The Improvement of Left Atrial Function after Twelve Weeks of Supervised Concurrent Training in Patients with Heart Failure with Mid-Range Ejection Fraction: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
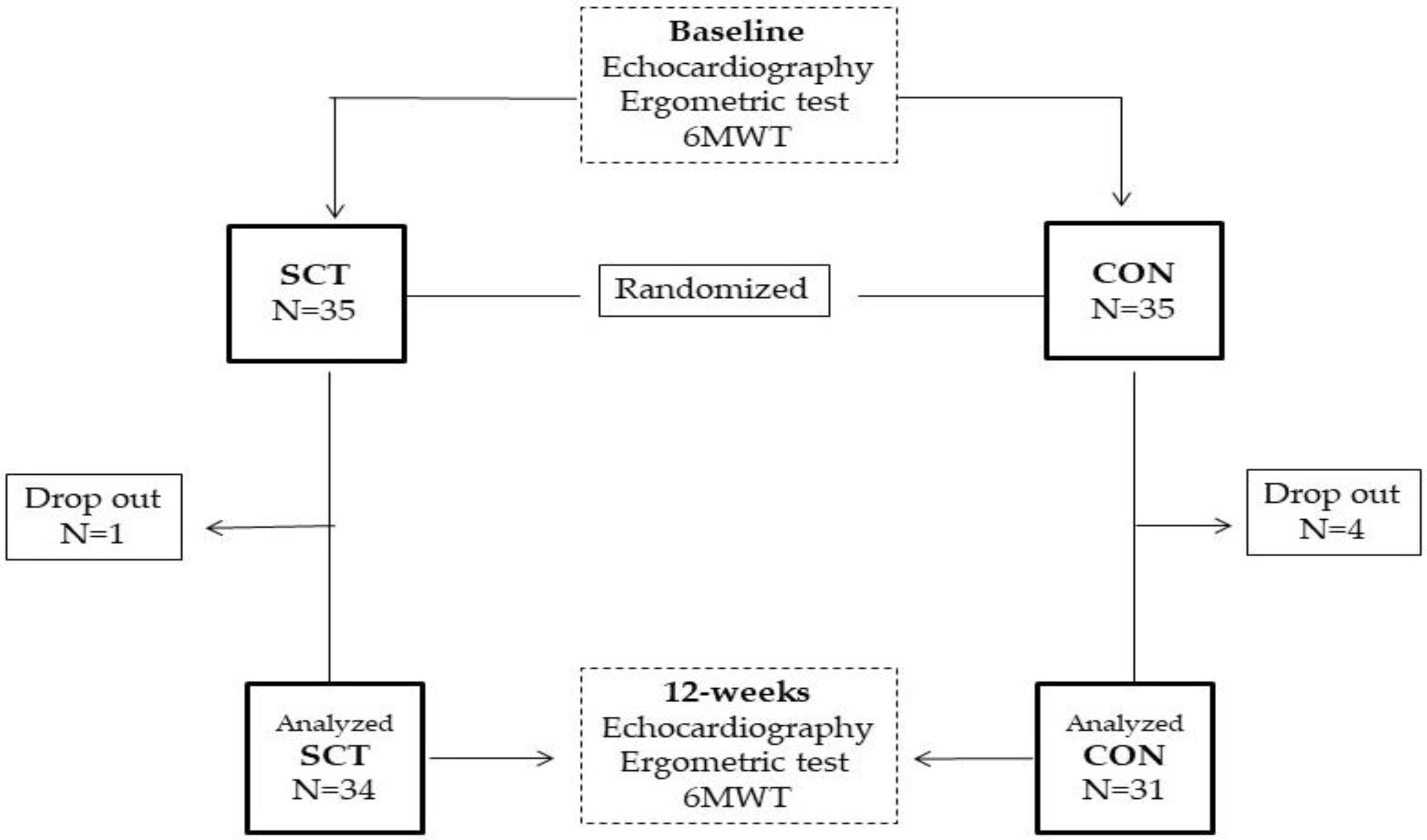
2.2. Study Design
2.3. Echocardiography Assessment
2.4. Exercise Training Programs
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
3.2. Between-Group Comparisons
4. Discussion
4.1. Main Results and Clinical Context
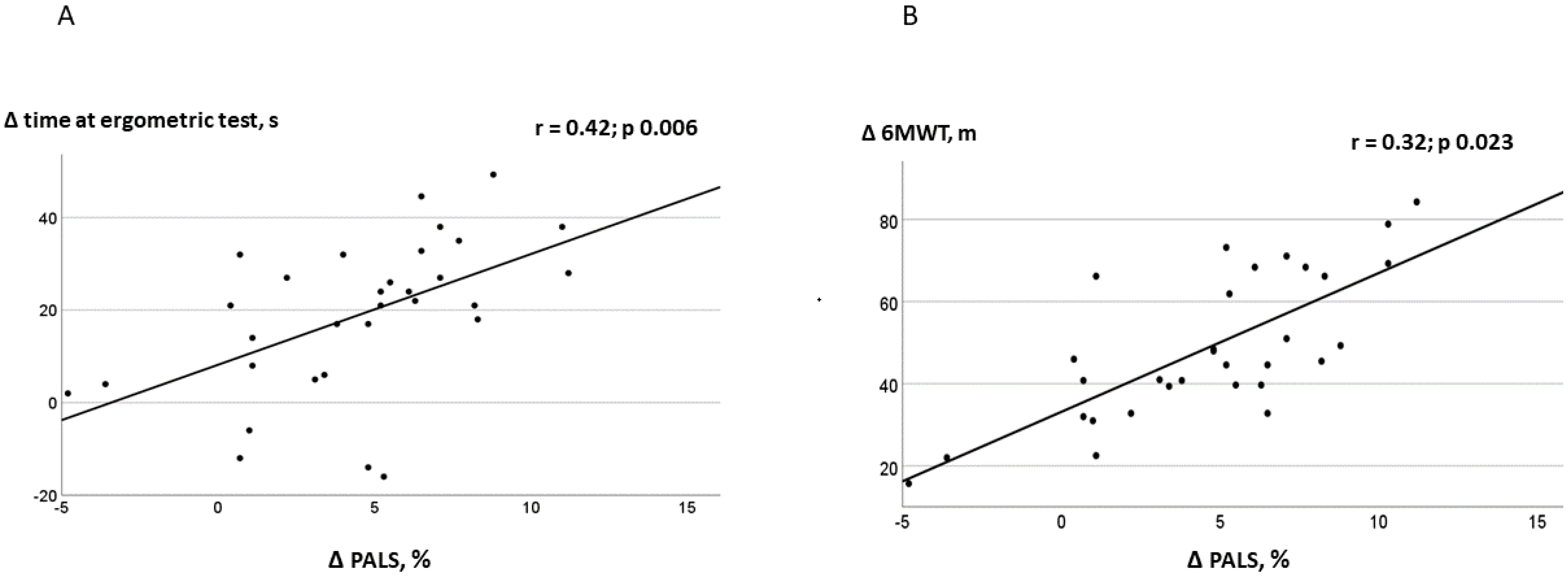
4.2. Relationship between LA Function and Exercise Tolerance
4.3. Study Strengths
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, J.H.; Chen, L. Exercise Training and Heart Failure: A Review of the Literature. Card. Fail. Rev. 2019, 5, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W. Left ventricular dysfunction: Causes, natural history, and hopes for reversal. Heart 2000, 84 (Suppl. S1), i15–i17. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Abhayaratna, W.P. Left Atrial Reverse Remodeling: Mechanisms, Evaluation, and Clinical Significance. JACC Cardiovasc. Imaging 2017, 10, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Carpenito, M.; Fanti, D.; Mega, S.; Benfari, G.; Bono, M.C.; Rossi, A.; Ribichini, F.L.; Grigioni, F. The Central Role of Left Atrium in Heart Failure. Front. Cardiovasc. Med. 2021, 8, 704762. [Google Scholar] [CrossRef]
- Ovchinnikov, A.G.; Potekhina, A.; Belyavskiy, E.; Gvozdeva, A.; Ageev, F. Left atrial dysfunction as the major driver of heart failure with preserved ejection fraction syndrome. J. Clin. Ultrasound. 2022, 50, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Lee, A.P.; Yu, C.M. Left atrial function in heart failure with impaired and preserved ejection fraction. Curr. Opin. Cardiol. 