Long-Term Prognosis of Different Subtypes of Left Ventricular Noncompaction Cardiomyopathy Patients: A Retrospective Study in China
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Variables
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Primary Outcomes
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef]
- Towbin, J.A.; Lorts, A.; Jefferies, J.L. Left ventricular non-compaction cardiomyopathy. Lancet 2015, 386, 813–825. [Google Scholar] [CrossRef]
- Arbustini, E.; Weidemann, F.; Hall, J.L. Left ventricular noncompaction: A distinct cardiomyopathy or a trait shared by different cardiac diseases? J. Am. Coll. Cardiol. 2014, 64, 1840–1850. [Google Scholar] [CrossRef]
- Stanton, C.; Bruce, C.; Connolly, H.; Brady, P.; Syed, I.; Hodge, D.; Asirvatham, S.; Friedman, P. Isolated left ventricular noncompaction syndrome. Am. J. Cardiol. 2009, 104, 1135–1138. [Google Scholar] [CrossRef]
- Aras, D.; Tufekcioglu, O.; Ergun, K.; Ozeke, O.; Yildiz, A.; Topaloglu, S.; Deveci, B.; Sahin, O.; Kisacik, H.L.; Korkmaz, S. Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. J. Card. Fail. 2006, 12, 726–733. [Google Scholar] [CrossRef]
- Henderson, D.J.; Anderson, R.H. The development and structure of the ventricles in the human heart. Pediatr. Cardiol. 2009, 30, 588–596. [Google Scholar] [CrossRef]
- Wu, M. Mechanisms of Trabecular Formation and Specification During Cardiogenesis. Pediatr. Cardiol. 2018, 39, 1082–1089. [Google Scholar] [CrossRef]
- Gati, S.; Chandra, N.; Bennett, R.L.; Reed, M.; Kervio, G.; Panoulas, V.F.; Ghani, S.; Sheikh, N.; Zaidi, A.; Wilson, M.; et al. Increased left ventricular trabeculation in highly trained athletes: Do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart 2013, 99, 401–408. [Google Scholar] [CrossRef]
- Ross, S.B.; Jones, K.; Blanch, B.; Puranik, R.; McGeechan, K.; Barratt, A.; Semsarian, C. A systematic review and meta-analysis of the prevalence of left ventricular non-compaction in adults. Eur. Heart J. 2020, 41, 1428–1436. [Google Scholar] [CrossRef]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef]
- Jenni, R.; Oechslin, E.; Schneider, J.; Attenhofer Jost, C.; Kaufmann, P.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef]
- Stöllberger, C.; Gerecke, B.; Finsterer, J.; Engberding, R. Refinement of echocardiographic criteria for left ventricular noncompaction. Int. J. Cardiol. 2013, 165, 463–467. [Google Scholar] [CrossRef]
