Minimal Access Aortic Valve Surgery
Abstract
1. Introduction
2. Minimal Access Options
2.1. Approach
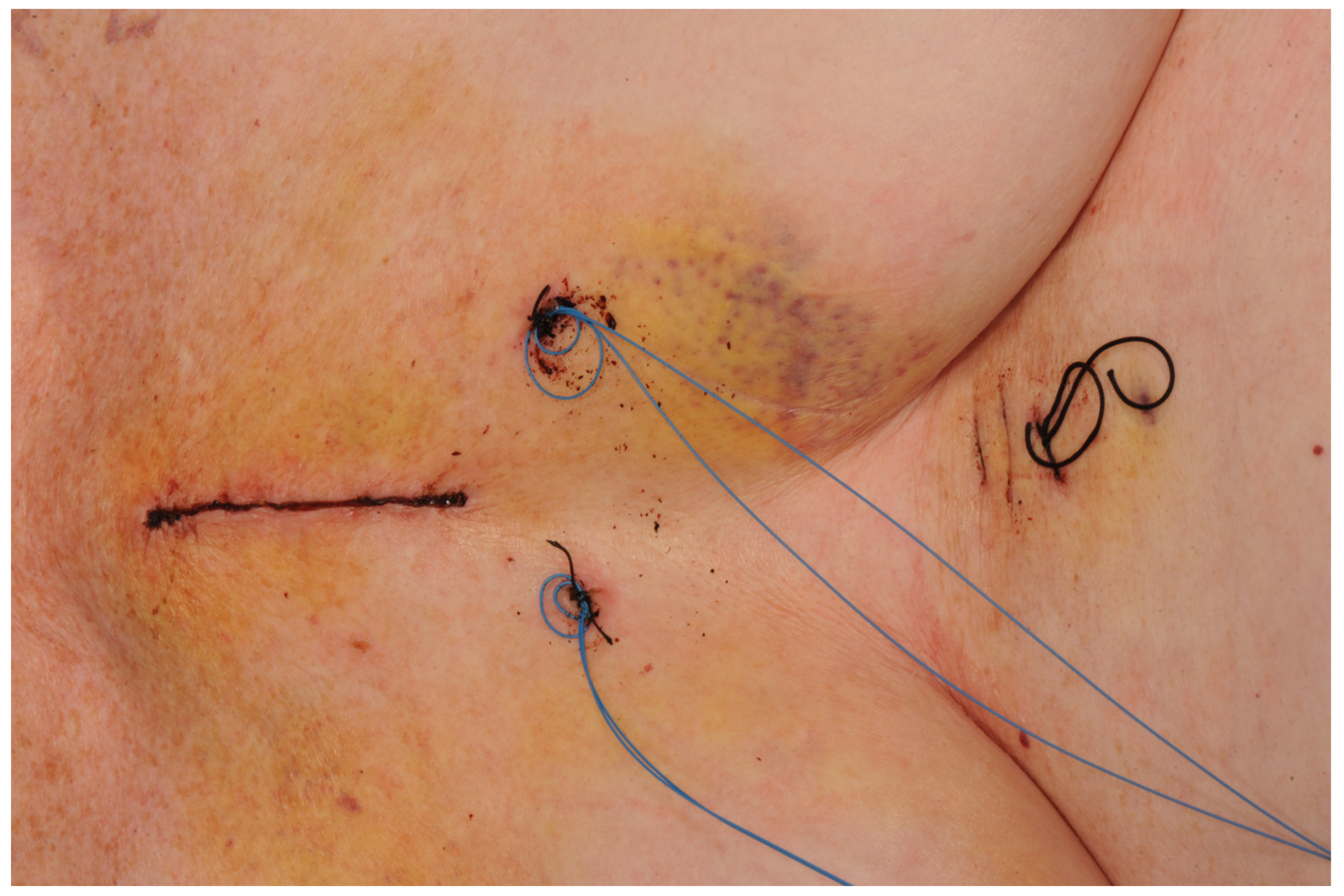
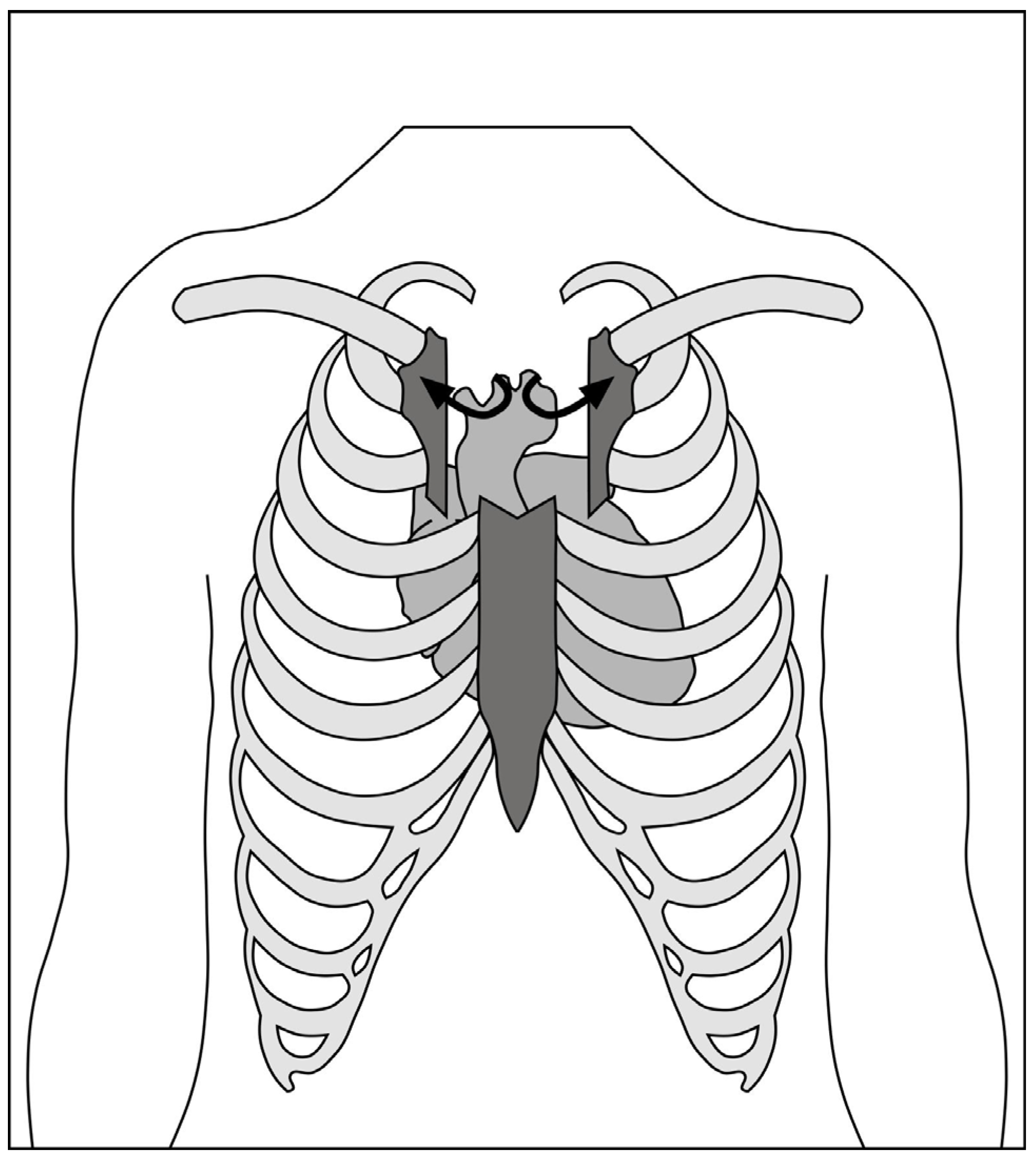
2.1.1. Hemi-Sternotomy
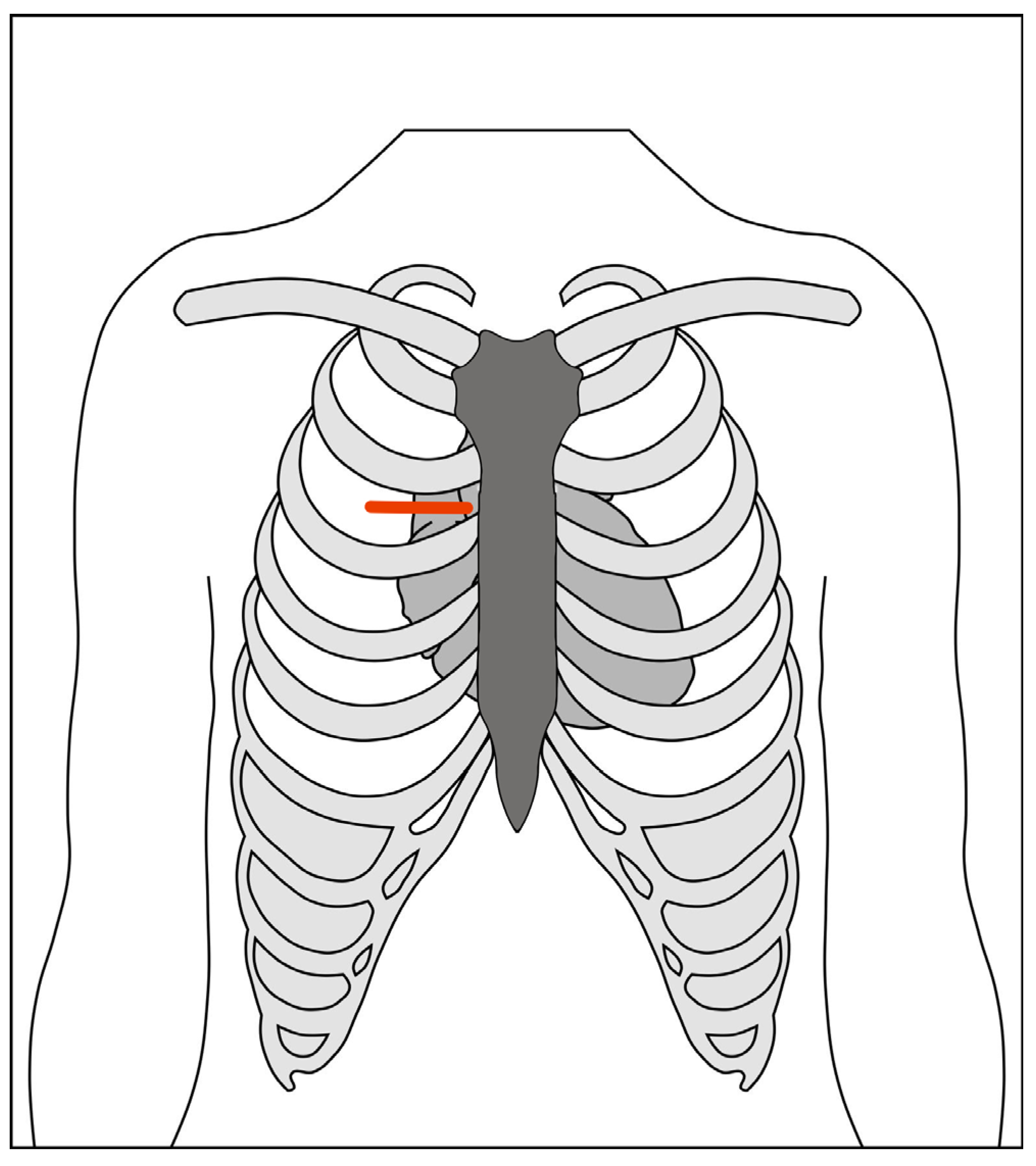
2.1.2. Right Anterior Mini-Thoracotomy
2.1.3. Hybrid Approach (Sternotomy + Transcatheter Aortic Valve Implantation)
2.2. Cannulation and Cardiopulmonary Bypass
2.2.1. Central
2.2.2. Peripheral
2.2.3. Hypothermia and Systemic Hyperkalaemia
2.2.4. Venting (and Imaging)
- The right superior pulmonary vein;
- The pulmonary artery;
- Trans-aortically.
2.3. Adjuncts
2.3.1. Rapid Deployment Valves
2.3.2. Automatic Suture/Knotting Devices
2.3.3. Transvenous Pacing, Cannulation, and Venting
- Transvenous pacing can be floated using an inflatable balloon tip into the right ventricle for endocardial pacing if the anterior right ventricle is not accessible;
