An In Vivo Model of Estrogen Supplementation Concerning the Expression of Ca2+-Dependent Exchangers and Mortality, Vitality and Survival After Myocardial Infarction in Ovariectomized Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Induction of Myocardial Infarction
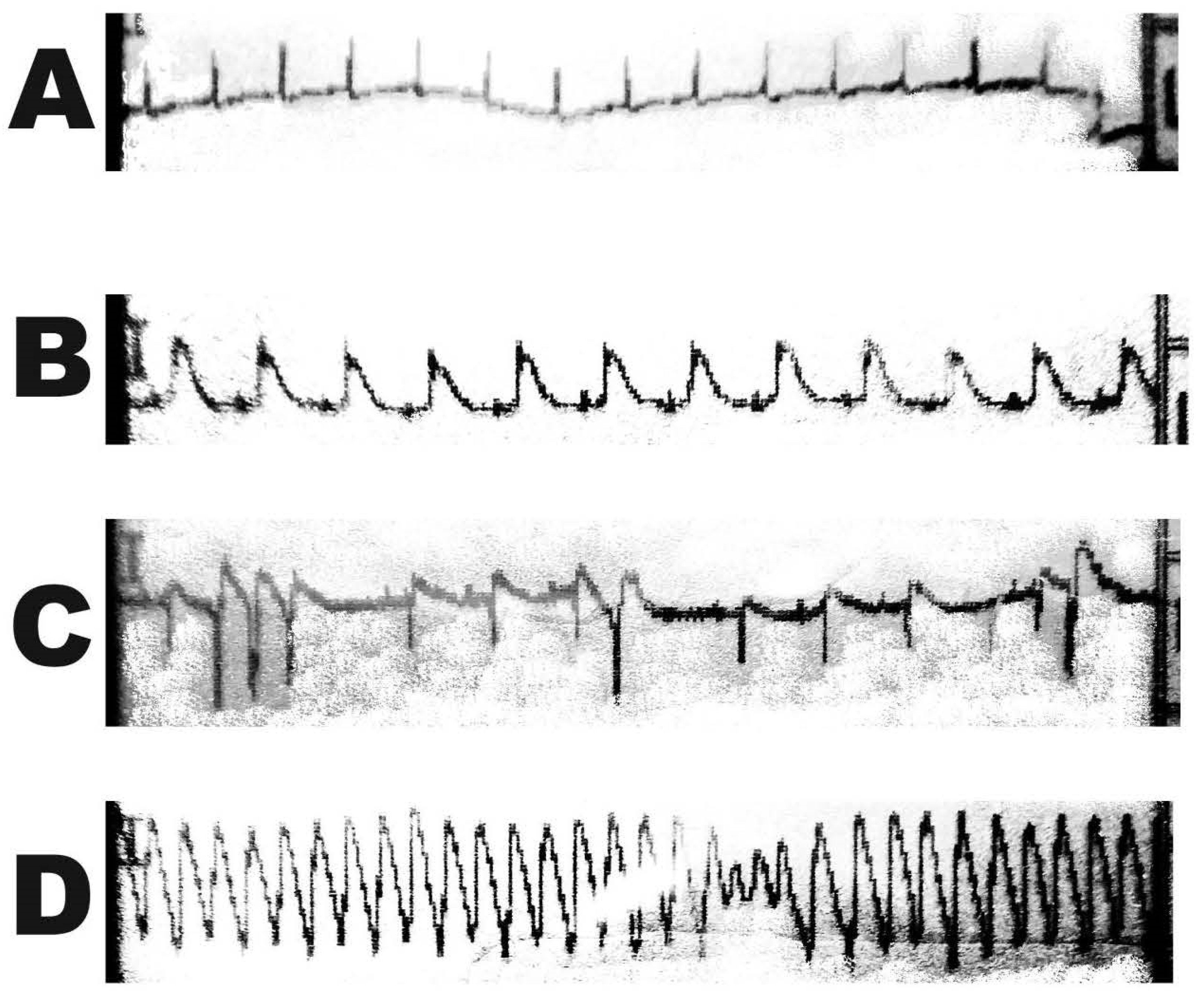
2.3. Vitality Test—Graded Treadmill Run
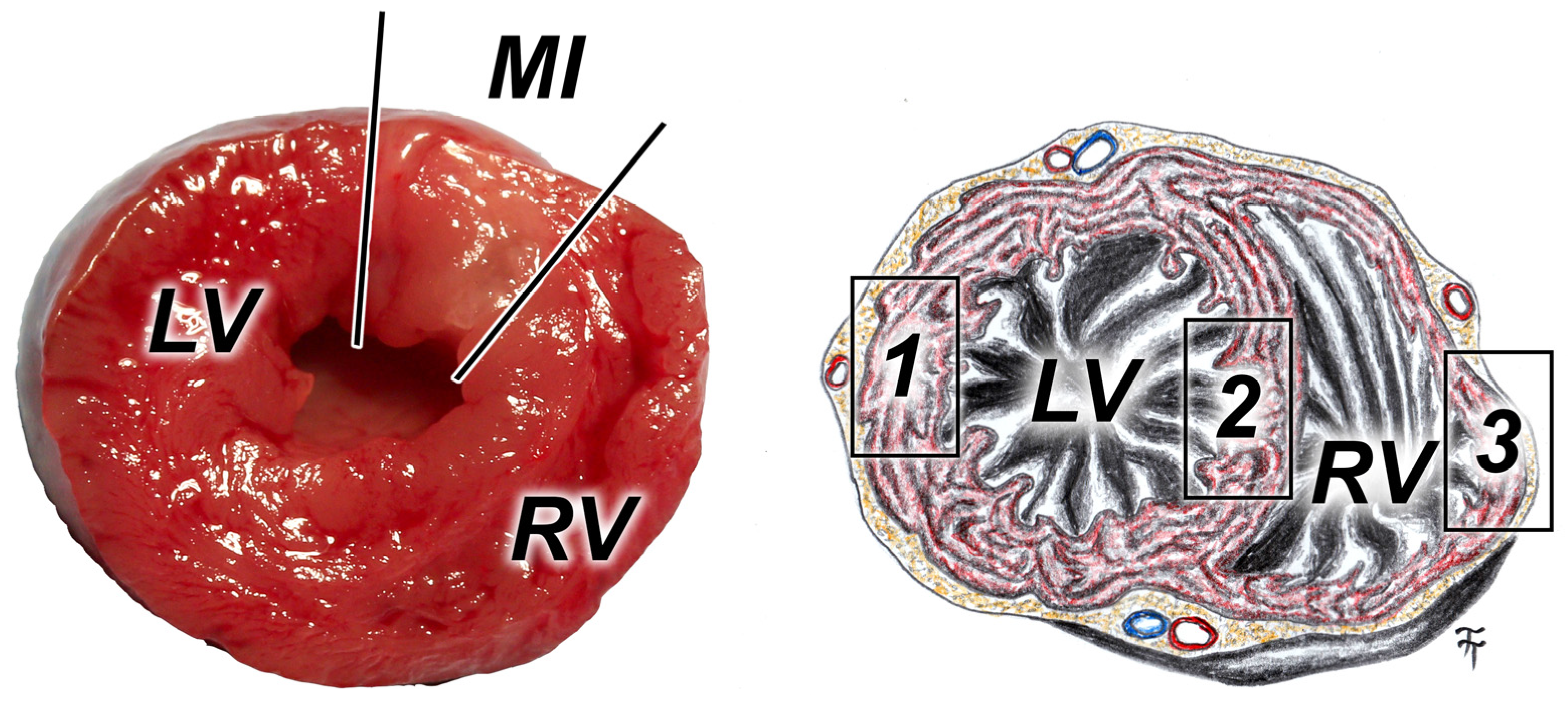
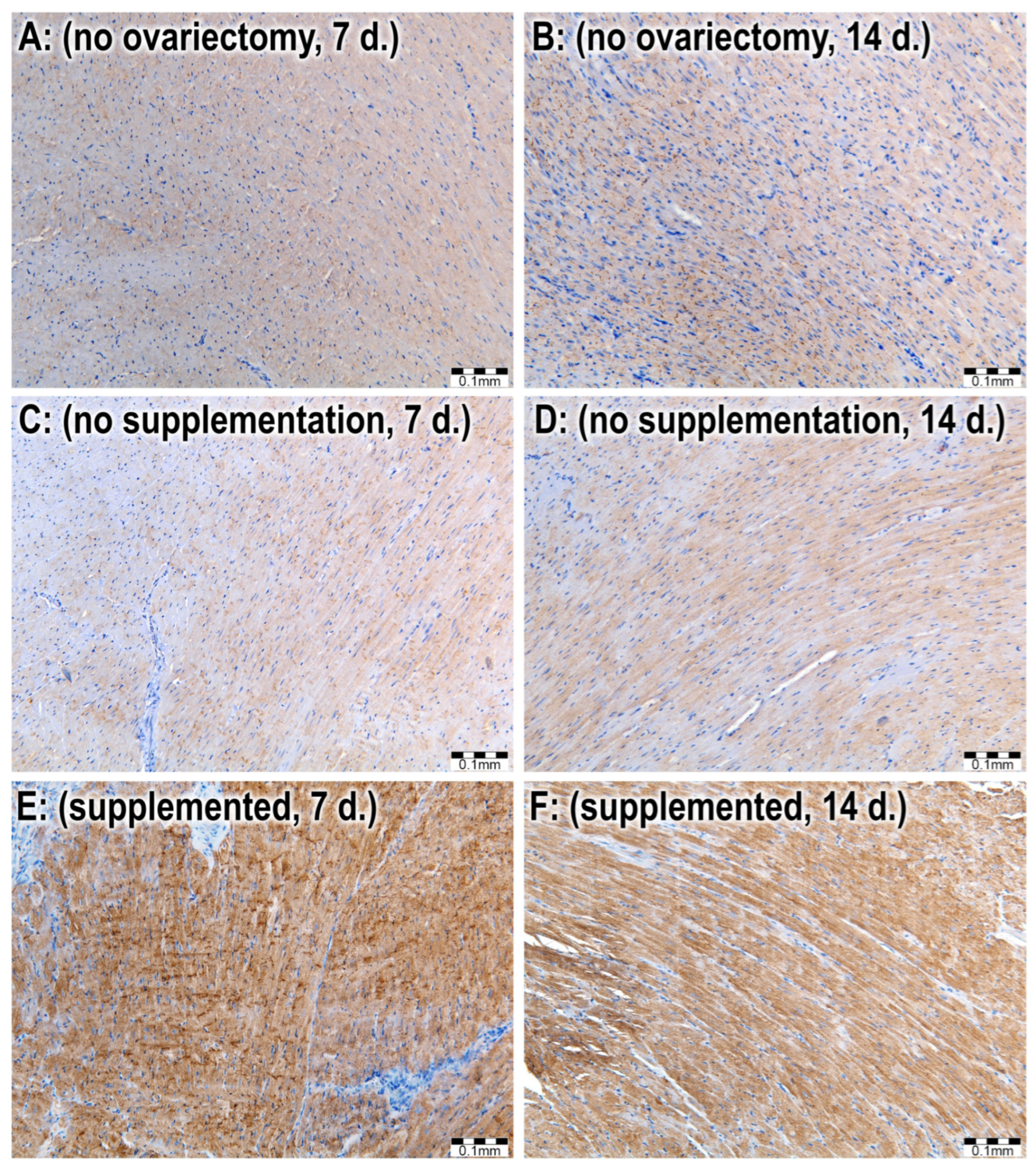
2.4. Immunohistological Analyses
2.5. RNA Isolation and mRNA Gene Level Expression
2.6. Statistical Analyses
3. Results
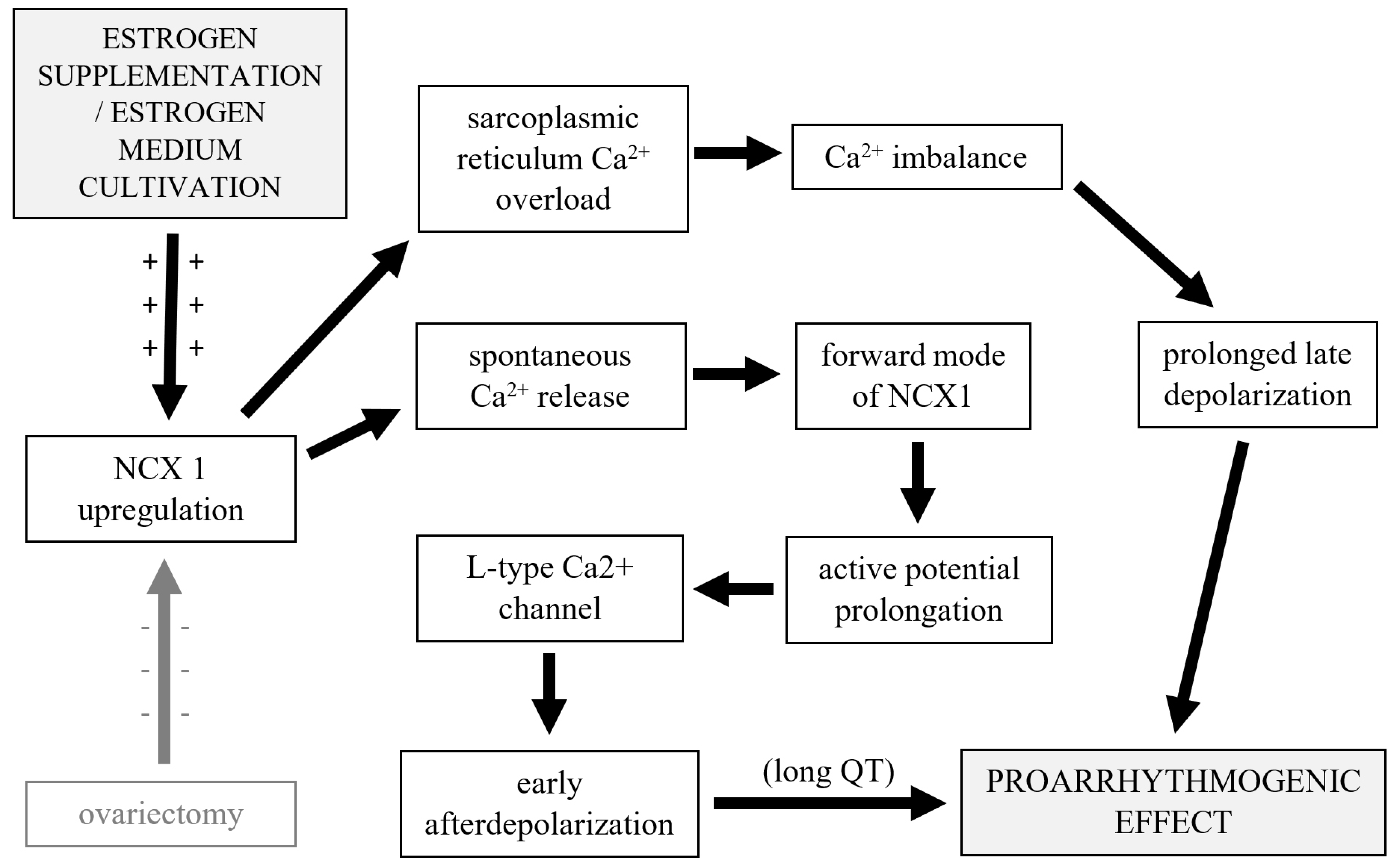
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conte, C.; Antonelli, G.; Melica, M.E.; Tarocchi, M.; Romagnani, P.; Peired, A.J. Role of Sex Hormones in Prevalent Kidney Diseases. Int. J. Mol. Sci. 2023, 24, 8244. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Saguner, A.M.; Gasperetti, A.; Akdis, D.; Brunckhorst, C.; Duru, F. The Link Between Sex Hormones and Susceptibility to Cardiac Arrhythmias: From Molecular Basis to Clinical Implications. Front. Cardiovasc. Med. 2021, 8, 644279. [Google Scholar] [CrossRef] [PubMed]
- Narla, R.R.; Ott, S.M. Bones and the sex hormones. Kidney Int. 2018, 94, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Santos-Marcos, J.A.; Mora-Ortiz, M.; Tena-Sempere, M.; Lopez-Miranda, J.; Camargo, A. Interaction between gut microbiota and sex hormones and their relation to sexual dimorphism in metabolic diseases. Biol. Sex. Differ. 2023, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wen, Z.; Deng, K.; Li, R.; Yu, Q.; Xiao, S.-M. Relationships of sex hormones with muscle mass and muscle strength in male adolescents at different stages of puberty. PLoS ONE 2021, 16, e0260521. [Google Scholar] [CrossRef]
- Prajapati, C.; Koivumäki, J.; Pekkanen-Mattila, M.; Aalto-Setälä, K. Sex differences in heart: From basics to clinics. Eur. J. Med. Res. 2022, 27, 241. [Google Scholar] [CrossRef]
- Adams, R.A.; Liu, Z.; Hsieh, C.; Marko, M.; Lederer, W.J.; Jafri, M.S.; Mannella, C. Structural Analysis of Mitochondria in Cardiomyocytes: Insights into Bioenergetics and Membrane Remodeling. Curr. Issues Mol. Biol. 2023, 45, 6097–6115. [Google Scholar] [CrossRef]
- Lee, W.; Zamudio-Ochoa, A.; Buchel, G.; Podlesniy, P.; Gutierrez, N.M.; Puigròs, M.; Calderon, A.; Tang, H.-Y.; Li, L.; Mikhalchenko, A.; et al. Molecular basis for maternal inheritance of human mitochondrial DNA. Nat. Genet. 2023, 55, 1632–1639. [Google Scholar] [CrossRef]
- Galow, A.M.; Brenmoehl, J.; Hoeflich, A. Synergistic effects of hormones on structural and functional maturation of cardiomyocytes and implications for heart regeneration. Cell Mol. Life Sci. 2023, 80, 240. [Google Scholar] [CrossRef]
