | 11 (29.72%) | 18 (76.92%) | 28 (71.79%) | 0.001 |
| Hypercholesterolaemia | 29 (78.37%) | 31 (79.48%) | 32 (82.05%) | 0.680 |
| Body mass index. kg/m2 | 26.73 ± 3.14 | 28.06 ± 4.73 | 29.05 ± 4.51 | 0.238 |
| NGT | 16 (43.24%) | 15 (40.54%) | 4 (10.25%) | 0.002 |
| IFG | 3 (8.10%) | 4 (10.81%) | 3 (7.69%) | 0.888 |
| IGT | 6 (16.21%) | 2 (5.40%) | 0 (0%) | 0.015 |
| Clinical presentation | ||||
| NSTEMI | 11 (29.72%) | 10 (25.64%) | 9 (23.07%) | 0.510 |
| STEMI | 12 (32.43%) | 12 (30.76%) | 11 (28.20%) | 0.793 |
| UAP | 14 (37.83%) | 17 (43.58%) | 19 (48.71%) | 0.632 |
| Number of vessels treated | 1.65 ± 0.84 | 2 ± 0.93 | 2.03 ± 0.92 | 0.167 |
| Laboratory findings | ||||
| Creatinine | 1.05 ± 0.25 | 1.28 ± 0.44 | 1.31 ± 0.36 | 0.020 |
| GFR (CKD-EPI Equations) mL/min/1.73 m2 | 73.90 ± 19.23 | 64.04 ± 22.96 | 65.29 ± 23.37 | 0.229 |
| IMT > 1.5 mm | 9 (24.32%) | 5 (12.82%) | 7 (17.94%) | 0.371 |
| Previous cardiovascular events | 10 (27.02%) | 14 (35.89%) | 17 (43.58%) | 0.1321 |
| Total cholesterol. mg/dL | 160.64 ± 44.19 | 179.53 ± 57.71 | 169.41 ± 43.79 | 0.248 |
| LDL-C. mg/dL | 97.69 ± 38.74 | 111.34 ± 53.51 | 91.51 ± 35.26 | 0.129 |
| HDL-C. mg/dL | 45.02 ± 11.48 | 43.15 ± 14.78 | 38.39 ± 11.90 | 0.075 |
| Triglycerides. mg/dL | 85.35 ± 21.27 | 130.38 ± 33.11 | 197.10 ± 68.17 | <0.001 |
| Hs-CRP. mg/dL | 1.21 ± 1.23 | 1.54 ± 1.86 | 1.99 ± 3.09 | 0.326 |
| ESR | 17.38 ± 14.47 | 19.75 ± 16.97 | 20.21 ± 20.56 | 0.791 |
| Mean TyG index | 4.51 ± 0.15 | 4.95 ± 0.07 | 5.30 ± 0.12 | <0.001 |
| Uric acid | 5.63 ± 2.46 | 5.57 ± 1.55 | 5.71 ± 1.96 | 0.951 |
| Fibrinogen | 374.45 ± 85.29 | 360.53 ± 84.28 | 393.48 ± 116.07 | 0.324 |
| TroponinI | 10.85 ± 23.23 | 11.25 ± 19.70 | 11.05 ± 24.37 | 0.997 |
| FPG | 102.43 ± 24.99 | 119.84 ± 38.16 | 162.02 ± 49.19 | <0.001 |
| PPG | 127.48 ± 45.19 | 124.48 ± 40.35 | 156.37 ± 50.54 | 0.006 |
| Fasting insulin | 8.32 ± 5.84 | 13.15 ± 14.20 | 11.55 ± 12.55 | 0.184 |
| Post-prandial insulin | 28.10 ± 22.02 | 34.61 ± 37.40 | 20.13 ± 13.54 | 0.006 |
| HOMA-IR | 3.06 ± 1.41 | 4.68 ± 3.36 | 5.93 ± 6.2 | 0.032 |
| HbAlc | 5.83 ± 0.43 | 7 ± 1.19 | 7.3 ± 1.87 | 0.327 |
| Echocardiographic findings | ||||
| LVEF < 40% | 1 (2.7%) | 0(%) | 0 (0%) | 0.20 |
| LVEF 40–49% | 4 (10.81%) | 5 (12.82%) | 3 (7.69%) | 0.890 |
| LVEF ≥ 50% | 32 (86.48) | 34 (87.17) | 36 (92.30) | 0.917 |
| Medications at discharge | ||||
| Aspirin | 37 (100%) | 39 (100%) | 39 (100%) | 0.9658 |
| Clopidogrel/Ticagrelor | 32 (86.48%) | 34 (87.17%) | 32 (82.05%) | 0.5438 |
