Comprehensive Risk Assessment of LAD Disease Progression in CCTA: The CLAP Score Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. CCTA Acquisition and Analysis
2.3. Invasive Coronary Angiography and 3D-QCA Image Acquisition and Analysis
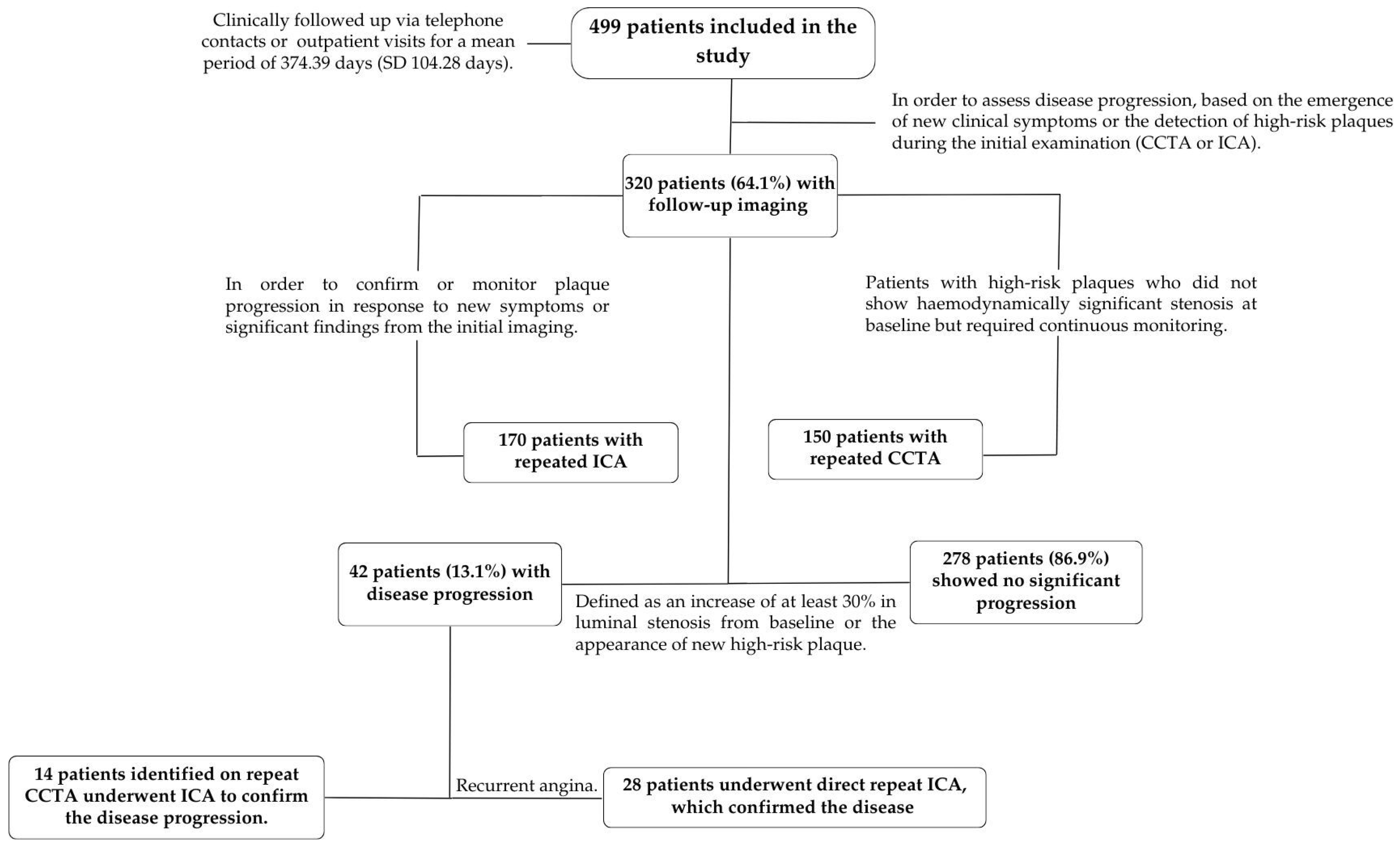
2.4. Follow-Up and Definitions
- Primary endpoints:
- (a)
- To evaluate the consistency and predictive value of LMBA measurements in determining the risk of significant stenosis in the proximal LAD when measured by CCTA and 3D-QCA.
