The Role of Advanced Glycation End-Product Levels Measured by Skin Autofluorescence in the Development of Mitral Annular Calcification
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGE | Advanced glycation end-product. |
| AF | Atrial fibrillation. |
| AML | Anterior mitral leaflet. |
| AUC | Area under the curve. |
| BMI | Body mass index. |
| CAD | Coronary artery disease. |
| CI | Confidence interval. |
| COPD | Chronic obstructive pulmonary disease. |
| CRI | Chronic renal insufficiency. |
| CRP | C-reactive protein. |
| DM | Diabetes Mellitus. |
| EF | Ejection fraction. |
| HF | Heart failure. |
| HT | Hypertension. |
| IL-6 | Interleukin 6. |
| IQR | Inter quartile range. |
| LA | Left atrium. |
| LV | Left ventricle. |
| MAC | Mitral annular calcification. |
| MR | Mitral regurgitation. |
| MS | Mitral stenosis. |
| MV | Mitral valve. |
| MVA | Mitral valve annulus. |
| OR | Odds ratio. |
| PML | Posterior mitral leaflet. |
| ROC | Receiver operating characteristics. |
| RAGE | Receptor of AGE. |
References
- Fox, C.S.; Vasan, R.S.; Parise, H.; Levy, D.; O’Donnell, C.J.; D’Agostino, R.B.; Benjamin, E.J. Mitral annular calcification predicts cardiovascular morbidity and mortality: The Framingham Heart Study. Circulation 2003, 107, 1492–1496. [Google Scholar] [CrossRef]
- Eleid, M.F.; Foley, T.A.; Said, S.M.; Pislaru, S.V.; Rihal, C.S. Severe mitral annular calcification: Multimodality imaging for therapeutic strategies and interventions. JACC Cardiovasc. Imaging 2016, 9, 1318–1337. [Google Scholar] [CrossRef]
- Massera, D.; Kizer, J.R.; Dweck, M.R. Mechanisms of mitral annular calcification. Trends Cardiovasc. Med. 2020, 30, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.F.; Pellerin, M.; Fuzellier, J.F.; Relland, J.Y. Extensive calcification of the mitral valve anulus: Pathology and surgical management. J. Thorac. Cardiovasc. Surg. 1996, 111, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Okura, H.; Nakada, Y.; Nogi, M.; Ishihara, S.; Okamura, A.; Okayama, S.; Saito, Y. Prevalence of mitral annular calcification and its association with mitral valvular disease. Echocardiography 2021, 38, 1907–1912. [Google Scholar] [CrossRef] [PubMed]
- Adler, Y.; Fisman, E.Z.; Shemesh, J.; Tanne, D.; Hovav, B.; Motro, M.; Tenenbaum, A. Usefulness of helical computed tomography in detection of mitral annular calcification as a marker of coronary artery disease. Int. J. Cardiol. 2005, 101, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Arimura, T.; Shiga, Y.; Kuwano, T.; Sugihara, M.; Miura, S.I. Association between mitral annulus calcification and subtypes of heart failure rehospitalization. Cardiol. J. 2021, 30, 256–265. [Google Scholar] [CrossRef]