- Coronary sinus cannulation via the right internal jugular was previously possible using the Proplege device (Edwards LifeSciences, Irvin, CA, USA). but as the strategies for anterograde cardioplegia alone showed good efficacy, it is no longer available;
- Pulmonary artery venting through a percutaneously floated catheter has again lost favour, as trans-aortic and direct pulmonary artery or pulmonary vein venting have been shown to be efficacious and safe.
2.3.4. Thoracoscopes
2.3.5. Robot Assistance
2.4. Special Circumstances
2.4.1. Concomitant Procedures
2.4.2. Re-Do Procedures
3. Minimal Access Pre-Operative Planning/Setup
- A CT scan pre-operatively can allow for assessment of the position of the aorta relative to the incision planned. If peripheral cannulation is intended, CT can also determine whether the femoral vessels are of an adequate calibre and the descending aorta is free of mobile atheroma that may preclude retrograde perfusion.
- Short-acting anaesthetic drugs should be considered to facilitate early extubation and enhanced recovery.
- A sheath in the right internal jugular vein can be introduced at the time of induction of anaesthesia if the usual access limits epicardial pacing wires.
- A bag of saline behind the shoulder blades can elevate and expand the chest, providing an improved approach to the mediastinum.
- External defibrillator pads are required to cardiovert ventricular fibrillation, as internal paddles cannot be applied to the heart.
- Trans-oesophageal echocardiography is mandated for minimally invasive aortic valve surgery, as the direct visualization of the right and left ventricular function is impaired.
- A double lumen tube or bronchial blocker for selective ventilation of the right lung can facilitate the early learning curve [65].
- Carbon dioxide field flooding can aid in de-airing at the end of the case, when cardiac massage is not possible and venting is limited. Passive and limited active de-airing can therefore be supplemented with displacement of air in the cardiac chambers with highly soluble CO2.