2014, 29, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Rossi, A.; Cannata, L.; Zocco, C.; Belyavskiy, E.; Radhakrishnan, A.K.; Feuerstein, A.; Morris, D.A.; Pieske-Kraigher, E.; Pieske, B.; et al. Left atrial strain predicts exercise capacity in heart failure independently of left ventricular ejection fraction. ESC Heart Fail. 2022, 9, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Modin, D.; Sengeløv, M.; Jørgensen, P.G.; Olsen, F.J.; Bruun, N.E.; Fritz-Hansen, T.; Andersen, D.M.; Jensen, J.S.; Biering-Sørensen, T. Prognostic Value of Left Atrial Functional Measures in Heart Failure with Reduced Ejection Fraction. J. Card. Fail. 2019, 25, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, L.; Gabrielli, L.; Andrea, R.; Falces, C.; Duchateau, N.; Perez-Villa, F.; Bijnens, B.; Sitges, M. Left atrial dysfunction relates to symptom onset in patients with heart failure and preserved left ventricular ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 62–67. [Google Scholar] [CrossRef]
- Sargento, L.; Vicente Simões, A.; Longo, S.; Lousada, N.; Palma Dos Reis, R. Left atrial function index predicts long-term survival in stable outpatients with systolic heart failure. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.B.; Roca, G.Q.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; Fang, J.C.; Zile, M.R.; Pitt, B.; Solomon, S.D.; et al. Prognostic Relevance of Left Atrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circ. Heart Fail. 2016, 9, e002763. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yang, W.; Yang, Y.; Yin, G.; Li, S.; Zhuang, B.; Xu, J.; He, J.; Wu, W.; Jiang, Y.; et al. Left atrial dysfunction may precede left atrial enlargement and abnormal left ventricular longitudinal function: A cardiac MR feature tracking study. BMC Cardiovasc Disord. 2022, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Gan, G.C.H.; Ferkh, A.; Boyd, A.; Thomas, L. Left atrial function: Evaluation by strain analysis. Cardiovasc. Diagn. Ther. 2018, 8, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Von Roeder, M.; Rommel, K.P.; Kowallick, J.T.; Blazek, S.; Besler, C.; Fengler, K.; Lotz, J.; Hasenfuß, G.; Lücke, C.; Gutberlet, M.; et al. Influence of Left Atrial Function on Exercise Capacity and Left Ventricular Function in Patients With Heart Failure and Preserved Ejection Fraction. Circ. Cardiovasc. Imaging 2017, 10, e005467. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, E.; Biagioli, P.; Mengoni, A.; Cerasa, M.F.; Lauciello, R.; Zuchi, C.; Bardelli, G.; Alunni, G.; Coiro, S.; Gronda, E.G.; et al. Left atrial reservoir function and outcome in heart failure with reduced ejection fraction: The importance of atrial strain by speckle tracking echocardiography. Circ. Cardiovasc. Imaging 2018, 11, e007696. [Google Scholar] [CrossRef] [PubMed]
- Castrichini, M.; Manca, P.; Nuzzi, V.; Barbati, G.; De Luca, A.; Korcova, R.; Stolfo, D.; Lenarda, A.D.; Merlo, M.; Sinagra, G. Sacubitril/valsartan induces global cardiac reverse remodeling in long-lasting heart failure with reduced ejection fraction: Standard and advanced echocardiographic evidences. J. Clin. Med. 2020, 9, 906. [Google Scholar] [CrossRef] [PubMed]
- Beckers, P.J.; Denollet, J.; Possemiers, N.M.; Wuyts, F.L.; Vrints, C.J.; Conraads, V.M. Combined endurance-resistance training vs. endurance training in patients with chronic heart failure: A prospective randomized study. Eur. Heart J. 2008, 29, 1858–1866. [Google Scholar] [CrossRef]
- Volterrani, M.; Caminiti, G.; Perrone, M.A.; Cerrito, A.; Franchini, A.; Manzi, V.; Iellamo, F. Effects of Concurrent, Within-Session, Aerobic and Resistance Exercise Training on Functional Capacity and Muscle Performance in Elderly Male Patients with Chronic Heart Failure. J. Clin. Med. 2023, 12, 750. [Google Scholar] [CrossRef]
- Conraads, V.M.; Beckers, P.; Vaes, J.; Martin, M.; Van Hoof, V.; De Maeyer, C.; Possemiers, N.; Wuyts, F.L.; Vrints, C.J. Combined endurance/resistance training reduces NT-proBNP levels in patients with chronic heart failure. Eur. Heart J. 2004, 25, 1797–1805. [Google Scholar] [CrossRef]
- Brooks, D.; Solway, S.; Gibbons, W.J. ATS statement on six-minute walk test. Am. J. Respir. Crit. Care Med. 2003, 167, 1287. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.E.; Weir, J.P. ASEP Procedures Recommendation I: Accurate Assessment of Muscular Strength and Power. J. Exerc. Physiol. 2001, 4, 1–21. [Google Scholar]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Reis Filho, J.R.; Cardoso, J.N.; Cardoso, C.M.; Pereira-Barretto, A.C. Reverse Cardiac Remodeling: A Marker of Better Prognosis in Heart Failure. Arq. Bras. Cardiol. 2015, 104, 502–506. [Google Scholar] [CrossRef]