- (b)
- To assess the incidence of major adverse cardiac events (MACE), defined as a composite of cardiovascular death (CD), myocardial infarction (MI), percutaneous coronary intervention (PCI), target lesion revascularization (TLR), and progression of atherosclerotic disease at the proximal LAD at follow-up.
- Secondary endpoints:
- (a)
- Determination of the individual components of MACE, in particular the progression of atherosclerotic disease at the proximal LAD and the occurrence of target lesion revascularization (TLR);
- (b)
- Identification of the clinical and anatomical predictors of proximal LAD stenosis.
- Statistical Analysis
3. Results
3.1. Primary Endpoints
- Consistency and predictive value of LMBA measurements in CCTA and 3D-QCA modalities
- Clinical outcome and predictors of MACE
- a.
- Individual Counts for Each Endpoint.
- b.
- Predictors of MACE
3.2. Secondary Endpoints
- Detailed Analysis of Predictors for Individual MACE Components
- Clinical and anatomical predictors of proximal LAD stenosis
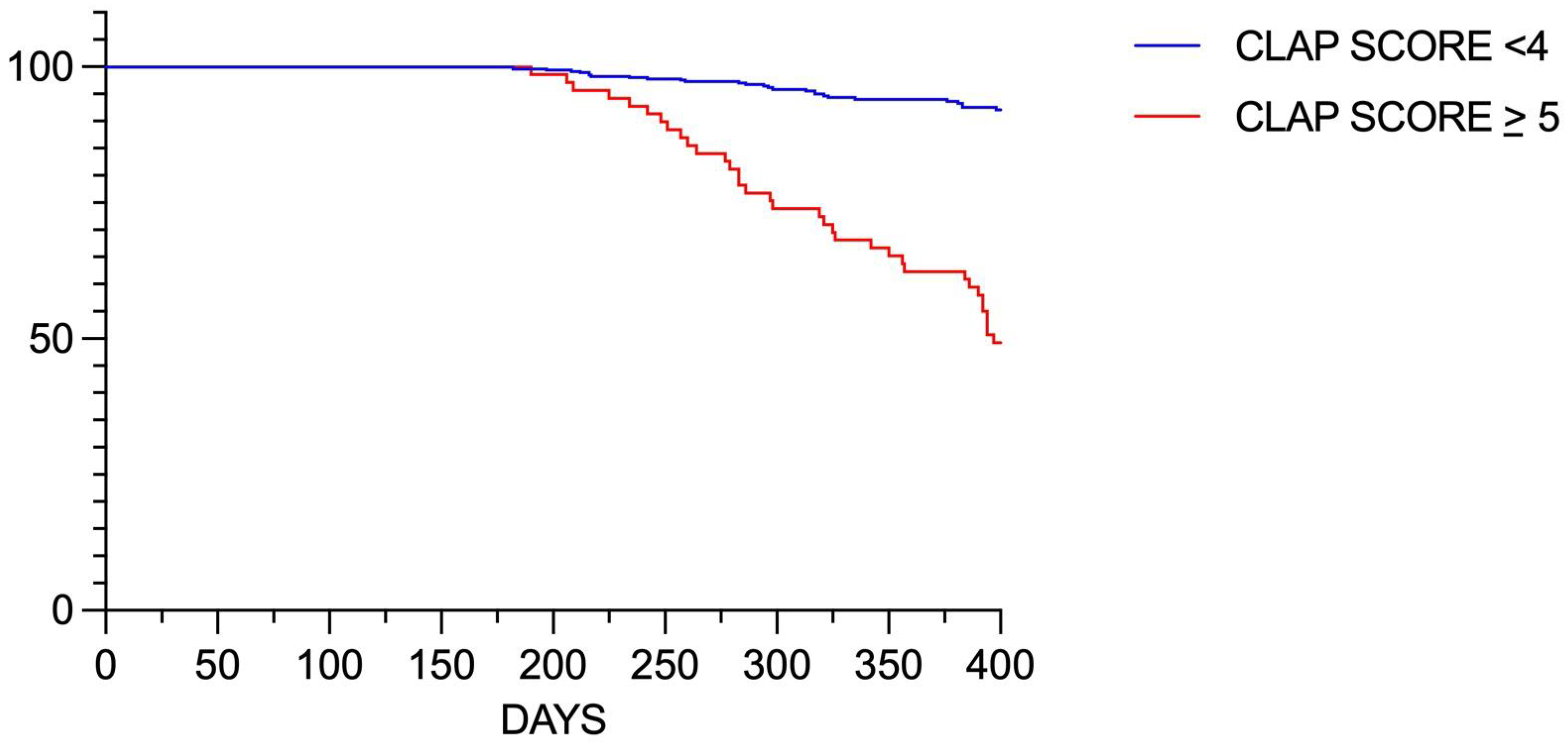
3.3. Predictive Score for Disease Progression in the LAD
- Sensitivity analysis
4. Discussion
- Study limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medrano-Gracia, P.; Ormiston, J.; Webster, M.; Beier, S.; Young, A.; Ellis, C.; Wang, C.; Smedby, Ö.; Cowan, B. A computational atlas of normal coronary artery anatomy. EuroIntervention 2016, 12, 845–854. [Google Scholar] [CrossRef]
- Wasilewski, J.; Niedziela, J.; Osadnik, T.; Duszańska, A.; Sraga, W.; Desperak, P.; Myga-Porosiło, J.; Jackowska, Z.; Nowakowski, A.; Głowacki, J. Predominant location of coronary artery atherosclerosis in the left anterior descending artery. The impact of septal perforators and the myocardial bridging effect. Kardiochirurgia Torakochirurgia Pol. 2015, 12, 379–385. [Google Scholar] [CrossRef]
- Murasato, Y.; Meno, K.; Mori, T.; Tanenaka, K. Impact of coronary bifurcation angle on the pathogenesis of atherosclerosis and clinical outcome of coronary bifurcation intervention—A scoping review. PLoS ONE 2022, 17, e0273157. [Google Scholar] [CrossRef]
- Capodanno, D.; Gargiulo, G.; Longo, G.; Tamburino, C. Three-Dimensional Angle Assessment and Plaque Distribution Classification in Left Main Disease: Impact of Geometry on Outcome. Rev. Cardiovasc. Med. 2015, 16, 131–139. [Google Scholar] [CrossRef]
- Bertolone, D.T.; Gallinoro, E.; Esposito, G.; Paolisso, P.; Bermpeis, K.; De Colle, C.; Fabbricatore, D.; Mileva, N.; Valeriano, C.; Munhoz, D.; et al. Contemporary Management of Stable Coronary Artery Disease. High Blood Press. Cardiovasc. Prev. 2022, 29, 207–219. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, L.; Fan, Z. Coronary CT angiography in calcified coronary plaques: Comparison of diagnostic accuracy between bifurcation angle measurement and coronary lumen assessment for diagnosing significant coronary stenosis. Int. J. Cardiol. 2016, 203, 78–86. [Google Scholar] [CrossRef]