| β-blocker | 28 (75.67%) | 30 (76.92%) | 35 (89.74%) | 0.1121 |
| ACEI/ARB | 32 (86.48%) | 29 (74.35%) | 32 (82.05%) | 0.6117 |
| Statin | 28 (75.67%) | 30 (76.92%) | 31 (79.48%) | 0.6887 |
| CCB | 5 (13.51%) | 4 (10.25%) | 3 (7.69%) | 0.4114 |
| Nitrate | 7 (18.91%) | 7 (17.94%) | 4 (10.25%) | 0.2968 |
| Insulin | 10 (27.02%) | 14 (35.89%) | 17 (43.58%) | 0.1321 |
| Tertile 1 (n = 37) | Tertile 2 (n = 39) | Tertile 3 (n = 39) | p Value < 0.05 | |
|---|---|---|---|---|
| MACCEs | 12 (32.43%) | 22 (56.41%) | 29 (74.35%) | 0.0002 |
| Unstable angina pectoris | 5 (13.51%) | 11 (28.20%) | 15 (38.46%) | 0.0144 |
| Acute myocardial infarction | 1 (2.70%) | 2 (5.12%) | 6 (15.38%) | 0.0385 |
| Heart failure | 4 (10.81%) | 3 (7.69%) | 4 (5.12%) | 0.9098 |
| Cardiac arrest | 2 (5.40%) | 5 (12.82%) | 2 (5.12%) | 0.9467 |
| Coronary revascularization | 4 (10.81%) | 7 (17.94%) | 8 (20.51%) | 0.2569 |
| Stroke | 0 (0%) | 1 (2.56%) | 2 (5.12%) | 0.1609 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macaione, F.; Di Lisi, D.; Madaudo, C.; D’agostino, A.; Adorno, D.; Sucato, V.; Novo, G.; Evola, S. The Impact of Triglyceride-Glucose (TyG) Index on Future Cardio and Cerebrovascular Events in Patients with Acute Coronary Syndrome, During 3 Years of Follow-Up. J. Cardiovasc. Dev. Dis. 2024, 11, 354. https://doi.org/10.3390/jcdd11110354
Macaione F, Di Lisi D, Madaudo C, D’agostino A, Adorno D, Sucato V, Novo G, Evola S. The Impact of Triglyceride-Glucose (TyG) Index on Future Cardio and Cerebrovascular Events in Patients with Acute Coronary Syndrome, During 3 Years of Follow-Up. Journal of Cardiovascular Development and Disease. 2024; 11(11):354. https://doi.org/10.3390/jcdd11110354
Chicago/Turabian StyleMacaione, Francesca, Daniela Di Lisi, Cristina Madaudo, Alessandro D’agostino, Daniele Adorno, Vincenzo Sucato, Giuseppina Novo, and Salvatore Evola. 2024. "The Impact of Triglyceride-Glucose (TyG) Index on Future Cardio and Cerebrovascular Events in Patients with Acute Coronary Syndrome, During 3 Years of Follow-Up" Journal of Cardiovascular Development and Disease 11, no. 11: 354. https://doi.org/10.3390/jcdd11110354
APA StyleMacaione, F., Di Lisi, D., Madaudo, C., D’agostino, A., Adorno, D., Sucato, V., Novo, G., & Evola, S. (2024). The Impact of Triglyceride-Glucose (TyG) Index on Future Cardio and Cerebrovascular Events in Patients with Acute Coronary Syndrome, During 3 Years of Follow-Up. Journal of Cardiovascular Development and Disease, 11(11), 354. https://doi.org/10.3390/jcdd11110354