- Messinis, I.E. Ovarian feedback, mechanism of action and possible clinical implications. Hum. Reprod. Update 2006, 12, 557–571. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G protein-coupled oestrogen receptor GPER in health and disease: An update. Nat. Rev. Endocrinol. 2023, 19, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Prossnitz, E.R. G-Protein-Coupled Estrogen Receptor (GPER) and Sex-Specific Metabolic Homeostasis. In Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity; Mauvais-Jarvis, F., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 427–453. Available online: http://link.springer.com/10.1007/978-3-319-70178-3_20 (accessed on 20 February 2024).
- Rossi, A.; Pizzo, P.; Filadi, R. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.J.; Howlett, S.E. Sex differences in mechanisms of cardiac excitation–contraction coupling. Pflüg Arch—Eur. J. Physiol. 2013, 465, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Angel Zarain-Herzberg, Á.; Fragoso-Medina, J. SERCA2a: Its role in the development of heart failure and as a potential therapeutic target. Res. Rep. Clin. Cardiol. 2014, 5, 43–55. [Google Scholar] [CrossRef]
- Thibault, S.; Long, V.; Fiset, C. Higher Na+-Ca2+ Exchanger Function and Triggered Activity Contribute to Male Predisposition to Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 10724. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex. Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Nicholson, C.J.; Sweeney, M.; Robson, S.C.; Taggart, M.J. Estrogenic vascular effects are diminished by chronological aging. Sci. Rep. 2017, 7, 12153. [Google Scholar] [CrossRef]
- Niță, A.R.; Knock, G.A.; Heads, R.J. Signalling mechanisms in the cardiovascular protective effects of estrogen: With a focus on rapid/membrane signalling. Curr. Res. Physiol. 2021, 4, 103–118. [Google Scholar] [CrossRef]
- Qian, C.; Liu, J.; Liu, H. Targeting Estrogen Receptor Signaling for Treating Heart Failure. Heart Fail Rev. 2023, 29, 125–131. Available online: https://link.springer.com/10.1007/s10741-023-10356-9 (accessed on 20 February 2024). [CrossRef]
- White, R.E.; Gerrity, R.; Barman, S.A.; Han, G. Estrogen and oxidative stress: A novel mechanism that may increase the risk for cardiovascular disease in women. Steroids 2010, 75, 788–793. [Google Scholar] [CrossRef]
- Chen, Y.; Lüttmann, F.F.; Schoger, E.; Schöler, H.R.; Zelarayán, L.C.; Kim, K.-P.; Haigh, J.J.; Kim, J.; Braun, T. Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science 2021, 373, 1537–1540. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Poss, K.D. Cardiac Regenerative Capacity and Mechanisms. Annu. Rev. Cell Dev. Biol. 2012, 28, 719–741. [Google Scholar] [CrossRef] [PubMed]