- Kesarwani, M.; Nakanishi, R.; Choi, T.-Y.; Shavelle, D.M.; Budoff, M.J. Evaluation of Plaque Morphology by 64-Slice Coronary Computed Tomographic Angiography Compared to Intravascular Ultrasound in Nonocclusive Segments of Coronary Arteries. Acad. Radiol. 2017, 24, 968–974. [Google Scholar] [CrossRef]
- Hecht, H.S.; Achenbach, S.; Kondo, T.; Narula, J. High-Risk Plaque Features on Coronary CT Angiography. JACC Cardiovasc. Imaging 2015, 8, 1336–1339. [Google Scholar] [CrossRef]
- Glodny, B.; Helmel, B.; Trieb, T.; Schenk, C.; Taferner, B.; Unterholzner, V.; Strasak, A.; Petersen, J. A method for calcium quantification by means of CT coronary angiography using 64-multidetector CT: Very high correlation with Agatston and volume scores. Eur. Radiol. 2009, 19, 1661–1668. [Google Scholar] [CrossRef]
- Mihic, P.Z.; Arambasic, J.; Mlinarevic, D.; Saric, S.; Labor, M.; Bosnjak, I.; Mihaljevic, I.; Curcic, I.B.; Juric, I. Coronary Tortuosity Index vs. Angle Measurement Method for the Quantification of the Tortuosity of Coronary Arteries in Non-Obstructive Coronary Disease. Diagnostics 2023, 14, 35. [Google Scholar] [CrossRef]
- Gradaus, R.; Mathies, K.; Breithardt, G.; Böcker, D. Clinical assessment of a new real time 3D quantitative coronary angiography system: Evaluation in stented vessel segments. Catheter. Cardiovasc. Interv. 2006, 68, 44–49. [Google Scholar] [CrossRef]
- Schlundt, C.; Kreft, J.; Fuchs, F.; Achenbach, S.; Daniel, W.; Ludwig, J. Three-dimensional on-line reconstruction of coronary bifurcated lesions to optimize side-branch stenting. Catheter. Cardiovasc. Interv. 2006, 68, 249–253. [Google Scholar] [CrossRef]
- Gollapudi, R.R.; Valencia, R.; Lee, S.S.; Wong, G.B.; Teirstein, P.S.; Price, M.J. Utility of three-dimensional reconstruction of coronary angiography to guide percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2007, 69, 479–482. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.; Members of the TRIPOD Group. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. Eur. Urol. 2015, 67, 1142–1151. [Google Scholar] [CrossRef]
- Hoffmann, U.; Truong, Q.A.; Schoenfeld, D.A.; Chou, E.T.; Woodard, P.K.; Nagurney, J.T.; Pope, J.H.; Hauser, T.H.; White, C.S.; Weiner, S.G.; et al. Coronary CT angiography versus standard evaluation in acute chest pain. N. Engl. J. Med. 2012, 367, 299–308. [Google Scholar] [CrossRef]
- Moon, S.H.; Byun, J.H.; Kim, J.W.; Kim, S.H.; Kim, K.N.; Jung, J.J.; Kang, D.H.; Yang, J.H.; Choi, J.Y.; Jang, I.S.; et al. Clinical usefulness of the angle between left main coronary artery and left anterior descending coronary artery for the evaluation of obstructive coronary artery disease. PLoS ONE 2018, 13, e0202249. [Google Scholar] [CrossRef]