4. Outcomes
4.1. Pain
4.2. Respiratory Mechanics
4.3. Quality of Life
4.4. Complications
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Faculty Opinions recommendation of Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 496161. [Google Scholar] [CrossRef]
- Kumar, R.K.; Tandon, R. Rheumatic fever & rheumatic heart disease: The last 50 years. Indian J. Med. Res. 2013, 137, 643–658. [Google Scholar]
- Manjunath, C.; Srinivas, P.; Ravindranath, K.; Dhanalakshmi, C. Incidence and patterns of valvular heart disease in a tertiary care high-volume cardiac center: A single center experience. Indian Heart J. 2014, 66, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Burwash, I.G.; Legget, M.E.; Munt, B.I.; Fujioka, M.; Healy, N.L.; Schwaegler, R.G. Prospective Study of Asymptomatic Valvular Aortic Stenosis Clinical, Echocardiographic, and Exercise Predictors of Outcome. Circulation 1997, 95, 2262–2270. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Sarano, M.E.; Nishimura, R.A.; Malouf, J.F.; Bailey, K.R.; Scott, C.G.; Barnes, M.E.; Tajik, A.J. Outcome of 622 Adults with Asymptomatic, Hemodynamically Significant Aortic Stenosis During Prolonged Follow-Up. Circulation 2005, 111, 3290–3295. [Google Scholar] [CrossRef] [PubMed]
- Gohlke-Bärwolf, C.; Minners, J.; Jander, N.; Gerdts, E.; Wachtell, K.; Ray, S.; Pedersen, T.R. Natural History of Mild and of Moderate Aortic Stenosis—New Insights From a Large Prospective European Study. Curr. Probl. Cardiol. 2013, 38, 365–409. [Google Scholar] [CrossRef]
- Iung, B.; Vahanian, A. Degenerative calcific aortic stenosis: A natural history. Heart 2012, 98. Available online: http://heart.bmj.com/content/98/Suppl_4/iv7.full.pdf+html (accessed on 6 May 2018). [CrossRef] [PubMed]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.-L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef]
- Carabello, B.A. Introduction to Aortic Stenosis. Circ. Res. 2013, 113, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Cachier, A.; Baron, G.; Messika-Zeitoun, D.; Delahaye, F.; Tornos, P.; Vahanian, A. Decision-making in elderly patients with severe aortic stenosis: Why are so many denied surgery? Eur. Heart J. 2005, 26, 2714–2720. [Google Scholar] [CrossRef] [PubMed]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F. Percutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Bourantas, C.V.; Serruys, P.W. Evolution of Transcatheter Aortic Valve Replacement. Circ. Res. 2014, 114, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Elbeery, J.R.; Chitwood, W.R. Minimally invasive cardiac surgery. Heart surgery for the 21st century. N. C. Med. J. 1997, 58, 374–377. [Google Scholar]
- Westaby, S.; Benetti, F.J. Less Invasive Coronary Surgery: Consensus From the Oxford Meeting. Ann. Thorac. Surg. 1996, 62, 924–931. [Google Scholar] [CrossRef]
- Lee, B.Y.; Gleason, T.G.; Sonnad, S.S. Quality of life after aortic valve replacement. Expert Rev. Pharmacoecon. Outcomes Res. 2004, 4, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Mikus, E.; Calvi, S.; Campo, G.; Pavasini, R.; Paris, M.; Raviola, E.; Del Giglio, M. Full Sternotomy, Hemisternotomy, and Mini-thoracotomy for Aortic Valve Surgery: Is There a Difference? Ann. Thorac. Surg. 2018, 106, 1782–1788. [Google Scholar] [CrossRef]
- Liu, J.; Sidiropoulos, A.; Konertz, W. Minimally invasive aortic valve replacement (AVR) compared to standard AVR. Eur. J. Cardio-Thorac. Surg. 1999, 16, S80–S83. [Google Scholar] [CrossRef]
- Autschbach, R.; Walther, T.; Falk, V.; Diegeler, A.; Metz, S.; Mohr, F.W. S-shaped in comparison to L-shaped partial sternotomy for less invasive aortic valve replacement. Eur. J. Cardiothorac. Surg. 1998, 14 (Suppl. 1), S117–S121. [Google Scholar] [CrossRef]
- Perrotta, S.; Lentini, S. Ministernotomy approach for surgery of the aortic root and ascending aorta. Interact. Cardiovasc. Thorac. Surg. 2009, 9, 849–858. [Google Scholar] [CrossRef]