- Elmariah, S.; Budoff, M.J.; Delaney, J.A.; Hamirani, Y.; Eng, J.; Fuster, V.; O’Brien, K.D. Risk factors associated with the incidence and progression of mitral annulus calcification: The multi-ethnic study of atherosclerosis. Am. Heart J. 2013, 166, 904–912. [Google Scholar] [CrossRef]
- Willens, H.J.; Chirinos, J.A.; Schob, A.; Veerani, A.; Perez, A.J.; Chakko, S. The relation between mitral annular calcification and mortality in patients undergoing diagnostic coronary angiography. Echocardiography 2006, 23, 717–722. [Google Scholar] [CrossRef]
- Bedeir, K.; Kaneko, T.; Aranki, S. Current and evolving strategies in the management of severe mitral annular calcification. J. Thorac. Cardiovasc. Surg. 2019, 157, 555–566. [Google Scholar] [CrossRef]
- Abramowitz, Y.; Jilaihawi, H.; Chakravarty, T.; Mack, M.J.; Makkar, R.R. Mitral annulus calcification. J. Am. Coll. Cardiol. 2015, 66, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, J.; Ho, C.T.; Li, S. Management of Maillard reaction-derived reactive carbonyl species and advanced glycation end products by tea and tea polyphenols. Food Sci. Hum. Wellness 2022, 11, 557–567. [Google Scholar] [CrossRef]
- Reddy, V.P.; Aryal, P.; Darkwah, E.K. Advanced glycation end products in health and disease. Microorganisms 2022, 10, 1848. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Beyaz, A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov. Today 2006, 11, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Amornrit, W.; Santiyanont, R. Effect of Amaranthus on advanced glycation end-products induced cytotoxicity and proinflammatory cytokine gene expression in SH-SY5Y cells. Molecules 2015, 20, 17288–17308. [Google Scholar] [CrossRef]
- Shen, C.Y.; Wu, C.H.; Lu, C.H.; Kuo, Y.M.; Li, K.J.; Hsieh, S.C.; Yu, C.L. Advanced glycation end products of bovine serum albumin suppressed Th1/Th2 cytokine but enhanced monocyte IL-6 gene expression via MAPK-ERK and MyD88 transduced NF-κB p50 signaling pathways. Molecules 2019, 24, 2461. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef]
- Hegab, Z.; Gibbons, S.; Neyses, L.; Mamas, M.A. Role of advanced glycation end products in cardiovascular disease. World J. Cardiol. 2012, 4, 90. [Google Scholar] [CrossRef]
- Arsov, S.; Graaff, R.; van Oeveren, W.; Stegmayr, B.; Sikole, A.; Rakhorst, G.; Smit, A.J. Advanced glycation end-products and skin autofluorescence in end-stage renal disease: A review. Clin. Chem. Lab. Med. 2014, 52, 11–20. [Google Scholar] [CrossRef]
- Lee, J.; Yun, J.S.; Ko, S.H. Advanced glycation end products and their effect on vascular complications in type 2 Diabetes mellitus. Nutrients 2022, 14, 3086. [Google Scholar] [CrossRef]
- Takata, T.; Sakasai-Sakai, A.; Ueda, T.; Takeuchi, M. Intracellular toxic advanced glycation end-products in cardiomyocytes may cause cardiovascular disease. Sci. Rep. 2019, 9, 2121. [Google Scholar] [CrossRef] [PubMed]