- Verjans, R.; van Bilsen, M.; Schroen, B. Reviewing the Limitations of Adult Mammalian Cardiac Regeneration: Noncoding RNAs as Regulators of Cardiomyogenesis. Biomolecules 2020, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Ryczkowska, K.; Adach, W.; Janikowski, K.; Banach, M.; Bielecka-Dabrowa, A. Menopause and women’s cardiovascular health: Is. it really an obvious relationship? Arch. Med. Sci. 2023, 19, 458–466. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxid. Med. Cell. Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Machuki, J.O.; Wu, Q.; Shi, M.; Fu, L.; Adekunle, A.O.; Tao, X.; Xu, C.; Hu, X.; Yin, Z.; et al. Estrogen and calcium handling proteins: New discoveries and mechanisms in cardiovascular diseases. Am. J. Physiol-Heart Circ. Physiol. 2020, 318, H820–H829. [Google Scholar] [CrossRef]
- Lira-Silva, E.; del Valle Mondragón, L.; Pérez-Torres, I.; Posadas-Sánchez, R.; Gómez, F.J.R.; Posadas-Romero, C.; Vargas-Barrón, J.; Pavón, N. Possible implication of estrogenic compounds on heart disease in menopausal women. Biomed. Pharmacother. 2023, 162, 114649. [Google Scholar] [CrossRef]
- Pinkerton, J.V. Hormone Therapy for Postmenopausal Women. N. Engl. J. Med. 2020, 382, 446–455. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Possenti, R.; Mahata, S.K.; Fischer-Colbrie, R.; Loh, Y.P.; Salton, S.R.J. The Extended Granin Family: Structure, Function, and Biomedical Implications. Endocr. Rev. 2011, 32, 755–797. [Google Scholar] [CrossRef]
- deAlmeida, A.; Sedmera, D. Fibroblast Growth Factor-2 regulates proliferation of cardiac myocytes in normal and hypoplastic left ventricles in the developing chick. Cardiol. Young 2009, 19, 159–169. [Google Scholar] [CrossRef]
- Fu, S.; Ping, P.; Wang, F.; Luo, L. Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure. J. Biol. Eng. 2018, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; de Bold, A.J. The heart as an endocrine organ. Endocr. Connect. 2014, 3, R31–R44. [Google Scholar] [CrossRef] [PubMed]
- Widyantoro, B.; Emoto, N.; Nakayama, K.; Anggrahini, D.W.; Adiarto, S.; Iwasa, N.; Yagi, K.; Miyagawa, K.; Rikitake, Y.; Suzuki, T.; et al. Endothelial Cell–Derived Endothelin-1 Promotes Cardiac Fibrosis in Diabetic Hearts Through Stimulation of Endothelial-to-Mesenchymal Transition. Circulation 2010, 121, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Gorbe, A.; Giricz, Z.; Szunyog, A.; Csont, T.; Burley, D.S.; Baxter, G.F.; Ferdinandy, P. Role of cGMP-PKG signaling in the protection of neonatal rat cardiac myocytes subjected to simulated ischemia/reoxygenation. Basic. Res. Cardiol. 2010, 105, 643–650. [Google Scholar] [CrossRef]