- Sun, Z. Coronary CT angiography in coronary artery disease: Correlation between virtual intravascular endoscopic appearances and left bifurcation angulation and coronary plaques. BioMed Res. Int. 2013, 2013, 732059. [Google Scholar] [CrossRef]
- Ziyrek, M.; Sertdemir, A.L.; Duran, M. Effect of Coronary Artery Bifurcation Angle on Atherosclerotic Lesion Localization Distance to the Bifurcation Site. J. Saudi Heart Assoc. 2020, 32, 399–407. [Google Scholar] [CrossRef]
- Cui, Y.; Zeng, W.; Yu, J.; Lu, J.; Hu, Y.; Diao, N.; Liang, B.; Han, P.; Shi, H. Quantification of left coronary bifurcation angles and plaques by coronary computed tomography angiography for prediction of significant coronary stenosis: A preliminary study with dual-source CT. PLoS ONE 2017, 12, e0174352. [Google Scholar] [CrossRef]
- Sun, Z.; Chaichana, T. An investigation of correlation between left coronary bifurcation angle and hemodynamic changes in coronary stenosis by coronary computed tomography angiography-derived computational fluid dynamics. Quant. Imaging Med. Surg. 2017, 7, 537–548. [Google Scholar] [CrossRef]
- Temov, K.; Sun, Z. Coronary computed tomography angiography investigation of the association between left main coronary artery bifurcation angle and risk factors of coronary artery disease. Int. J. Cardiovasc. Imaging 2016, 32, 129–137. [Google Scholar] [CrossRef]
- Yahia, M.; Farid, W.; Lotfy, M.; Osama, M.; El Deep, H.A. Association between bifurcation angle of the left main coronary artery and severity of stenosis of the proximal left anterior descending artery. Cardiovasc. J. Afr. 2023, 34, 93–97. [Google Scholar] [CrossRef]
- Juan, Y.-H.; Tsay, P.-K.; Shen, W.-C.; Yeh, C.-S.; Wen, M.-S.; Wan, Y.-L. Comparison of the Left Main Coronary Bifurcating Angle among Patients with Normal, Non-significantly and Significantly Stenosed Left Coronary Arteries. Sci. Rep. 2017, 7, 1515. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Li, S.; Lv, J. Endothelial Dysfunction and Diabetic Cardiomyopathy. Front. Endocrinol. 2022, 13, 851941. [Google Scholar] [CrossRef]
- Raymond, T.; Raymond, R.; Lincoff, A.M. Management of the patient with diabetes and coronary artery disease: A contemporary review. Future Cardiol. 2013, 9, 387–403. [Google Scholar] [CrossRef]
- Tommasino, A.; Burzotta, F.; Trani, C.; Giammarinaro, M.; Schiavoni, G. Impact of metabolic syndrome on angiographic and clinical outcome after stenting. Am. J. Cardiol. 2008, 101, 1679. [Google Scholar] [CrossRef]
- Tommasino, A.; Burzotta, F.; Trani, C.; Giammarinaro, M.; Schiavoni, G. Metabolic syndrome is a poor predictor of outcome after coronary interventions in high-risk patients. Hypertens. Res. 2008, 31, 2097. [Google Scholar] [CrossRef]