- Egaña-Gorroño, L.; López-Díez, R.; Yepuri, G.; Ramirez, L.S.; Reverdatto, S.; Gugger, P.F.; Schmidt, A.M. Receptor for advanced glycation end products (RAGE) and mechanisms and therapeutic opportunities in diabetes and cardiovascular disease: Insights from human subjects and animal models. Front. Cardiovasc. Med. 2020, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; Cardelo, M.P.; de la Cruz, S.; Alcala-Diaz, J.F.; Roncero-Ramos, I.; Guler, I.; Lopez-Miranda, J. Reduction in circulating advanced glycation end products by mediterranean diet is associated with increased likelihood of type 2 diabetes remission in patients with coronary heart disease: From the cordioprev study. Mol. Nutr. Food Res. 2021, 65, 1901290. [Google Scholar] [CrossRef]
- Seo, J.; Jeong, H.; Cho, I.; Hong, G.R.; Ha, J.W.; Shim, C.Y. Sex Differences in Mitral Annular Calcification and the Clinical Implications. Front. Cardiovasc. Med. 2021, 8, 736040. [Google Scholar] [CrossRef]
- Suzuki, D.; Hoshide, S.; Kario, K. AGEs and renal sodium handling: Association with hypertension. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2022, 45, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Rabizadeh, S.; Mirahmad, M.; Hajmiri, M.S.; Nakhjavani, M.; Hemmatabadi, M.; Shirzad, N. The association between advanced glycation end products (AGEs) and ABC (hemoglobin A1C, blood pressure, and low-density lipoprotein cholesterol) control parameters among patients with type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2022, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, L.R.P.; Sá, M.P.B.O.; Perazzo, Á.M.; Escorel Neto, A.C.; Gomes, R.A.F.; Weymann, A.; Zhigalov, K.; Ruhparwar, A.; Lima, R.C. Mitral Annular Calcification: Association with Atherosclerosis and Clinical Implications. Curr. Atheroscler. Rep. 2020, 22, 9. [Google Scholar] [CrossRef]
- Sanchis, P.; Rivera, R.; Fortuny, R.; Río, C.; Mas-Gelabert, M.; Gonzalez-Freire, M.; Grases, F.; Masmiquel, L. Role of Advanced Glycation End Products on Aortic Calcification in Patients with Type 2 Diabetes Mellitus. J. Clin. Med. 2020, 9, 1751. [Google Scholar] [CrossRef]
- Deng, G.; Zhang, L.; Wang, C.; Wang, S.; Xu, J.; Dong, J.; Kang, Q.; Zhai, X.; Zhao, Y.; Shan, Z. AGEs-RAGE axis causes endothelial-to-mesenchymal transition in early calcific aortic valve disease via TGF-β1 and BMPR2 signaling. Exp. Gerontol. 2020, 141, 111088. [Google Scholar] [CrossRef]
- Chen, H.Y.; Engert, J.C.; Thanassoulis, G. Risk factors for valvular calcification. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 96–102. [Google Scholar] [CrossRef]