- Prysyazhna, O.; Eaton, P. Redox Regulation of cGMP-Dependent Protein Kinase Iα in the Cardiovascular System. Front. Pharmacol. 2015, 6, e00139. Available online: http://journal.frontiersin.org/Article/10.3389/fphar.2015.00139/abstract (accessed on 20 February 2024). [CrossRef]
- Ottolini, M.; Sonkusare, S.K. The Calcium Signaling Mechanisms in Arterial Smooth Muscle and Endothelial Cells. In Comprehensive Physiology, 1st ed.; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 1831–1869. Available online: https://onlinelibrary.wiley.com/doi/10.1002/cphy.c200030 (accessed on 20 February 2024).
- Schlossmann, J.; Desch, M. IRAG and novel PKG targeting in the cardiovascular system. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H672–H682. [Google Scholar] [CrossRef]
- Gilbert, G.; Demydenko, K.; Dries, E.; Puertas, R.D.; Jin, X.; Sipido, K.; Roderick, H.L. Calcium Signaling in Cardiomyocyte Function. Cold Spring Harb. Perspect. Biol. 2020, 12, a035428. [Google Scholar] [CrossRef] [PubMed]
- Niggli, E.; Ullrich, N.D.; Gutierrez, D.; Kyrychenko, S.; Poláková, E.; Shirokova, N. Posttranslational modifications of cardiac ryanodine receptors: Ca2+ signaling and EC-coupling. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2013, 1833, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.; Meizoso-Huesca, A.; Seng, C.; Lamboley, C.R.; Singh, D.P.; Launikonis, B.S. Ryanodine receptor activity and store-operated Ca2+ entry: Critical regulators of Ca2+ content and function in skeletal muscle. J. Physiol. 2023, 601, 4183–4202. [Google Scholar] [CrossRef]
- Yamada, Y.; Namba, K.; Fujii, T. Cardiac muscle thin filament structures reveal calcium regulatory mechanism. Nat. Commun. 2020, 11, 153. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Grossman, D.C.; Curry, S.J.; Owens, D.K.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; Krist, A.H.; et al. Hormone Therapy for the Primary Prevention of Chronic Conditions in Postmenopausal Women: US Preventive Services Task Force Recommendation Statement. JAMA 2017, 318, 2224. [Google Scholar] [PubMed]
- Gál, P.; Toporcer, T.; Vidinský, B.; Mokrý, M.; Grendel, T.; Novotný, M.; Sokolský, J.; Bobrov, N.; Toporcerová, S.; Sabo, J.; et al. Postsurgical Administration of Estradiol Benzoate Decreases Tensile Strength of Healing Skin Wounds in Ovariectomized Rats. J. Surg. Res. 2008, 147, 117–122. [Google Scholar] [CrossRef]
- Jin, Y.; Lee, M.; Park, Y. Synergistic attenuation of ovariectomy-induced bone loss by combined use of fish oil and 17β-oestradiol. Br. J. Nutr. 2017, 117, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Gál, P.; Vidinský, B.; Toporcer, T.; Mokrý, M.; Mozeš, Š.T.; Longauer, F.; Sabo, J. Histological assessment of the effect of laser irradiation on skin wound healing in rats. Photomed. Laser Ther. 2006, 24, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Vidinský, B.; Gál, P.; Toporcer, T.; Longauer, F.; Lenhardt, Ľ.; Bobrov, N.; Sabo, J. Histological study of the first seven days of skin wound healing in rats. Acta Vet. Brno 2006, 75, 197–202. [Google Scholar] [CrossRef]
