“Troponinosis”, the Cardiologist’s Curse—When Clinic–Laboratory Interaction Unveils the Mystery: A Case Report
Abstract
:1. Introduction
2. Detailed Case Description
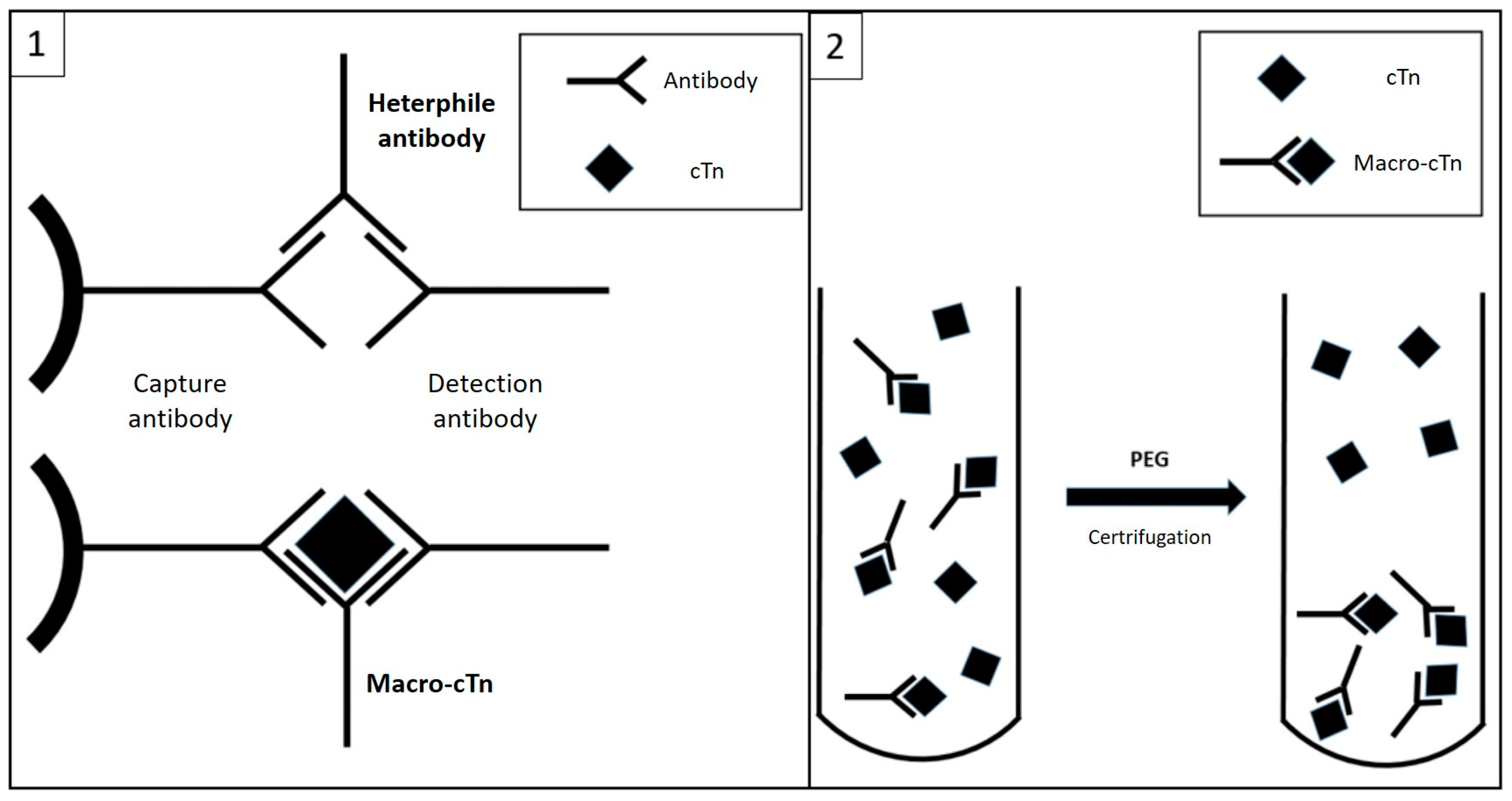
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apple, F.S.; Sandoval, Y.; Jaffe, A.S.; Ordonez-Llanos, J.; IFCC Task Force on Clinical Applications of Cardiac Bio-Markers. Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care. Clin. Chem. 2017, 63, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 76, 1383–1415. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.P.; Raber, I.; Chapman, A.R.; Sandoval, Y.; Apple, F.S.; Mills, N.L.; Januzzi, J.L., Jr. Myocardial Injury in the Era of High-Sensitivity Cardiac Troponin Assays: A Practical Approach for Clinicians. JAMA Cardiol. 2019, 4, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Mair, J.; Lindahl, B.; Müller, C.; Giannitsis, E.; Huber, K.; Möckel, M.; Plebani, M.; Thygesen, K.; Jaffe, A.S. What to do when you question cardiac troponin values. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Saragò, M.; Fiore, D.; De Rosa, S.; Amaddeo, A.; Pulitanò, L.; Bozzarello, C.; Iannello, A.M.; Sammarco, G.; Indolfi, C.; Rizzuto, A. Acute acalculous cholecystitis and cardiovascular disease, which came first? After two hundred years still the classic chicken and eggs debate: A review of literature. Ann. Med. Surg. 2022, 78, 103668. [Google Scholar] [CrossRef] [PubMed]
- Wauthier, L.; Plebani, M.; Favresse, J. Interferences in immunoassays: Review and practical algorithm. Clin. Chem. Lab. Med. 2022, 60, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Christenson, R.H.; Jacobs, E.; Uettwiller-Geiger, D.; Estey, M.P.; Lewandrowski, K.; Koshy, T.I.; Kupfer, K.; Li, Y.; Wesenberg, J.C. Comparison of 13 Commercially Available Cardiac Troponin Assays in a Multicenter North American Study. J. Appl. Lab. Med. 2017, 2, 134. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Montagnana, M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann. Transl. Med. 2016, 4, 194. [Google Scholar] [CrossRef] [PubMed]
- Lakusic, N.; Merkas, I.S.; Lucinger, D.; Mahovic, D. Heterophile antibodies, false-positive troponin, and acute coronary syndrome: A case report indicating a pitfall in clinical practice. Eur. Heart J.-Case Rep. 2021, 5, ytab018. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.M.; O’Byrne, L.; Finn, J.; Grimes, H.; Daly, K.M. False-positive cardiac troponin I in a routine clinical population. Am. J. Cardiol. 2002, 89, 1212–1215. [Google Scholar] [CrossRef]
- Collinson, P. Macrotroponin—Analytical Anomaly or Clinical Confounder. Clin. Chem. 2022, 68, 1229–1231. [Google Scholar] [CrossRef]