- Movahed, M.R.; Saito, Y.; Ahmadi-Kashani, M.; Ebrahimi, R. Mitral annulus calcification is associated with valvular and cardiac structural abnormalities. Cardiovasc. Ultrasound 2007, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Parise, H.; Vasan, R.S.; Levy, D.; O’Donnell, C.J.; D’Agostino, R.B.; Plehn, J.F.; Benjamin, E.J. Mitral annular calcification is a predictor for incident atrial fibrillation. Atherosclerosis 2004, 173, 291–294. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, W.T.; Efird, J.T.; Nazarian, S.; Alonso, A.; Heckbert, S.R.; Soliman, E.Z. Mitral annular calcification and incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis. Europace 2015, 17, 358–363. [Google Scholar] [CrossRef]
- Kass, D.A.; Shapiro, E.P.; Kawaguchi, M.; Capriotti, A.R.; Scuteri, A.; deGroof, R.C.; Lakatta, E.G. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation 2012, 104, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Little, W.C.; Zile, M.R.; Kitzman, D.W.; Hundley, W.G.; O’Brien, T.X.; Degroof, R.C. The effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failure. J. Card. Fail. 2005, 11, 191–195. [Google Scholar] [CrossRef]
- Zieman, S.J.; Melenovsky, V.; Clattenburg, L.; Corretti, M.C.; Capriotti, A.; Gerstenblith, G.; Kass, D.A. Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. J. Hypertens. 2007, 25, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, S.; Hartog, J.W.; Hummel, Y.M.; Posma, J.L.; van Wijk, L.M.; van Veldhuisen, D.J.; Voors, A.A. Effects of alagebrium, an advanced glycation end-product breaker, in patients with chronic heart failure: Study design and baseline characteristics of the BENEFICIAL trial. Eur. J. Heart Fail. 2010, 12, 294–300. [Google Scholar] [CrossRef]
- Oudegeest-Sander, M.H.; Rikkert, M.G.O.; Smits, P.; Thijssen, D.H.; van Dijk, A.P.; Levine, B.D.; Hopman, M.T. The effect of an advanced glycation end-product crosslink breaker and exercise training on vascular function in older individuals: A randomized factorial design trial. Exp. Gerontol. 2013, 48, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Hastings, J.L.; Carrick-Ranson, G.; Shafer, K.M.; Shibata, S.; Bhella, P.S.; Levine, B.D. Cardiovascular effects of 1 year of alagebrium and endurance exercise training in healthy older individuals. Circ. Heart Fail. 2013, 6, 1155–1164. [Google Scholar] [CrossRef]
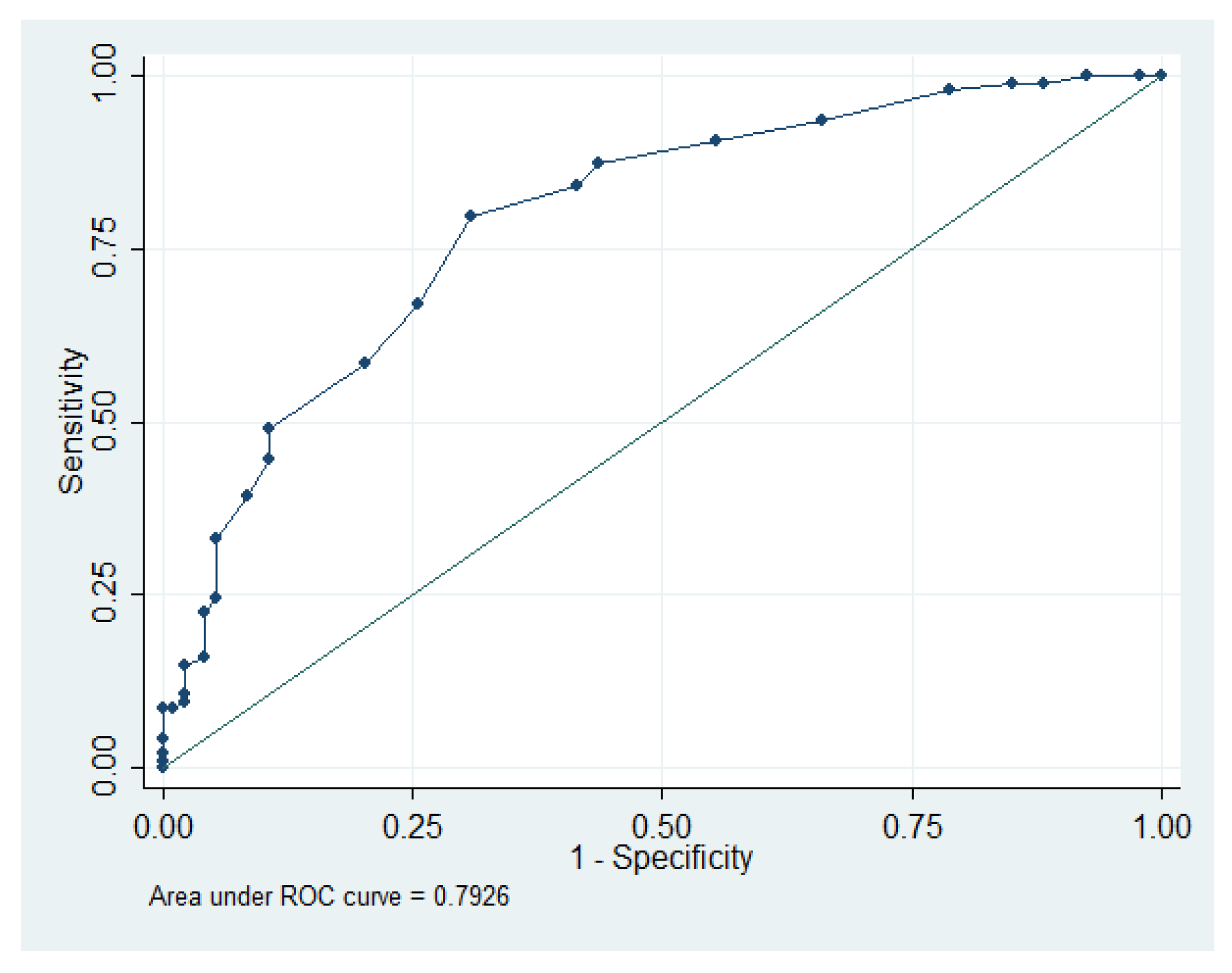
|
| Variable | MAC-Positive | MAC-Negative | p Value |
|---|---|---|---|
| Number of patients | 94 | 94 | |
| Gender (female) | 55 (58.5%) | 40 (42.6%) | 0.02 |
| DM | 46 (48.9%) | 47 (50%) | 0.5 |
| Hypertension | 78 (83%) | 67 (71.3%) | 0.041 |
| Hyperlipidemia | 57 (60.6%) | 59 (62.8%) | 0.4 |
| Smoking | 33 (35.1%) | 35 (37.2%) | 0.4 |
| Coronary artery disease | 54 (57.4%) | 52 (55.3%) | 0.4 |
| Stroke | 11 (11.7%) | 3 (3.2%) | 0.02 |
| COPD | 21 (22.3%) | 9 (9.6%) | 0.01 |
| Atrial fibrillation | 14 (14.9%) | 8 (8.5%) | 0.1 |
| MR > 1 degree | 29 (30.9%) | 9 (9.6%) | <0.001 |