- Colaiori, I.; Izzo, R.; Barbato, E.; Franco, D.; Di Gioia, G.; Rapacciuolo, A.; Bartunek, J.; Mancusi, C.; Losi, M.A.; Strisciuglio, T.; et al. Severity of Coronary Atherosclerosis and Risk of Diabetes Mellitus. J. Clin. Med. 2019, 8, 1069. [Google Scholar] [CrossRef]
- Shigemoto, E.; Iwata, A.; Futami, M.; Kato, Y.; Yamashita, M.; Imaizumi, S.; Kuwano, T.; Ike, A.; Sugihara, M.; Saku, K.; et al. Influence of chronic kidney disease on coronary plaque components in coronary artery disease patients with both diabetes mellitus and hypertension. Heart Vessel. 2019, 34, 1065–1075. [Google Scholar] [CrossRef]
- Achenbach, S. What makes a plaque rupture? A simple answer seems too much to ask for. EuroIntervention 2023, 18, 952–954. [Google Scholar] [CrossRef]
- Geerlings-Batt, J.; Sun, Z. Evaluation of the Relationship between Left Coronary Artery Bifurcation Angle and Coronary Artery Disease: A Systematic Review. J. Clin. Med. 2022, 11, 5143. [Google Scholar] [CrossRef]
- Katakia, Y.T.; Kanduri, S.; Bhattacharyya, R.; Ramanathan, S.; Nigam, I.; Kuncharam, B.V.R.; Majumder, S. Angular difference in human coronary artery governs endothelial cell structure and function. Commun. Biol. 2022, 5, 1044. [Google Scholar] [CrossRef]
- Chaichana, T.; Sun, Z.; Jewkes, J. Computation of hemodynamics in the left coronary artery with variable angulations. J. Biomech. 2011, 44, 1869–1878. [Google Scholar] [CrossRef]
- Konishi, T.; Yamamoto, T.; Funayama, N.; Nishihara, H.; Hotta, D. Relationship between left coronary artery bifurcation angle and restenosis after stenting of the proximal left anterior descending artery. Coron. Artery Dis. 2016, 27, 449–459. [Google Scholar] [CrossRef]
- Dzavik, V.; Kharbanda, R.; Ivanov, J.; Ing, D.J.; Bui, S.; Mackie, K.; Ramsamujh, R.; Barolet, A.; Schwartz, L.; Seidelin, P.H. Predictors of long-term outcome after crush stenting of coronary bifurcation lesions: Importance of the bifurcation angle. Am. Heart J. 2006, 152, 762–769. [Google Scholar] [CrossRef]
- Ki, Y.-J.; Jung, J.H.; Han, J.-K.; Hong, S.; Cho, J.H.; Gwon, H.-C.; Lee, S.Y.; Rhew, J.Y.; Chae, J.K.; Chae, I.-H.; et al. Clinical Implications of Bifurcation Angles in Left Main Bifurcation Intervention Using a Two-Stent Technique. J. Interv. Cardiol. 2020, 2020, 2475930. [Google Scholar] [CrossRef]
- Stone, P.H.; Saito, S.; Takahashi, S.; Makita, Y.; Nakamura, S.; Kawasaki, T.; Takahashi, A.; Katsuki, T.; Nakamura, S.; Namiki, A.; et al. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: The PREDICTION Study. Circulation 2012, 126, 172–181. [Google Scholar] [CrossRef]
- Ekmejian, A.A.; Carpenter, H.J.; Ciofani, J.L.; Gray, B.H.M.; Allahwala, U.K.; Ward, M.; Escaned, J.; Psaltis, P.J.; Bhindi, R. Advances in the Computational Assessment of Disturbed Coronary Flow and Wall Shear Stress: A Contemporary Review. J. Am. Heart Assoc. 2024, 13, e037129. [Google Scholar] [CrossRef]