- Xue, J.; Zeng, W.; Han, Y.; John, S.; Ottolia, M.; Jiang, Y. Structural mechanisms of the human cardiac sodium-calcium exchanger NCX1. Nat. Commun. 2023, 14, 6181. [Google Scholar] [CrossRef]
- Lariccia, V.; Macrì, M.L.; Matteucci, A.; Maiolino, M.; Amoroso, S.; Magi, S. Effects of ticagrelor on the sodium/calcium exchanger 1 (NCX1) in cardiac derived H9c2 cells. Eur. J. Pharmacol. 2019, 850, 158–166. [Google Scholar] [CrossRef]
- Lotteau, S.; Zhang, R.; Hazan, A.; Grabar, C.; Gonzalez, D.; Aynaszyan, S.; Philipson, K.D.; Ottolia, M.; Goldhaber, J.I. Acute Genetic Ablation of Cardiac Sodium/Calcium Exchange in Adult Mice: Implications for Cardiomyocyte Calcium Regulation, Cardioprotection, and Arrhythmia. J. Am. Heart Assoc. 2021, 10, e019273. [Google Scholar] [CrossRef]
- Ujihara, Y.; Iwasaki, K.; Takatsu, S.; Hashimoto, K.; Naruse, K.; Mohri, S.; Katanosaka, Y. Induced NCX1 overexpression attenuates pressure overload-induced pathological cardiac remodelling. Cardiovasc. Res. 2016, 111, 348–361. [Google Scholar] [CrossRef]
- Gök, C.; Main, A.; Gao, X.; Kerekes, Z.; Plain, F.; Kuo, C.W.; Robertson, A.D.; Fraser, N.J.; Fuller, W. Insights into the molecular basis of the palmitoylation and depalmitoylation of NCX1. Cell Calcium 2021, 97, 102408. [Google Scholar] [CrossRef]
- Gambardella, J.; Trimarco, B.; Iaccarino, G.; Santulli, G. New Insights in Cardiac Calcium Handling and Excitation-Contraction Coupling. Adv. Exp. Med. Biol. 2018, 1067, 373–385. [Google Scholar] [PubMed]
- Chou, A.C.; Ju, Y.T.; Pan, C.Y. Calmodulin Interacts with the Sodium/Calcium Exchanger NCX1 to Regulate Activity. PLoS ONE 2015, 10, e0138856. [Google Scholar] [CrossRef] [PubMed]
- Javidanpour, S.; Dianat, M.; Badavi, M.; Mard, S.A. The inhibitory effect of rosmarinic acid on overexpression of NCX1 and stretch- induced arrhythmias after acute myocardial infarction in rats. Biomed. Pharmacother. 2018, 102, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Doleschal, B.; Primessnig, U.; Wölkart, G.; Wolf, S.; Schernthaner, M.; Lichtenegger, M.; Glasnov, T.N.; Kappe, C.O.; Mayer, B.; Antoons, G.; et al. TRPC3 contributes to regulation of cardiac contractility and arrhythmogenesis by dynamic interaction with NCX1. Cardiovasc. Res. 2015, 106, 163–173. [Google Scholar] [CrossRef]
- Poteser, M.; Schleifer, H.; Lichtenegger, M.; Schernthaner, M.; Stockner, T.; Kappe, C.O.; Glasnov, T.N.; Romanin, C.; Groschner, K. PKC-dependent coupling of calcium permeation through transient receptor potential canonical 3 (TRPC3) to calcineurin signaling in HL-1 myocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 10556–10561. [Google Scholar] [CrossRef]