- Jacquier, A.; Thuny, F.; Jop, B.; Giorgi, R.; Cohen, F.; Gaubert, J.Y.; Vidal, V.; Bartoli, J.M.; Habib, G.; Moulin, G. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur. Heart J. 2010, 31, 1098–1104. [Google Scholar] [CrossRef]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef]
- Vaidya, V.R.; Lyle, M.; Miranda, W.R.; Farwati, M.; Isath, A.; Patlolla, S.H.; Hodge, D.O.; Asirvatham, S.J.; Kapa, S.; Deshmukh, A.J.; et al. Long-Term Survival of Patients with Left Ventricular Noncompaction. J. Am. Heart Assoc. 2021, 10, e015563. [Google Scholar] [CrossRef]
- Casas, G.; Limeres, J.; Oristrell, G.; Gutierrez-Garcia, L.; Andreini, D.; Borregan, M.; Larrañaga-Moreira, J.M.; Lopez-Sainz, A.; Codina-Solà, M.; Teixido-Tura, G.; et al. Clinical Risk Prediction in Patients with Left Ventricular Myocardial Noncompaction. J. Am. Coll. Cardiol. 2021, 78, 643–662. [Google Scholar] [CrossRef]
- Yang, Z.G.; Liu, Z.J.; Zhang, X.X.; Wang, L. Prognostic factors associated with left ventricular non-compaction: A PRISMA-compliant meta-analysis. Medicine 2022, 101, e30337. [Google Scholar] [CrossRef]
- Stämpfli, S.F.; Donati, T.G.; Hellermann, J.; Anwer, S.; Erhart, L.; Gruner, C.; Kaufmann, B.A.; Gencer, B.; Haager, P.K.; Müller, H.; et al. Right ventricle and outcome in left ventricular non-compaction cardiomyopathy. J. Cardiol. 2020, 75, 20–26. [Google Scholar] [CrossRef]
- Klaassen, S.; Probst, S.; Oechslin, E.; Gerull, B.; Krings, G.; Schuler, P.; Greutmann, M.; Hürlimann, D.; Yegitbasi, M.; Pons, L.; et al. Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation 2008, 117, 2893–2901. [Google Scholar] [CrossRef]
- Sedaghat-Hamedani, F.; Haas, J.; Zhu, F.; Geier, C.; Kayvanpour, E.; Liss, M.; Lai, A.; Frese, K.; Pribe-Wolferts, R.; Amr, A.; et al. Clinical genetics and outcome of left ventricular non-compaction cardiomyopathy. Eur. Heart J. 2017, 38, 3449–3460. [Google Scholar] [CrossRef]
- Rojanasopondist, P.; Nesheiwat, L.; Piombo, S.; Porter, G.A., Jr.; Ren, M.; Phoon, C.K.L. Genetic Basis of Left Ventricular Noncompaction. Circ. Genom. Precis. Med. 2022, 15, e003517. [Google Scholar] [CrossRef]
- Towbin, J.A.; Beasley, G. Left Ventricular Noncompaction and Vigorous Physical Activity: What Is the Connection? J. Am. Coll. Cardiol. 2020, 76, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, E.; Jenni, R. Left ventricular non-compaction revisited: A distinct phenotype with genetic heterogeneity? Eur. Heart J. 2011, 32, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Pinto, Y.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Böhm, M.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef]