- O’Donohoe, T.J.; Ketheesan, N.; Schrale, R.G. Anti-troponin antibodies following myocardial infarction. J. Cardiol. 2017, 69, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, Z.; Dargan, J.; Gaze, D.; Firoozi, S.; Collinson, P.; Shanmugam, N. False-positive troponin elevation due to an immunoglobulin-G-cardiac troponin T complex: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.V.; Marshall, G.A. High incidence of macrotroponin I with a high-sensitivity troponin I assay. Clin. Chem. Lab. Med. 2016, 54, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.; Tse, R.; Gladding, P.; Kyle, C. Effect of macrotroponin in a cohort of community patients with elevated cardiac troponin. Clin. Chem. 2022, 68, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Nevraumont, A.; Deltombe, M.; Favresse, J.; Guillaume, L.; Chapelle, V.; Twerenbold, R.; Gruson, D. Interferences with cardiac biomarker assays: Understanding the clinical impact. Eur. Heart J. 2022, 43, 2286–2288. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Bayart, J.L.; Gruson, D.; Bernardini, S.; Clerico, A.; Perrone, M. The underestimated issue of non-reproducible cardiac troponin I and T results: Case series and systematic review of the literature. Clin. Chem. Lab. Med. 2021, 59, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.; Hammarsten, O.; Kyle, C. Which method to detect macrotroponin? Clin. Chem. Lab. Med. 2022, 60, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.; Aspin, L.; Heron, R.C.; Ha, L.; Kyle, C. Discrepancy between Cardiac Troponin Assays Due to Endogenous Antibodies. Clin. Chem. 2020, 66, 445–454. [Google Scholar] [CrossRef] [PubMed]


| Clinical Event | Laboratory Test | |
|---|---|---|
| Week 0 | Inferior STEMI | Hs-TnI 15,993 ng/L (peak) CK-MB 314 ng/mL (peak) |
| Week 4 | Chest pain-emergency department | Hs-TnI 727 ng/L |
| Week 6 | Chest pain-emergency department | Hs-TnI 854 – 868 ng/L CK-MB 1.37 ng/mL |
| Week 7 | Physical examination-asymptomatic | Hs-TnI 886 ng/L |
| Week 10 | Cholangitis | Hs-TnI 1261 ng/L CK-MB 1.66 ng/mL |
| Week 12 | AMI type 2-suspected vasospatic angina | Hs-TnI 1316 ng/L CK-MB 3.11 ng/mL |
| Week 28 | Physical examination-asymptomatic | Hs-TnI 723 ng/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosi, D.; Canovi, S.; Pennacchioni, A.; Demola, P.; Corradini, M.; Guiducci, V.; Colla, R.; Navazio, A. “Troponinosis”, the Cardiologist’s Curse—When Clinic–Laboratory Interaction Unveils the Mystery: A Case Report. J. Cardiovasc. Dev. Dis. 2023, 10, 378. https://doi.org/10.3390/jcdd10090378
Bosi D, Canovi S, Pennacchioni A, Demola P, Corradini M, Guiducci V, Colla R, Navazio A. “Troponinosis”, the Cardiologist’s Curse—When Clinic–Laboratory Interaction Unveils the Mystery: A Case Report. Journal of Cardiovascular Development and Disease. 2023; 10(9):378. https://doi.org/10.3390/jcdd10090378
Chicago/Turabian StyleBosi, Davide, Simone Canovi, Andrea Pennacchioni, Pierluigi Demola, Mattia Corradini, Vincenzo Guiducci, Rossana Colla, and Alessandro Navazio. 2023. "“Troponinosis”, the Cardiologist’s Curse—When Clinic–Laboratory Interaction Unveils the Mystery: A Case Report" Journal of Cardiovascular Development and Disease 10, no. 9: 378. https://doi.org/10.3390/jcdd10090378
APA StyleBosi, D., Canovi, S., Pennacchioni, A., Demola, P., Corradini, M., Guiducci, V., Colla, R., & Navazio, A. (2023). “Troponinosis”, the Cardiologist’s Curse—When Clinic–Laboratory Interaction Unveils the Mystery: A Case Report. Journal of Cardiovascular Development and Disease, 10(9), 378. https://doi.org/10.3390/jcdd10090378