- Ezeani, M. TRP Channels Mediated Pathological Ca2+-Handling and Spontaneous Ectopy. Front. Cardiovasc. Med. 2019, 6, 83. [Google Scholar] [CrossRef]
- Menick, D.R.; Li, M.S.; Chernysh, O.; Renaud, L.; Kimbrough, D.; Kasiganesan, H.; Mani, S.K. Transcriptional pathways and potential therapeutic targets in the regulation of Ncx1 expression in cardiac hypertrophy and failure. Adv. Exp. Med. Biol. 2013, 961, 125–135. [Google Scholar]
- Ottolia, M.; John, S.; Hazan, A.; Goldhaber, J.I. The Cardiac Na+-Ca2+ Exchanger: From Structure to Function. Compr. Physiol. 2021, 12, 2681–2717. [Google Scholar] [PubMed]
- Edwards, A.G.; Mørk, H.; Stokke, M.K.; Lipsett, D.B.; Sjaastad, I.; Richard, S.; Sejersted, O.M.; Louch, W.E. Sarcoplasmic Reticulum Calcium Release Is Required for Arrhythmogenesis in the Mouse. Front. Physiol. 2021, 12, 744730. [Google Scholar] [CrossRef]
- Herrmann, S.; Lipp, P.; Wiesen, K.; Stieber, J.; Nguyen, H.; Kaiser, E.; Ludwig, A. The cardiac sodium–calcium exchanger NCX1 is a key player in the initiation and maintenance of a stable heart rhythm. Cardiovasc. Res. 2013, 99, 780–788. [Google Scholar] [CrossRef]
- Kistamás, K.; Veress, R.; Horváth, B.; Bányász, T.; Nánási, P.P.; Eisner, D.A. Calcium Handling Defects and Cardiac Arrhythmia Syndromes. Front. Pharmacol. 2020, 11, 72. [Google Scholar] [CrossRef]
- Landstrom, A.P.; Dobrev, D.; Wehrens, X.H.T. Calcium Signaling and Cardiac Arrhythmias. Circ. Res. 2017, 120, 1969–1993. [Google Scholar] [CrossRef] [PubMed]
- Njegic, A.; Wilson, C.; Cartwright, E.J. Targeting Ca2 + Handling Proteins for the Treatment of Heart Failure and Arrhythmias. Front. Physiol. 2020, 11, 1068. [Google Scholar] [CrossRef] [PubMed]
- Papp, R.; Bett, G.C.L.; Lis, A.; Rasmusson, R.L.; Baczkó, I.; Varró, A.; Salama, G. Genomic upregulation of cardiac Cav1.2α and NCX1 by estrogen in women. Biol. Sex. Differ. 2017, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Aronsen, J.M.; Louch, W.E.; Sjaastad, I. Cardiomyocyte Ca2+ dynamics: Clinical perspectives. Scand. Cardiovasc. J. 2016, 50, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Kistamás, K.; Hézső, T.; Horváth, B.; Nánási, P.P. Late sodium current and calcium homeostasis in arrhythmogenesis. Channels 2021, 15, 1–19. [Google Scholar] [CrossRef]
- Lerman, B.B.; Markowitz, S.M.; Cheung, J.W.; Thomas, G.; Ip, J.E. Ventricular Tachycardia Due to Triggered Activity. JACC Clin. Electrophysiol. 2023, 10, 379–401. [Google Scholar] [CrossRef]
- Drici, M.D.; Burklow, T.R.; Haridasse, V.; Glazer, R.I.; Woosley, R.L. Sex Hormones Prolong the QT Interval and Downregulate Potassium Channel Expression in the Rabbit Heart. Circulation 1996, 94, 1471–1474. [Google Scholar] [CrossRef]