- Cardoso, B.; Jeewa, A.; Minn, S.; Ashkanase, J.; Lynch, A.; Jean-St-Michel, E. Left Ventricular Noncompaction Cardiomyopathy: Left Ventricular Dilation and Dysfunction at Baseline Portend the Risk of Death or Heart Transplantation. Can. J. Cardiol. 2022, 38, 754–762. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Sharma, S.; Drezner, J.A.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International recommendations for electrocardiographic interpretation in athletes. Eur. Heart J. 2018, 39, 1466–1480. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Aung, N.; Doimo, S.; Ricci, F.; Sanghvi, M.M.; Pedrosa, C.; Woodbridge, S.P.; Al-Balah, A.; Zemrak, F.; Khanji, M.Y.; Munroe, P.B.; et al. Prognostic Significance of Left Ventricular Noncompaction: Systematic Review and Meta-Analysis of Observational Studies. Circ. Cardiovasc. Imaging 2020, 13, e009712. [Google Scholar] [CrossRef]
- Gerard, H.; Iline, N.; Martel, H.; Nguyen, K.; Richard, P.; Donal, E.; Eicher, J.C.; Huttin, O.; Selton-Suty, C.; Raud-Raynier, P.; et al. Prognosis of Adults with Isolated Left Ventricular Non-Compaction: Results of a Prospective Multicentric Study. Front. Cardiovasc. Med. 2022, 9, 856160. [Google Scholar] [CrossRef]

| Baseline Characteristics | Dilated LVNC (n = 64) | Isolated LVNC (n = 37) | p Value |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age of diagnosis, years | 51.2 ± 16.4 | 39.4 ± 13.9 | <0.01 |
| Age ≥ 60 y | 22 (34.4) | 4 (10.8) | <0.01 |
| Male | 41 (64.1) | 16 (43.2) | 0.04 |
| Body mass index, kg/m2 | 22.8 ± 3.5 | 23.9 ± 3.5 | 0.19 |
| Diagnosis of HF | 41 (64.1) | 18 (48.6) | 0.13 |
| NYHA cardiac function class | |||
| I or II | 33 (51.6) | 33 (89.2) | <0.001 |
| III or IV | 31 (48.4) | 4 (10.8) | |
| Hypertension | 16 (25.0) | 13 (35.1) | 0.28 |
| Diabetes mellitus | 8 (12.5) | 4 (10.8) | >0.99 |
| Dyslipidemia | 25 (39.1) | 6 (16.2) | 0.02 |
| Coronary heart disease | 8 (12.5) | 1 (2.7) | 0.15 |
| Cigarette consumption | 29 (45.3) | 11 (29.7) | 0.12 |
| Alcohol intake | 29 (45.3) | 12 (32.4) | 0.20 |
| Electrocardiography features | |||
| Abnormal ECG | 48 (75.0) | 12 (32.4) | <0.001 |
| LBBB | 14 (21.9) | 2 (5.4) | 0.03 |
| AVB | 2 (3.1) | 1 (2.7) | >0.99 |
| Atrial fibrillation | 9 (14.1) | 2 (5.4) | 0.18 |
| Atrial flutter | 1 (1.6) | 0 (0.0) | >0.99 |
| Supraventricular tachycardia | 3 (4.7) | 3 (8.1) | 0.67 |
| Ventricular tachycardia | 11 (17.2) | 1 (2.7) | 0.05 |