- Nikpour, M.; Mohebbi, A. Predicting coronary artery occlusion risk from noninvasive images by combining CFD-FSI, cGAN and CNN. Sci. Rep. 2024, 14, 22693. [Google Scholar] [CrossRef]
- Zhang, D.; Dou, K. Coronary Bifurcation Intervention: What Role Do Bifurcation Angles Play? J. Interv. Cardiol. 2015, 28, 236–248. [Google Scholar] [CrossRef]
- Nakazawa, G.; Yazdani, S.K.; Finn, A.V.; Vorpahl, M.; Kolodgie, F.D.; Virmani, R. Pathological findings at bifurcation lesions: The impact of flow distribution on atherosclerosis and arterial healing after stent implantation. J. Am. Coll Cardiol. 2010, 55, 1679–1687. [Google Scholar] [CrossRef]
- Katritsis, D.; Kaiktsis, L.; Chaniotis, A.; Pantos, J.; Efstathopoulos, E.P.; Marmarelis, V. Wall shear stress: Theoretical considerations and methods of measurement. Prog. Cardiovasc. Dis. 2007, 49, 307–329. [Google Scholar] [CrossRef]
- Perktold, K.; Peter, R.; Resch, M.; Langs, G. Pulsatile non-newtonian blood flow in three-dimensional carotid bifurcation models: A numerical study of flow phenomena under different bifurcation angles. J. Biomed. Eng. 1991, 13, 507–515. [Google Scholar] [CrossRef]
- Park, S.-J.; Park, S.-J.; Ahn, J.-M.; Ahn, J.-M.; Kang, D.-Y.; Kang, D.-Y.; Yun, S.-C.; Yun, S.-C.; Ahn, Y.-K.; Ahn, Y.-K.; et al. Preventive percutaneous coronary intervention versus optimal medical therapy alone for the treatment of vulnerable atherosclerotic coronary plaques (PREVENT): A multicentre, open-label, randomised controlled trial. Lancet 2024, 403, 1753–1765. [Google Scholar] [CrossRef]
- Mol, J.-Q.; Bom, M.J.; Damman, P.; Knaapen, P.; van Royen, N. Pre-Emptive OCT-Guided Angioplasty of Vulnerable Intermediate Coronary Lesions: Results from the Prematurely Halted PECTUS-Trial. J. Interv. Cardiol. 2020, 2020, 8821525. [Google Scholar] [CrossRef]
- Kinoshita, D.; Suzuki, K.; Usui, E.; Hada, M.; Yuki, H.; Niida, T.; Minami, Y.; Lee, H.; McNulty, I.; Ako, J.; et al. High-Risk Plaques on Coronary Computed Tomography Angiography: Correlation with Optical Coherence Tomography. JACC Cardiovasc. Imaging 2024, 17, 382–391. [Google Scholar] [CrossRef]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.L.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A.; et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef]
- Nakanishi, R.; Li, D.; Blaha, M.J.; Whelton, S.P.; Darabian, S.; Flores, F.R.; Dailing, C.; Blumenthal, R.S.; Nasir, K.; Berman, D.S.; et al. All-cause mortality by age and gender based on coronary artery calcium scores. Eur. Heart J.-Cardiovasc. Imaging 2016, 17, 1305–1314. [Google Scholar] [CrossRef]