- Pham, T.V.; Sosunov, E.A.; Gainullin, R.Z.; Danilo, P.; Rosen, M.R. Impact of Sex and Gonadal Steroids on Prolongation of Ventricular Repolarization and Arrhythmias Induced by I K -Blocking Drugs. Circulation 2001, 103, 2207–2212. [Google Scholar] [CrossRef]
- Kim, J.J.; Němec, J.; Papp, R.; Strongin, R.; Abramson, J.J.; Salama, G. Bradycardia alters Ca2+ dynamics enhancing dispersion of repolarization and arrhythmia risk. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H848–H860. [Google Scholar] [CrossRef]
- Machuki, J.O.; Zhang, H.Y.; Geng, J.; Fu, L.; Adzika, G.K.; Wu, L.; Shang, W.; Wu, J.; Kexue, L.; Zhao, Z.; et al. Estrogen regulation of cardiac cAMP-L-type Ca2+ channel pathway modulates sex differences in basal contraction and responses to β2AR-mediated stress in left ventricular apical myocytes. Cell Commun. Signal 2019, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, G.; Papp, R.; DeFranco, D.B.; Zeng, F.; Salama, G. Oestrogen upregulates L-type Ca2+ channels via oestrogen-receptor-α by a regional genomic mechanism in female rabbit hearts. J. Physiol. 2012, 590, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, E.; Chan, T.O.; Zhang, X.Q.; Song, J.; Shang, X.; Koch, W.J.; Feldman, A.M.; Cheung, J.Y. Induced Overexpression of Na+/Ca2+ Exchanger Does Not Aggravate Myocardial Dysfunction Induced by Transverse Aortic Constriction. J. Card. Fail 2013, 19, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, M.; Castaldo, P.; Lariccia, V.; Piccirillo, S.; Amoroso, S.; Magi, S. Essential role of the Na+-Ca2+ exchanger (NCX) in glutamate-enhanced cell survival in cardiac cells exposed to hypoxia/reoxygenation. Sci. Rep. 2017, 7, 13073. [Google Scholar] [CrossRef]
- Torrente, A.G.; Zhang, R.; Zaini, A.; Giani, J.F.; Kang, J.; Lamp, S.T.; Philipson, K.D.; Goldhaber, J.I. Burst pacemaker activity of the sinoatrial node in sodium–calcium exchanger knockout mice. Proc. Natl. Acad. Sci. USA 2015, 112, 9769–9774. [Google Scholar] [CrossRef]
- Li, T.; Xiao, P.; Qiu, D.; Yang, A.; Chen, Q.; Lin, J.; Liu, Y.; Chen, J.; Zeng, Z. NCX1/Ca2+ promotes autophagy and decreases bortezomib activity in multiple myeloma through non-canonical NFκB signaling pathway. Cell Commun. Signal 2024, 22, 258. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Wanitchakool, P.; Schreiber, R.; Wuelling, M.; Vortkamp, A.; Kunzelmann, K. Anoctamin-6 controls bone mineralization by activating the calcium transporter NCX1. J. Biol. Chem. 2015, 290, 6270–6280. [Google Scholar] [CrossRef]

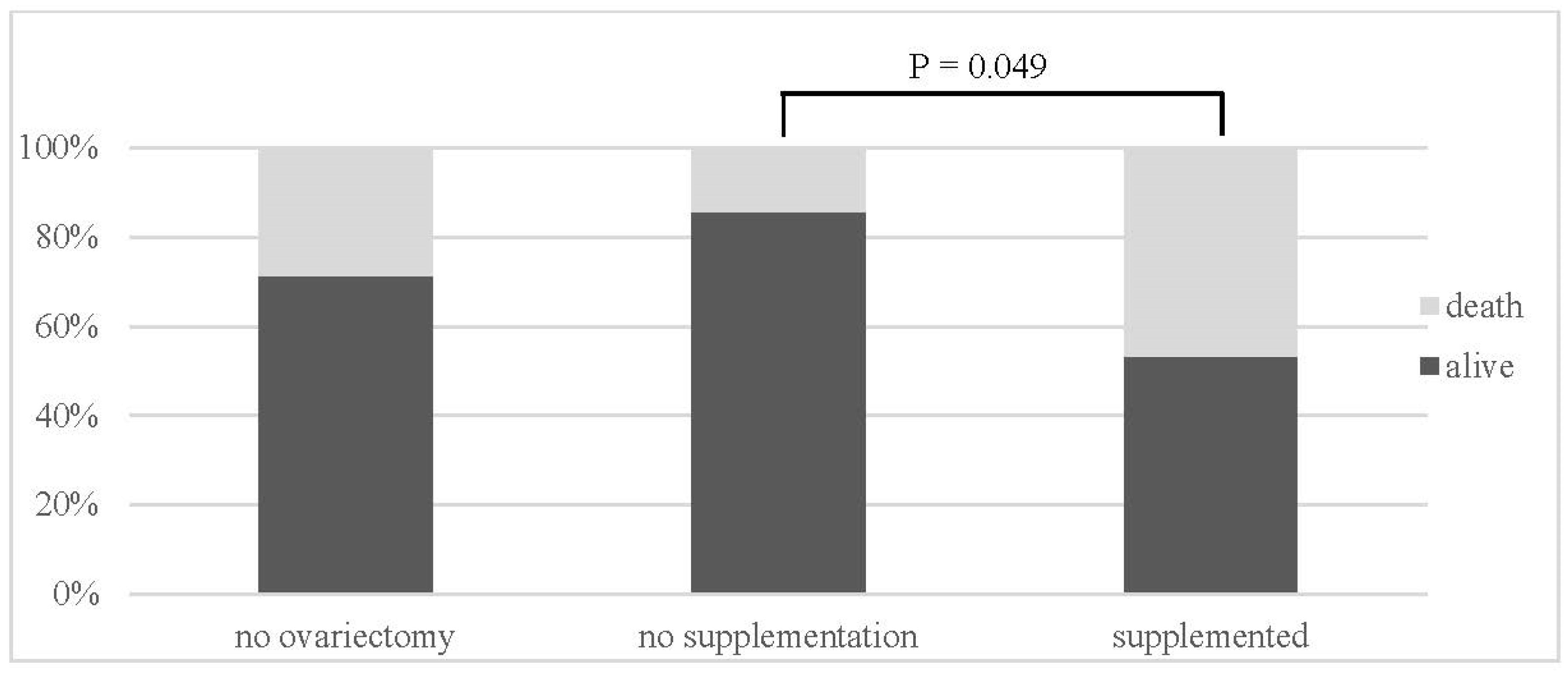

| Group | Alive/Death | |
|---|---|---|
| no ovariectomy (NN) | 10 (71%)/4 (29%) | |