| Premature ventricular contraction | 12 (18.8) | 4 (10.8) | 0.29 |
| Bradycardia | 1 (1.6) | 1 (2.7) | >0.99 |
| ST-T changes | 10 (15.6) | 3 (8.1) | 0.36 |
| Echocardiography features | |||
| LVEDD, mm | 69.0 (62.0–77.0) | 53.0 (48.5–56.0) | <0.001 |
| LVESD, mm | 61.0 (52.0–67.0) | 35.0 (31.0–41.5) | <0.001 |
| LVEF, % | 27.0 (22.0–34.0) | 58.0 (50.0–66.0) | <0.001 |
| IVS, mm | 7.0 (6.3–8.8) | 8.0 (7.0–9.0) | 0.10 |
| LVPW, mm | 8.0 (7.0–9.0) | 8.0 (7.0–10.0) | 0.31 |
| LA enlargement | 61 (95.3) | 14 (27.5) | <0.001 |
| MV regurgitation | |||
| None to mild | 37 (57.8) | 36 (97.3) | <0.001 |
| Moderate to severe | 27 (42.2) | 1 (2.7) | |
| Laboratory examinations | |||
| Lipids profile, mmol/L | |||
| Total cholesterol | 3.89 (3.22–4.66) | 4.55 (3.29–4.99) | 0.20 |
| Triglycerides | 1.10 (0.73–1.54) | 1.39 (0.75–2.11) | 0.25 |
| HDL-C | 0.99 (0.78–1.14) | 1.01 (0.82–1.20) | 0.85 |
| LDL-C | 2.43 (1.90–3.34) | 2.43 (1.83–2.80) | 0.46 |
| cTnI elevation § | 19 (36.5) | 4 (16.7) | 0.08 |
| BNP or NT-proBNP elevation ※ | 60 (93.8) | 14 (37.8) | <0.001 |
| Medications, No. (%) | |||
| ACEI/ARB/ARNI | 25 (39.1) | 13 (35.1) | 0.70 |
| Beta blocker | 26 (40.6) | 13 (35.1) | 0.59 |
| MRA | 16 (25.0) | 6 (16.2) | 0.30 |
| Diuretics | 11 (17.2) | 4 (10.8) | 0.39 |
| Antiarrhythmic drugs | 2 (3.1) | 0 (0.0) | 0.53 |
| Antiplatelet drugs | 5 (7.8) | 2 (5.4) | >0.99 |
| Anticoagulant drugs | 7 (10.9) | 2 (5.4) | 0.48 |
| Clinical Outcomes | Overall (n = 88) | Dilated LVNC (n = 54) | Isolated LVNC (n = 34) | Overall Incidence Rate (Events Per 100 Person-Years) |
|---|---|---|---|---|
| Follow-up time, year | 5.24 (1.55–8.62) | 5.11 (1.34–8.34) | 6.79 (2.46–9.66) | - |
| Primary endpoint | ||||
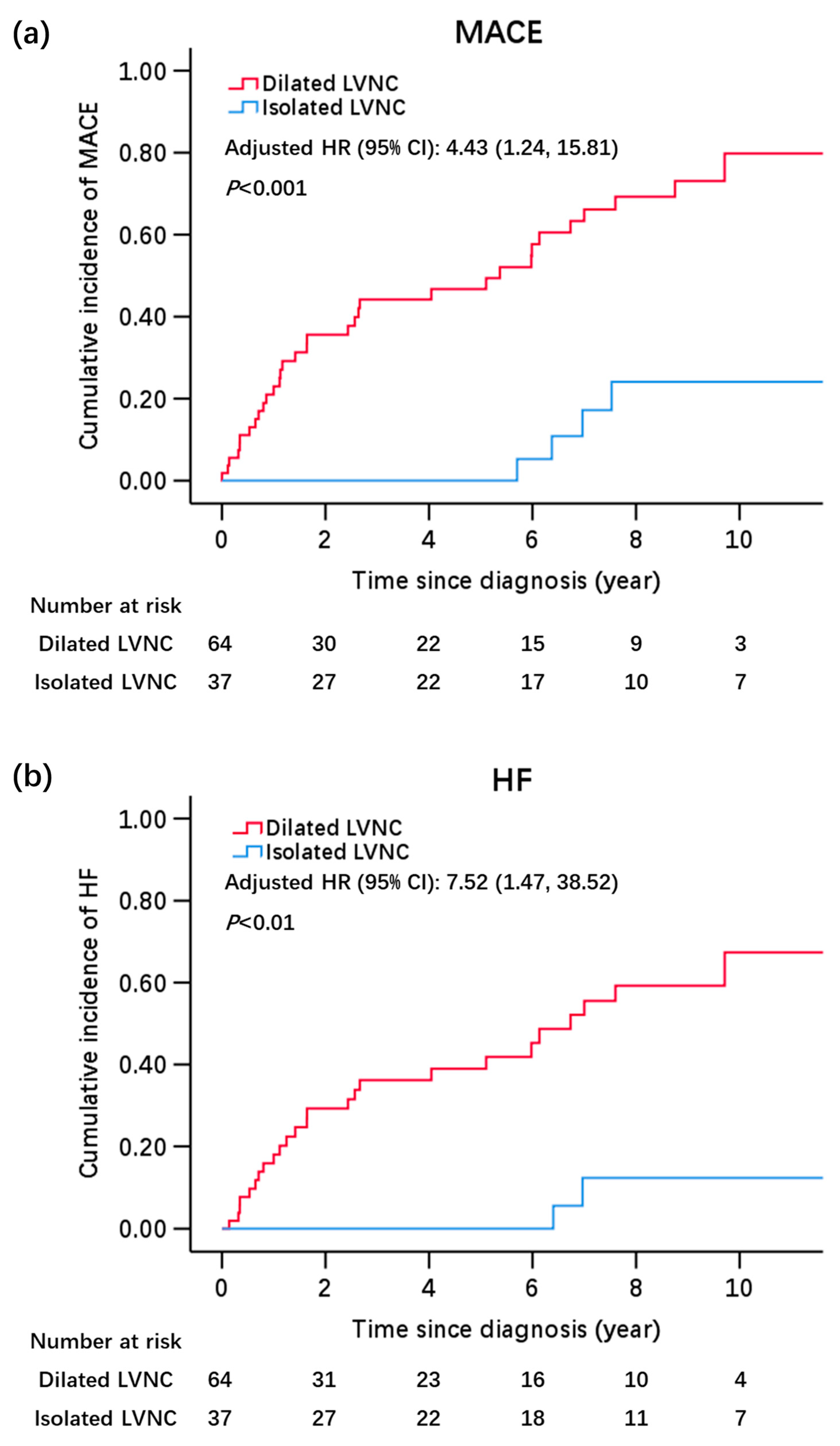
| MACE | 38 (43.2) | 34 (63.0) | 4 (11.8) | 9.20 |
| Cardiovascular mortality | 8 (9.1) | 8 (14.8) | 0 (0.0) | 1.94 |
| HF | 28 (31.8) | 26 (48.1) | 2 (5.9) | 6.78 |
| Rehospitalization for HF | 25 (28.4) | 23 (42.6) | 2 (5.9) | - |
| CRT implantation | 2 (2.3) | 2 (3.7) | 0 (0.0) | - |
| Heart transplantation | 1 (1.1) | 1 (1.9) | 0 (0.0) | - |
| Severe ventricular arrhythmia | 2 (2.3) | 2 (3.7) | 0 (0.0) | 0.48 |
| Ventricular fibrillation | 1 (1.1) | 1 (1.9) | 0 (0.0) | - |
| Appropriate ICD discharge | 1 (1.1) | 1 (1.9) | 0 (0.0) | - |
| Systemic embolism | 9 (10.2) | 6 (11.1) | 3 (8.8) | 2.18 |
| Embolic stroke | 6 (6.8) | 4 (7.4) | 2 (5.9) | - |
| Peripheral artery embolism | 4 (4.5) | 3 (5.6) | 1 (2.9) | - |
| Secondary endpoint | ||||