- Tsugu, T.; Tanaka, K.; Nagatomo, Y.; Belsack, D.; Devos, H.; Buls, N.; Cosyns, B.; Argacha, J.-F.; De Maeseneer, M.; De Mey, J. Impact of coronary bifurcation angle on computed tomography derived fractional flow reserve in coronary vessels with no apparent coronary artery disease. Eur. Radiol. 2023, 33, 1277–1285. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Koo, B.-K.; Park, J.; Kim, J.; Hwang, D.; Rhee, T.-M.; Kim, H.Y.; Jung, H.W.; Kim, K.-J.; et al. Prognostic Implications of Plaque Characteristics and Stenosis Severity in Patients with Coronary Artery Disease. J. Am. Coll Cardiol. 2019, 73, 2413–2424. [Google Scholar] [CrossRef]
- Bittencourt, M.S. Prognostic Value of Coronary Atherosclerotic Burden, Its Plaque Components and Estimation of Coronary Flow by Coronary Computed Tomography. Circ. Cardiovasc. Imaging 2024, 17, e016524. [Google Scholar] [CrossRef]
- Dzaye, O.; Razavi, A.C.; Blaha, M.J.; Mortensen, M.B. Evaluation of coronary stenosis versus plaque burden for atherosclerotic cardiovascular disease risk assessment and management. Curr. Opin. Cardiol. 2021, 36, 769–775. [Google Scholar] [CrossRef]
- Ahmed, A.I.; Han, Y.; Al Rifai, M.; Alnabelsi, T.; Nabi, F.; Chang, S.M.; Chamsi-Pasha, M.A.; Nasir, K.; Mahmarian, J.J.; Cainzos-Achirica, M.; et al. Added prognostic value of plaque burden to computed tomography angiography and myocardial perfusion imaging. Atherosclerosis 2021, 334, 9–16. [Google Scholar] [CrossRef]
- Arrey-Mbi, T.B.; Klusewitz, S.M.; Villines, T.C. Correction to: Long-Term Prognostic Value of Coronary Computed Tomography Angiography. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 51. [Google Scholar] [CrossRef]
- Chang, H.-J.; Lin, F.Y.; Lee, S.-E.; Andreini, D.; Bax, J.; Cademartiri, F.; Chinnaiyan, K.; Chow, B.J.; Conte, E.; Cury, R.C.; et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J. Am. Coll Cardiol. 2018, 71, 2511–2522. [Google Scholar] [CrossRef]
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results From the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020, 141, 1452–1462. [Google Scholar] [CrossRef]
- Douglas, P.S.; Hoffmann, U.; Patel, M.R.; Mark, D.B.; Al-Khalidi, H.R.; Cavanaugh, B.; Cole, J.; Dolor, R.J.; Fordyce, C.B.; Huang, M.; et al. Outcomes of anatomical versus functional testing for coronary artery disease. N. Engl. J. Med. 2015, 372, 1291–1300. [Google Scholar] [CrossRef]
- Lee, S.-E.; Chang, H.-J.; Rizvi, A.; Hadamitzky, M.; Kim, Y.-J.; Conte, E.; Andreini, D.; Pontone, G.; Volpato, V.; Budoff, M.J.; et al. Rationale and design of the Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography IMaging (PARADIGM) registry: A comprehensive exploration of plaque progression and its impact on clinical outcomes from a multicenter serial coronary computed tomographic angiography study. Am. Heart J. 2016, 182, 72–79. [Google Scholar] [CrossRef]


| Overall Population (n = 499) | Mean (SD) or Frequency |
|---|---|
| Age (yrs) | 64.39 ± 9.9 |
| Male | 391 (78.3%) |
| Smoke | 99 (19.8%) |
| Hypertension | 365 (73.1%) |