- Malaisrie, S.C.; Barnhart, G.R.; Farivar, R.S.; Mehall, J.; Hummel, B.; Rodriguez, E.; Anderson, M.; Lewis, C.; Hargrove, C.; Ailawadi, G.; et al. Current era minimally invasive aortic valve replacement: Techniques and practice. J. Thorac. Cardiovasc. Surg. 2014, 147, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.; Falk, V.; Mohr, F.W. Minimally Invasive Surgery for Valve Disease. Curr. Probl. Cardiol. 2006, 31, 399–437. [Google Scholar] [CrossRef] [PubMed]
- Kirmani, B.H.; Jones, S.G.; Malaisrie, S.C.; Chung, D.A.; Williams, R.J. Limited versus full sternotomy for aortic valve replacement. Cochrane Database Syst. Rev. 2017, 4, CD011793. [Google Scholar] [CrossRef]
- Akowuah, E.; Goodwin, A.T.; Owens, W.A.; Hancock, H.C.; Maier, R.; Kasim, A.; Mason, J. Manubrium-limited ministernotomy versus conventional sternotomy for aortic valve replacement (MAVRIC): Study protocol for a randomised controlled trial. Trials 2017, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Hancock, H.C.; Maier, R.H.; Kasim, A.; Mason, J.; Murphy, G.; Goodwin, A.; Owens, W.A.; Akowuah, E. Mini-sternotomy versus conventional sternotomy for aortic valve replacement: A randomised controlled trial. BMJ Open 2021, 11, e041398. [Google Scholar] [CrossRef]
- Mächler, H.E.; Bergmann, P.; Anelli-Monti, M.; Dacar, D.; Rehak, P.; Knez, I.; Salaymeh, L.; Mahla, E.; Rigler, B. Minimally invasive versus conventional aortic valve operations: A prospective study in 120 patients. Ann. Thorac. Surg. 1999, 67, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Gofus, J.; Vobornik, M.; Koblizek, V.; Smolak, P.; Myjavec, A.; Vojacek, J.; Pojar, M. Pulmonary function and quality of life after aortic valve replacement through ministernotomy: A prospective randomized study. Kardiol. Pol. 2020, 78, 1278–1280. [Google Scholar] [CrossRef]
- Aris, A.; Cámara, M.L.; Montiel, J.; Delgado, L.J.; Galán, J.; Litvan, H. Ministernotomy versus median sternotomy for aortic valve replacement: A prospective, randomized study. Ann. Thorac. Surg. 1999, 67, 1583–1587. [Google Scholar] [CrossRef]
- Rodríguez-Caulo, E.A.; Guijarro-Contreras, A.; Otero-Forero, J.; Mataró, M.J.; Sánchez-Espín, G.; Guzón, A.; Porras, C.; Such, M.; Ordóñez, A.; Melero-Tejedor, J.M.; et al. Quality of life, satisfaction and outcomes after ministernotomy versus full sternotomy isolated aortic valve replacement (QUALITY-AVR): Study protocol for a randomised controlled trial. Trials 2018, 19, 1–8. [Google Scholar] [CrossRef]
- Bonacchi, M.; Prifti, E.; Giunti, G.; Frati, G.; Sani, G. Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Ann. Thorac. Surg. 2002, 73, 460–465. [Google Scholar] [CrossRef]
- Borger, M.A.; Moustafine, V.; Conradi, L.; Knosalla, C.; Richter, M.; Merk, D.R.; Doenst, T.; Hammerschmidt, R.; Treede, H.; Dohmen, P.; et al. A Randomized Multicenter Trial of Minimally Invasive Rapid Deployment Versus Conventional Full Sternotomy Aortic Valve Replacement. Ann. Thorac. Surg. 2015, 99, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Dzemali, O.; Wimmer-Greinecker, G.; Derra, P.; Doss, M.; Khan, M.F.; Moritz, A. Minimally invasive versus conventional aortic valve replacement: A prospective randomized trial. J. Heart Valve Dis. 2003, 12, 76–80. [Google Scholar] [PubMed]
- Calderon, J.; Richebe, P.; Guibaud, J.P.; Coiffic, A.; Branchard, O.; Asselineau, J.; Janvier, G. Prospective Randomized Study of Early Pulmonary Evaluation of Patients Scheduled for Aortic Valve Surgery Performed by Ministernotomy or Total Median Sternotomy. J. Cardiothorac. Vasc. Anesth. 2009, 23, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.A.; Abdelsamad, A.A.; Zakaria, G.; Omarah, M.M. Minimal vs Median Sternotomy for Aortic Valve Replacement. Asian Cardiovasc. Thorac. Ann. 2007, 15, 472–475. [Google Scholar] [CrossRef]
- Shneider, Y.u.A.; Tsoi, M.D.; Fomenko, M.S.; Pavlov, A.A.; Shilenko, P.A. Aortic valve replacement via J-shaped partial up-per sternotomy: Randomized trial, mid-term results. Khirurgiia 2020, 7, 25–30. [Google Scholar] [CrossRef]