| Age | 72.04 ± 7.26 | 70.04 ± 6.22 | 0.07 |
| BMI | 29.27 (26.55–31.97) | 28.9 (27.6–32.8) | 0.4 |
| DM year | 10 (7–15) | 10 (6–15) | 0.8 |
| HbA1c | 7.35 (6.7–8.21) | 7 (6.7–7.3) | 0.03 |
| AGEs | 3.2 (2.8–3.7) | 2.7 (2.4–2.9) | <0.001 |
| Ejection fraction | 55 (48.75–60) | 55 (50–60) | 0.18 |
| Left atrium diameter (cm) | 4.15 (3.87–4.5) | 3.8 (3.5–4.1) | <0.001 |
| Triglyceride | 120 (98–155 IQR) | 113 (93–142 IQR) | 0.1 |
| Low-density lipoprotein | 116.6 (89–133) | 94.5 (68–114.25) | 0.01 |
| Creatin | 0.95 (0.78–1.12) | 0.90 (0.78–1.04) | 0.4 |
| Univariate Regression Analysis | Multivariate Regression Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p Value | OR | 95% CI | p Value |
| DM year | 1.19 | 0.93–1.04 | 0.6 | |||
| Age | 1.03 | 0.99–1.07 | 0.074 | 0.99 | 0.88–1.11 | 0.8 |
| Gender (Female) | 1.90 | 1.06–3.39 | 0.029 | 2.22 | 0.64–7.61 | 0.2 |
| DM | 0.95 | 0.54–1.69 | 0.8 | - | - | - |
| BMI | 1.03 | 0.96–1.11 | 0.2 | - | - | - |
| Hypertension | 1.96 | 0.97–3.95 | 0.058 | 0.28 | 0.03–2.06 | 0.2 |
| Hyperlipidemia | 0.91 | 0.50–1.64 | 0.7 | - | - | - |
| Smoking | 0.91 | 0.50–1.65 | 0.7 | - | - | - |
| CAD | 1.09 | 0.61–1.94 | 0.7 | - | - | - |
| Stroke | 4.02 | 1.08–14.91 | 0.03 | 5.55 | 1.27–24.25 | 0.023 |
| COPD | 2.71 | 1.17–6.30 | 0.02 | 7.00 | 0.89–54.90 | 0.064 |
| AF | 1.88 | 0.74–4.72 | 0.178 | - | - | - |
| HbA1c | 1.89 | 1.13–3.15 | 0.01 | 1.40 | 0.63–3.13 | 0.4 |
| AGEs | 9.00 | 4.25–19.05 | <0.001 | 8.05 | 3.74–17.33 | <0.001 |
| EF | 0.96 | 0.93–1.00 | 0.107 | - | - | - |
| LA | 5.07 | 2.25–11.42 | <0.001 | 3.76 | 1.47–9.64 | 0.006 |
| MR > 1 degree | 4.21 | 1.86–9.51 | 0.001 | 4.07 | 0.95–17.47 | 0.059 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyraz, B.; Peker, T. The Role of Advanced Glycation End-Product Levels Measured by Skin Autofluorescence in the Development of Mitral Annular Calcification. J. Cardiovasc. Dev. Dis. 2023, 10, 406. https://doi.org/10.3390/jcdd10090406
Boyraz B, Peker T. The Role of Advanced Glycation End-Product Levels Measured by Skin Autofluorescence in the Development of Mitral Annular Calcification. Journal of Cardiovascular Development and Disease. 2023; 10(9):406. https://doi.org/10.3390/jcdd10090406
Chicago/Turabian StyleBoyraz, Bedrettin, and Tezcan Peker. 2023. "The Role of Advanced Glycation End-Product Levels Measured by Skin Autofluorescence in the Development of Mitral Annular Calcification" Journal of Cardiovascular Development and Disease 10, no. 9: 406. https://doi.org/10.3390/jcdd10090406
APA StyleBoyraz, B., & Peker, T. (2023). The Role of Advanced Glycation End-Product Levels Measured by Skin Autofluorescence in the Development of Mitral Annular Calcification. Journal of Cardiovascular Development and Disease, 10(9), 406. https://doi.org/10.3390/jcdd10090406