| Dyslipidemia | 280 (56.1%) |
| Diabetes Mellitus | 113 (22.6%) |
| Chronic kidney disease | 93 (18.6%) |
| 1-vessel disease | 252 (50.5%) |
| 2-vessels disease | 141 (28.3%) |
| 3-vessels disease | 96 (19.2%) |
| LM disease Proximal LAD disease | 110 (22.1%) 210 (42.1%) |
| LAD PCI | 110 (22.0%) |
| Variable | B | SE | Wald | p Value | Exp (B) | 95% LCI | 95% UCI |
|---|---|---|---|---|---|---|---|
| Diabetes | 1.077 | 0.281 | 14.72 | 0.031 | 2.938 | 1.540 | 4.627 |
| Chronic kidney disease | 0.537 | 0.422 | 1.62 | 0.041 | 1.709 | 1.310 | 6.720 |
| CCTA LMBA > 80° | 1.499 | 0.206 | 53.04 | <0.001 | 4.474 | 3.799 | 6.701 |
| High-risk Plaque | 0.833 | 0.234 | 12.68 | <0.01 | 2.298 | 1.449 | 3.644 |
| CAC Score | 0.048 | 0.017 | 8.15 | 0.004 | 1.05 | 1.02 | 1.08 |
| Obstructive CAD | 0.917 | 0.310 | 8.75 | 0.01 | 2.50 | 1.50 | 4.10 |
| Variable | B | SE | Wald | p Value | Exp (B) | 95% LCI | 95% UCI |
|---|---|---|---|---|---|---|---|
| LMBA > 80° | 1.672 | 0.258 | 42.08 | <0.001 | 5.325 | 4.029 | 6.701 |
| High-risk Plaque | 0.752 | 0.435 | 2.99 | <0.01 | 2.120 | 1.385 | 3.246 |
| CAC Score > 180 | 0.039 | 0.015 | 6.74 | 0.01 | 1.04 | 1.01 | 1.07 |
| Obstructive CAD | 0.788 | 0.274 | 8.23 | 0.02 | 2.20 | 1.40 | 3.50 |
| Diabetes | 1.321 | 0.269 | 24.18 | <0.001 | 3.745 | 2.691 | 4.713 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tommasino, A.; Dell’Aquila, F.; Redivo, M.; Pittorino, L.; Mattaroccia, G.; Tempestini, F.; Santucci, S.; Casenghi, M.; Giovannelli, F.; Rigattieri, S.; et al. Comprehensive Risk Assessment of LAD Disease Progression in CCTA: The CLAP Score Study. J. Cardiovasc. Dev. Dis. 2024, 11, 338. https://doi.org/10.3390/jcdd11110338
Tommasino A, Dell’Aquila F, Redivo M, Pittorino L, Mattaroccia G, Tempestini F, Santucci S, Casenghi M, Giovannelli F, Rigattieri S, et al. Comprehensive Risk Assessment of LAD Disease Progression in CCTA: The CLAP Score Study. Journal of Cardiovascular Development and Disease. 2024; 11(11):338. https://doi.org/10.3390/jcdd11110338
Chicago/Turabian StyleTommasino, Antonella, Federico Dell’Aquila, Marco Redivo, Luca Pittorino, Giulia Mattaroccia, Federica Tempestini, Stefano Santucci, Matteo Casenghi, Francesca Giovannelli, Stefano Rigattieri, and et al. 2024. "Comprehensive Risk Assessment of LAD Disease Progression in CCTA: The CLAP Score Study" Journal of Cardiovascular Development and Disease 11, no. 11: 338. https://doi.org/10.3390/jcdd11110338
APA StyleTommasino, A., Dell’Aquila, F., Redivo, M., Pittorino, L., Mattaroccia, G., Tempestini, F., Santucci, S., Casenghi, M., Giovannelli, F., Rigattieri, S., Berni, A., & Barbato, E. (2024). Comprehensive Risk Assessment of LAD Disease Progression in CCTA: The CLAP Score Study. Journal of Cardiovascular Development and Disease, 11(11), 338. https://doi.org/10.3390/jcdd11110338