- Dalen, M.; Oliveira Da Silva, C.; Sartipy, U.; Winter, R.; Franco-Cereceda, A.; Barimani, J.; Svenarud, P. Comparison of right ventricular function after ministernotomy and full sternotomy aortic valve replacement: A randomized study. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 790–797. [Google Scholar] [CrossRef]
- Vukovic, P.M.; Milojevic, P.; Stojanovic, I.; Micovic, S.; Zivkovic, I.; Peric, M.; Milicic, M.; Milacic, P.; Milojevic, M.; Bojic, M. The role of ministernotomy in aortic valve surgery—A prospective randomized study. J. Card. Surg. 2019, 34, 435–439. [Google Scholar] [CrossRef]
- Hancock, H.C.; Maier, R.H.; Kasim, A.S.; Mason, J.M.; Murphy, G.J.; Goodwin, A.T.; Akowuah, E.F. Mini-Sternotomy Versus Conventional Sternotomy for Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 73, 2491–2492. [Google Scholar] [CrossRef]
- Nair, S.K.; Sudarshan, C.D.; Thorpe, B.S.; Singh, J.; Pillay, T.; Catarino, P.; Sharples, L.D. Mini-Stern Trial: A randomized trial comparing mini-sternotomy to full median sternotomy for aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2018, 156, 2124–2132.e31. [Google Scholar] [CrossRef]
- Rao, P.N.; Kumar, A.S. Aortic valve replacement through right thoracotomy. Tex. Heart Inst. J. 1993, 20, 307–308. [Google Scholar]
- Cosgrove, D.M., 3rd; Sabik, J.F. Minimally invasive approach for aortic valve operations. Ann. Thorac. Surg. 1996, 62, 596–597. [Google Scholar] [CrossRef]
- Cohn, L.H. Minimally invasive aortic valve surgery: Technical considerations and results with the parasternal approach. J. Card. Surg. 1998, 13, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Detter, C.; Deuse, T.; Boehm, D.H.; Reichenspurner, H.; Reichart, B. Midterm Results and Quality of Life after Minimally Invasive vs. Conventional Aortic Valve Replacement. Thorac. Cardiovasc. Surg. 2002, 50, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Raza, S.; Altarabsheh, S.E.; Delozier, S.; Sharma, U.M.; Zia, A.; Khan, M.S.; Neudecker, M.; Markowitz, A.H.; Sabik, J.F.; et al. Minimally Invasive Approaches to Surgical Aortic Valve Replacement: A Meta-Analysis. Ann. Thorac. Surg. 2018, 106, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Mrcs, M.Y.S.; Hamilton, H.; Rahman, I.; Chien, L.; Rival, P.; Benedetto, U.; Young, C.; Caputo, M.; Angelini, G.D.; Vohra, H.A. Mini-sternotomy vs right anterior thoracotomy for aortic valve replacement. J. Card. Surg. 2020, 35, 1570–1582. [Google Scholar] [CrossRef]
- Ogami, T.; Yokoyama, Y.; Takagi, H.; Serna-Gallegos, D.; Ferdinand, F.D.; Sultan, I.; Kuno, T. Minimally invasive versus conventional aortic valve replacement: The network meta-analysis. J. Card. Surg. 2022, 37, 4868–4874. [Google Scholar] [CrossRef]
- Vohra, H.A.; Salmasi, M.Y.; Mohamed, F.; Shehata, M.; Bahrami, B.; Caputo, M.; Deshpande, R.; Bapat, V.; Bahrami, T.; Birdi, I.; et al. Consensus statement on aortic valve replacement via an anterior right minithoracotomy in the UK healthcare setting. Open. Heart 2023, 10, e002194. [Google Scholar] [CrossRef]
- Balmforth, D.; Harky, A.; Lall, K.; Uppal, R. Is ministernotomy superior to right anterior minithoracotomy in minimally invasive aortic valve replacement? Interact. Cardiovasc. Thorac. Surg. 2017, 25, 818–821. [Google Scholar] [CrossRef]
- Hassan, M.; Miao, Y.; Maraey, A.; Lincoln, J.; Brown, S.; Windsor, J.; Ricci, M. Minimally Invasive Aortic Valve Replacement: Cost-Benefit Analysis of Ministernotomy Versus Minithoracotomy Approach. J. Heart Valve Dis. 2015, 24, 531–539. [Google Scholar] [PubMed]
- Ghanta, R.K.; Lapar, D.J.; Kern, J.A.; Kron, I.L.; Speir, A.M.; Fonner, E.; Quader, M.; Ailawadi, G. Minimally invasive aortic valve replacement provides equivalent outcomes at reduced cost compared with conventional aortic valve replacement: A real-world multi-institutional analysis. J. Thorac. Cardiovasc. Surg. 2015, 149, 1060–1065. [Google Scholar] [CrossRef]
- Rodriguez, E.; Malaisrie, S.C.; Mehall, J.R.; Moore, M.; Salemi, A.; Ailawadi, G.; Economic Workgroup on Valvular Surgery. Right anterior thoracotomy aortic valve replacement is associated with less cost than sternotomy-based approaches: A multi-institution analysis of ‘real world’ data. J. Med. Econ. 2014, 17, 846–852. [Google Scholar] [CrossRef]