| no supplementation (OVX-N) | 12 (86%)/2 (14%) | p = 0.049 (OVX-N:OVX-S) |
| supplemented (OVX-S) | 16 (53%)/14 (47%) |
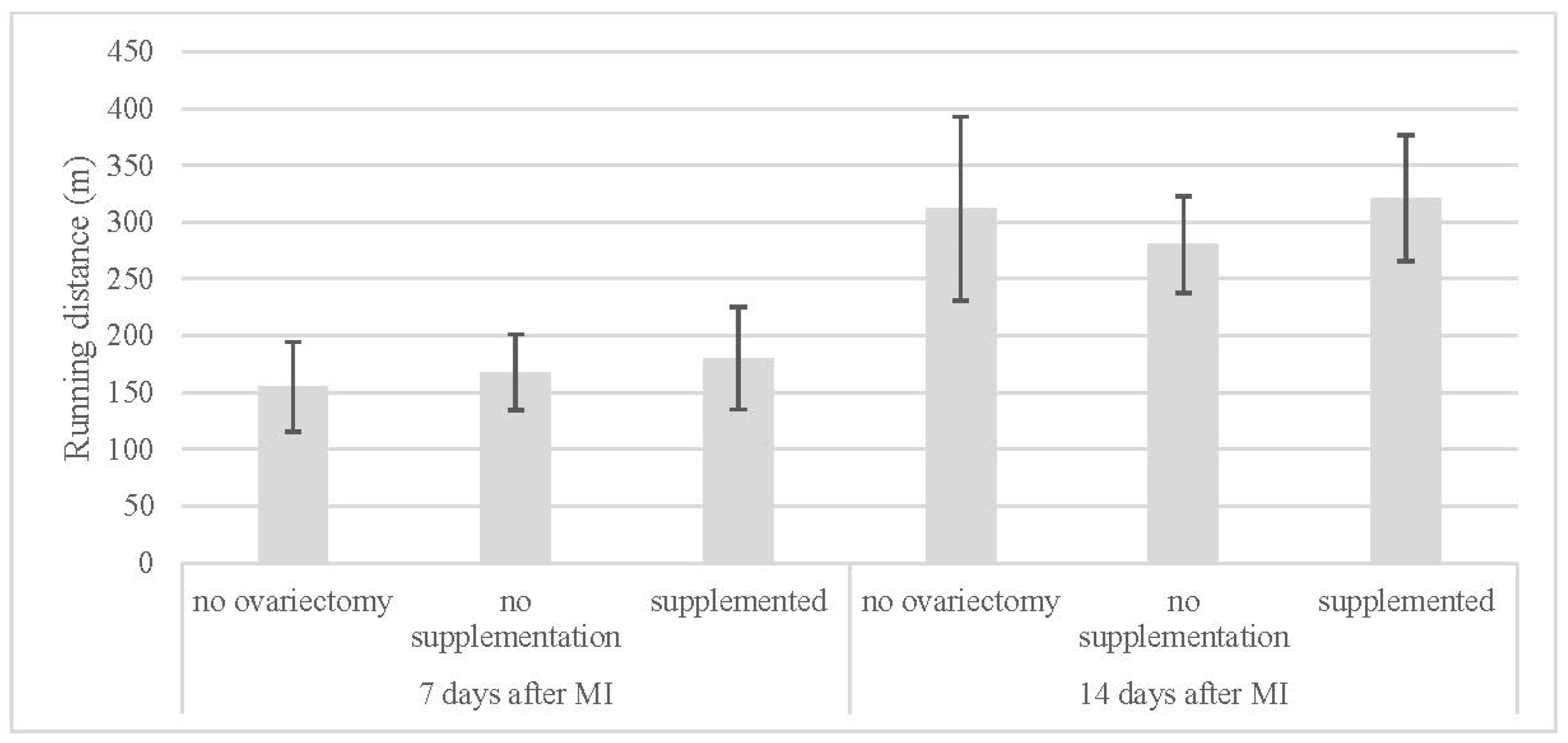
| Running Distance (m) | ||
|---|---|---|
| 7 Days After MI | 14 Days After MI | |
| no ovariectomy (NN) | 155 ± 40 | 312 ± 81 |
| no supplementation (OVX-N) | 168 ± 33 | 281 ± 43 |
| supplemented (OVX-S) | 180 ± 45 | 321 ± 56 |
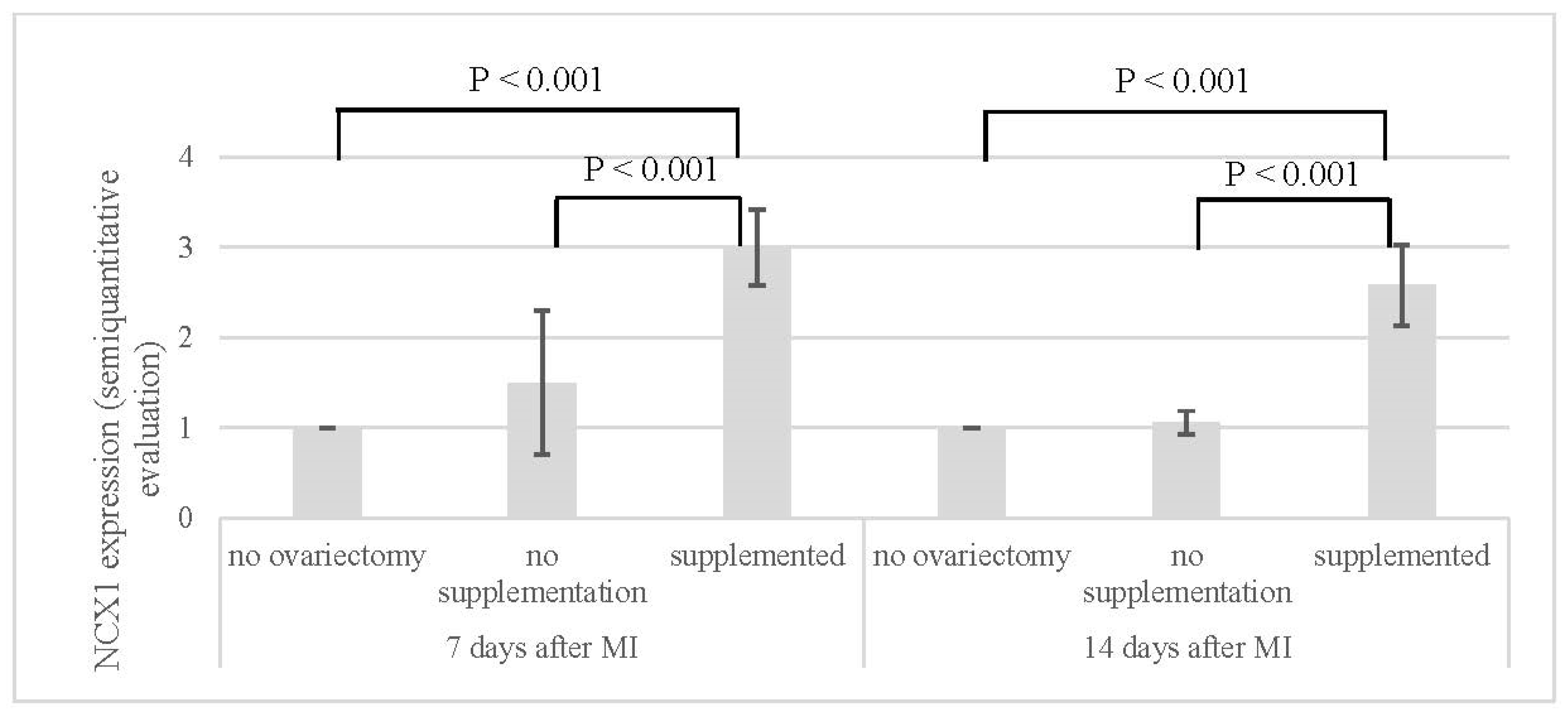
| NCX1 (Semi-Quantitative Evaluation) | ||
|---|---|---|
| 7 Days After MI | 14 Days After MI | |
| no ovariectomy (NN) | 1.0 ± 0.0 | 1.0 ± 0.0 |
| no supplementation (OVX-N) | 1.5 ± 0.8 | 1.1 ± 0.1 |
| supplemented (OVX-S) | 3.0 ± 0.4 | 2.6 ± 0.4 |
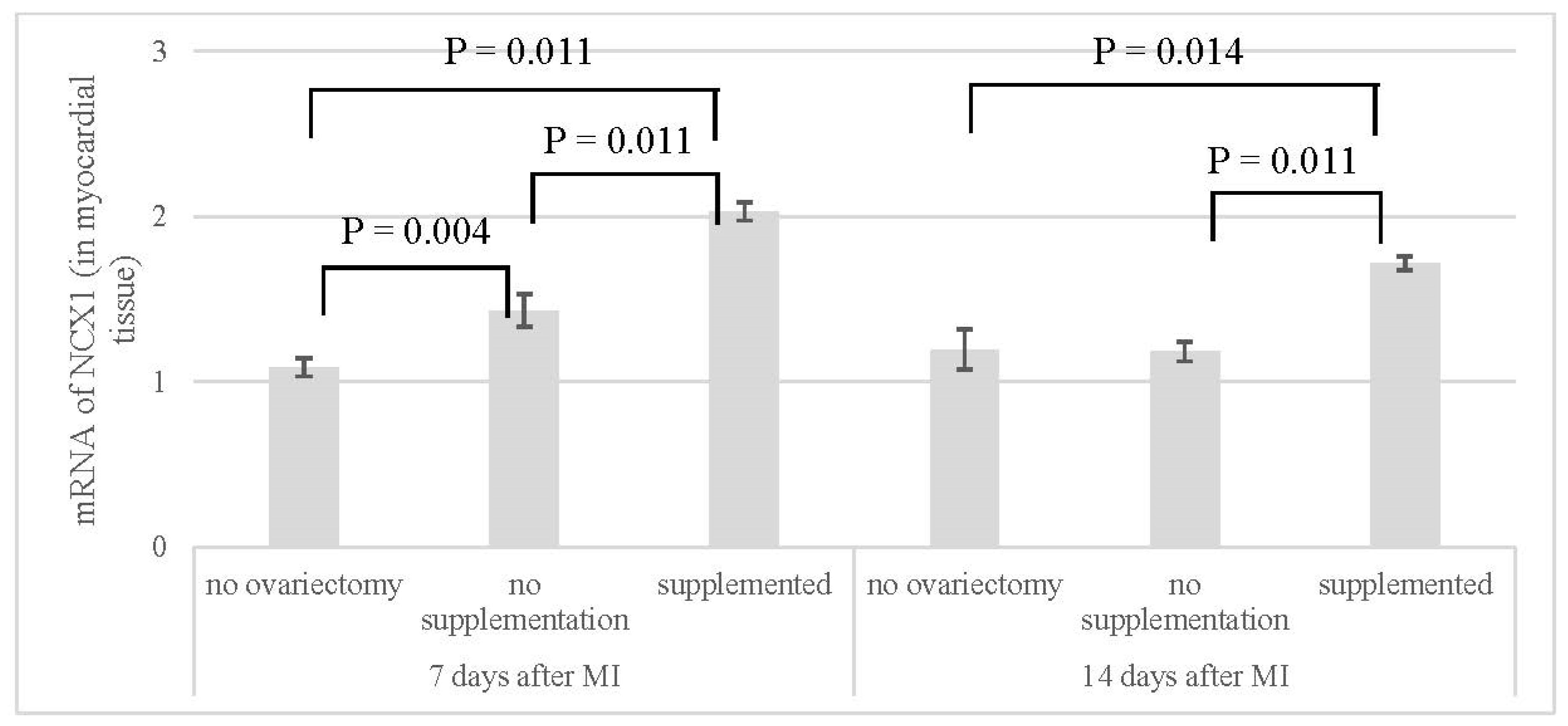
| mRNA of NCX1 | ||
|---|---|---|
| 7 Days After MI | 14 Days After MI | |
| no ovariectomy (NN) | 1.09 ± 0.06 | 1.20 ± 0.12 |
| no supplementation (OVX-N) | 1.43 ± 0.10 | 1.18 ± 0.06 |
| supplemented (OVX-S) | 2.03 ± 0.06 | 1.72 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toporcer, T.; Grendel, T.; Špaková, I.; Blichárová, A.; Verbóová, Ľ.; Benetinová, Z.; Čižmárová, B.; Rabajdová, M.; Toporcerová, S. An In Vivo Model of Estrogen Supplementation Concerning the Expression of Ca2+-Dependent Exchangers and Mortality, Vitality and Survival After Myocardial Infarction in Ovariectomized Rats. J. Cardiovasc. Dev. Dis. 2024, 11, 352. https://doi.org/10.3390/jcdd11110352
Toporcer T, Grendel T, Špaková I, Blichárová A, Verbóová Ľ, Benetinová Z, Čižmárová B, Rabajdová M, Toporcerová S. An In Vivo Model of Estrogen Supplementation Concerning the Expression of Ca2+-Dependent Exchangers and Mortality, Vitality and Survival After Myocardial Infarction in Ovariectomized Rats. Journal of Cardiovascular Development and Disease. 2024; 11(11):352. https://doi.org/10.3390/jcdd11110352
Chicago/Turabian StyleToporcer, Tomáš, Tomáš Grendel, Ivana Špaková, Alžbeta Blichárová, Ľudmila Verbóová, Zuzana Benetinová, Beata Čižmárová, Miroslava Rabajdová, and Silvia Toporcerová. 2024. "An In Vivo Model of Estrogen Supplementation Concerning the Expression of Ca2+-Dependent Exchangers and Mortality, Vitality and Survival After Myocardial Infarction in Ovariectomized Rats" Journal of Cardiovascular Development and Disease 11, no. 11: 352. https://doi.org/10.3390/jcdd11110352
APA StyleToporcer, T., Grendel, T., Špaková, I., Blichárová, A., Verbóová, Ľ., Benetinová, Z., Čižmárová, B., Rabajdová, M., & Toporcerová, S. (2024). An In Vivo Model of Estrogen Supplementation Concerning the Expression of Ca2+-Dependent Exchangers and Mortality, Vitality and Survival After Myocardial Infarction in Ovariectomized Rats. Journal of Cardiovascular Development and Disease, 11(11), 352. https://doi.org/10.3390/jcdd11110352