| All-cause mortality | 10 (11.4) | 9 (16.7) | 1 (2.9) | 2.42 |
| New onset or worsened arrhythmia | 14 (15.9) | 10 (18.5) | 4 (11.8) | 3.39 |
| Ventricular fibrillation | 1 (1.1) | 1 (1.9) | 0 (0.0) | - |
| Appropriate ICD discharge | 1 (1.1) | 1 (1.9) | 0 (0.0) | - |
| Paroxysmal ventricular tachycardia | 1 (1.1) | 1 (1.9) | 0 (0.0) | - |
| ICD implantation | 4 (4.5) | 4 (7.4) | 0 (0.0) | - |
| Pacemaker implantation | 1 (1.1) | 1 (1.9) | 0 (0.0) | - |
| Atrial fibrillation | 5 (5.7) | 3 (5.6) | 2 (5.9) | - |
| Supraventricular tachycardia | 2 (2.3) | 2 (3.7) | 0 (0.0) | - |
| Atrioventricular block | 2 (2.3) | 0 (0.0) | 2 (5.9) | - |
| Outcomes | Isolated LVNC (n = 34) | Dilated LVNC (n = 54) | p Value |
|---|---|---|---|
| MACE | |||
| HR (95% CI), Model I * | 1.0 | 6.00 (2.06, 17.51) | <0.01 |
| HR (95% CI), Model II † | 1.0 | 6.93 (2.29, 21.00) | <0.01 |
| HR (95% CI), Model III § | 1.0 | 4.43 (1.24, 15.81) | 0.02 |
| Heart failure | |||
| HR (95% CI), Model I * | 1.0 | 9.88 (2.28, 42.86) | <0.01 |
| HR (95% CI), Model II † | 1.0 | 12.76 (2.85, 57.13) | <0.01 |
| HR (95% CI), Model III § | 1.0 | 7.52 (1.47, 38.52) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Zhang, S.; Wang, Z.; Wu, M.; Gu, C.; Bai, R.; Liu, M.; Tian, Z.; Zhang, S. Long-Term Prognosis of Different Subtypes of Left Ventricular Noncompaction Cardiomyopathy Patients: A Retrospective Study in China. J. Cardiovasc. Dev. Dis. 2023, 10, 369. https://doi.org/10.3390/jcdd10090369
Gao S, Zhang S, Wang Z, Wu M, Gu C, Bai R, Liu M, Tian Z, Zhang S. Long-Term Prognosis of Different Subtypes of Left Ventricular Noncompaction Cardiomyopathy Patients: A Retrospective Study in China. Journal of Cardiovascular Development and Disease. 2023; 10(9):369. https://doi.org/10.3390/jcdd10090369
Chicago/Turabian StyleGao, Shiqi, Shuyuan Zhang, Zeyuan Wang, Ming Wu, Chengying Gu, Ruilian Bai, Meixi Liu, Zhuang Tian, and Shuyang Zhang. 2023. "Long-Term Prognosis of Different Subtypes of Left Ventricular Noncompaction Cardiomyopathy Patients: A Retrospective Study in China" Journal of Cardiovascular Development and Disease 10, no. 9: 369. https://doi.org/10.3390/jcdd10090369
APA StyleGao, S., Zhang, S., Wang, Z., Wu, M., Gu, C., Bai, R., Liu, M., Tian, Z., & Zhang, S. (2023). Long-Term Prognosis of Different Subtypes of Left Ventricular Noncompaction Cardiomyopathy Patients: A Retrospective Study in China. Journal of Cardiovascular Development and Disease, 10(9), 369. https://doi.org/10.3390/jcdd10090369