- Gumus, F.; Hasde, A.I.; Bermede, O.; Kilickap, M.; Durdu, M.S. Multiple Valve Implantation Through a Minimally Invasive Approach: Comparison of Standard Median Sternotomy and Right Anterior Thoracotomy. Heart Lung Circ. 2020, 29, 1418–1423. [Google Scholar] [CrossRef]
- Musumeci, F.; Lio, A.; Montalto, A.; Bergonzini, M.; Cammardella, A.G.; Comisso, M.; Nicolò, F.; Ranocchi, F. Minimally invasive treatment of multiple valve disease: A modified approach through a right lateral minithoracotomy. J. Card. Surg. 2019, 35, 135–139. [Google Scholar] [CrossRef]
- Zhao, D.; Wei, L.; Zhu, S.; Zhang, Z.; Liu, H.; Yang, Y.; Wang, Y.; Ji, Q.; Wang, C. Combined Mitral and Aortic Valve Procedure via Right Mini-Thoracotomy versus Full Median Sternotomy. Int. Heart J. 2019, 60, 336–344. [Google Scholar] [CrossRef]
- Manoly, I.; Hasan, R.; Brazier, A.; Farooq, V.; Thompson, T.; Karunaratne, D.; Naylor, H.; Fraser, D. Feasibility of hybrid off pump artery bypass grafting and transaortic transcatheter aortic valve implantation: A case series. Catheter. Cardiovasc. Interv. 2016, 89, 1273–1279. [Google Scholar] [CrossRef]
- Zubarevich, A.; Zhigalov, K.; Szczechowicz, M.; Thielmann, M.; Rabis, M.; Van den Eynde, J.; Wendt, D. Simultaneous trans-aortic transcatheter aortic valve implantation and off-pump coronary artery bypass: An effective hybrid approach. J. Card. Surg. 2021, 36, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Mayr, B.; Firschke, C.; Erlebach, M.; Bleiziffer, S.; Krane, M.; Joner, M.; Herold, U.; Nöbauer, C.; Lange, R.; Deutsch, M.-A. Transcatheter aortic valve implantation and off-pump coronary artery bypass surgery: An effective hybrid procedure in selected patients. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 102–107. [Google Scholar] [CrossRef]
- Tabata, M.; Khalpey, Z.; Shekar, P.S.; Cohn, L.H. Reoperative minimal access aortic valve surgery: Minimal mediastinal dissection and minimal injury risk. J. Thorac. Cardiovasc. Surg. 2008, 136, 1564–1568. [Google Scholar] [CrossRef]
- Santarpino, G.; Pfeiffer, S.; Concistrè, G.; Fischlein, T. Perceval S aortic valve implantation in mini-invasive surgery: The simple sutureless solution. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Morgant, M.-C.; Malapert, G.; Petrosyan, A.; Pujos, C.; Jazayeri, S.; Bouchot, O. Comparison of automated fastener device Cor-Knot versus manually-tied knot in minimally-invasive isolated aortic valve replacement surgery. J. Cardiovasc. Surg. 2020, 61, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Sazzad, F.; Ler, A.; Kuzemczak, M.; Ng, S.; Choong, A.M.; Kofidis, T. Automated Fastener vs Handtied Knots in Heart Valve Surgery: A Systematic Review and Meta-analysis. Ann. Thorac. Surg. 2020, 112, 970–980. [Google Scholar] [CrossRef]
- Folliguet, T.A.; Vanhuyse, F.; Magnano, D.; Laborde, F. Robotic Aortic Valve Replacement: Case Report. Heart Surg. Forum 2004, 7, E551–E553. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, V.; Wei, L.M.; Cook, C.C.; Hayanga, J.W.A.; Daggubati, R.; Sengupta, P.P.; Rankin, J.S. Robotic aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2021, 161, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Krueger, H.; Umminger, J.; Koigeldiyev, N.; Beckmann, E.; Haverich, A.; Martens, A. Minimally invasive valve sparing aortic root replacement (David procedure) is safe. Ann. Cardiothorac. Surg. 2015, 4, 148–153. [Google Scholar] [CrossRef]
- Glauber, M.; Miceli, A. Minimally Invasive Aortic Valve Surgery [Internet]. In Cardiac Surgery: A Complete Guide; Raja, S.G., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 421–428. [Google Scholar] [CrossRef]
- Weissman, C. Pulmonary Complications After Cardiac Surgery. Semin. Cardiothorac. Vasc. Anesth. 2004, 8, 185–211. [Google Scholar] [CrossRef]
- Moscoso Ludueña, M.; Rastan, A.J. Complications and conversions in minimally invasive aortic valve surgery. Ann. Cardiothorac. Surg. 2015, 4, 94–98. [Google Scholar]
- Di Eusanio, M. Minimally invasive aortic valve replacement. Ann. Cardiothorac. Surg. 2015, 4, 1–2. [Google Scholar]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Smith, C.R. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Tam, D.Y.; Azizi, P.M.; Fremes, S.E.; Chikwe, J.; Gaudino, M.; Wijeysundera, H.C. The cost-effectiveness of transcatheter aortic valve replacement in low surgical risk patients with severe aortic stenosis. Eur. Heart J.-Qual. Care Clin. Outcomes 2020, 7, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Tabata, M.; Umakanthan, R.; Cohn, L.H.; Iii, R.M.B.; Shekar, P.S.; Chen, F.Y.; Couper, G.S.; Aranki, S.F. Early and late outcomes of 1000 minimally invasive aortic valve operations. Eur. J. Cardio-Thorac. Surg. 2008, 33, 537–541. [Google Scholar] [CrossRef]
- Soppa, G.; Yates, M.; Viviano, A.; Smelt, J.; Valencia, O.; Van Besouw, J.P.; Jahangiri, M. Trainees can learn minimally invasive aortic valve replacement without compromising safety. Interact. Cardio Vasc. Thorac. Surg. 2015, 20, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Attia, R.Q.; Hickey, G.L.; Grant, S.W.; Bridgewater, B.; Roxburgh, J.C.; Kumar, P.; Young, C.P. Minimally Invasive Versus Conventional Aortic Valve Replacement: A Propensity-Matched Study From the UK National Data. Innovations 2016, 11, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bilkhu, R.; Borger, M.; Briffa, N.P.; Jahangiri, M. Sutureless aortic valve prostheses. Heart 2019, 105, s16–s20. [Google Scholar] [CrossRef]
- Berretta, P.; De Angelis, V.; Alfonsi, J.; Pierri, M.D.; Malvindi, P.G.; Zahedi, H.M.; Munch, C.; Di Eusanio, M. Enhanced recovery after minimally invasive heart valve surgery: Early and midterm outcomes. Int. J. Cardiol. 2022, 370, 98–104. [Google Scholar] [CrossRef] [PubMed]
| Full Sternotomy | Hemi-Sternotomy | Right Anterior Minithoracotomy | |
|---|---|---|---|
| Access | Unfettered view of whole mediastinum and whole heart | Good access to aortic root, limited to whole heart | Most challenging view |
| Sternal disruption | Whole sternum | To 2nd–4th intercostal spaces unilaterally or bilaterally | None, although costal cartilages are sometimes divided (may include right mammary artery ligation) |
| Cannulation | Full central | Variable—from full central to aortic arterial only | Typically requires peripheral cannulation |
| Instruments | Standard cardiac | Variable—can be standard or long-handled | Typically requires long-handled |
| Technical difficulty | Baseline | Learning curve easily traversed, including for trainee surgeons | Accepted to be technically challenging |
| Adjuncts Required | None | Variable—possible with standard equipment. Facilitated by rapid deployment valves, suture placement devices, and knot-tying devices | Facilitated by rapid deployment valves, suture placement devices, and knot-tying devices; Light source advantageous; Single lung ventilation. |
| Benefits (from most recent meta-analyses) * | Reduced intensive care and hospital length of stay; Reduced ventilation time | Reduced hospital length of stay; Reduced ventilation time; Lower stroke rate; Lower pacemaker rate | |
| Risks * | Increased operative time; Increased costs | Increased operative time; Increased costs (including vs. ministernotomy); Lung herniation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirmani, B.H.; Akowuah, E. Minimal Access Aortic Valve Surgery. J. Cardiovasc. Dev. Dis. 2023, 10, 281. https://doi.org/10.3390/jcdd10070281
Kirmani BH, Akowuah E. Minimal Access Aortic Valve Surgery. Journal of Cardiovascular Development and Disease. 2023; 10(7):281. https://doi.org/10.3390/jcdd10070281
Chicago/Turabian StyleKirmani, Bilal H., and Enoch Akowuah. 2023. "Minimal Access Aortic Valve Surgery" Journal of Cardiovascular Development and Disease 10, no. 7: 281. https://doi.org/10.3390/jcdd10070281
APA StyleKirmani, B. H., & Akowuah, E. (2023). Minimal Access Aortic Valve Surgery. Journal of Cardiovascular Development and Disease, 10(7), 281. https://doi.org/10.3390/jcdd10070281