- Wright, S.; Esfandiari, S.; Elmayergi, N.; Sasson, Z.; Goodman, J.M. Left atrial functional changes following short-term exercise training. Eur. J. Appl. Physiol. 2014, 114, 2667–2675. [Google Scholar] [CrossRef]
- McNamara, D.A.; Aiad, N.; Howden, E.; Hieda, M.; Link, M.S.; Palmer, D.; Samels, M.; Everding, B.; Ng, J.; Adams-Huet, B.; et al. Left Atrial Electromechanical Remodeling Following 2 Years of High-Intensity Exercise Training in Sedentary Middle-Aged Adults. Circulation 2019, 139, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Sareban, M.; Winkert, K.; Sperlich, B.; Berger, M.M.; Niebauer, J.; Steinacker, J.M.; Treff, G. Speckle tracking-derived bi-atrial strain before and after eleven weeks of training in elite rowers. Sci. Rep. 2018, 8, 14300. [Google Scholar] [CrossRef] [PubMed]
- Brás, P.G.; Gonçalves, A.V.; Branco, L.M.; Moreira, R.I.; Pereira-da-Silva, T.; Galrinho, A.; Timóteo, A.T.; Rio, P.; Leal, A.; Gameiro, F.; et al. Sacubitril/Valsartan Improves Left Atrial and Ventricular Strain and Strain Rate in Patients with Heart Failure with Reduced Ejection Fraction. Life 2023, 13, 995. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.G.; Hwang, I.C.; Lee, H.J.; Kim, S.H.; Yoon, Y.E.; Park, J.B.; Lee, S.P.; Kim, H.K.; Kim, Y.J.; Cho, G.Y. Reverse Remodeling Assessed by Left Atrial and Ventricular Strain Reflects Treatment Response to Sacubitril/Valsartan. JACC Cardiovasc. Imaging 2022, 15, 1525–1541. [Google Scholar] [CrossRef] [PubMed]
- Correale, M.; Magnesa, M.; Mazzeo, P.; Fortunato, M.; Tricarico, L.; Leopizzi, A.; Mallardi, A.; Mennella, R.; Croella, F.; Iacoviello, M.; et al. Left Atrial Functional Remodeling in Patients with Chronic Heart Failure Treated with Sacubitril/Valsartan. J. Clin. Med. 2023, 12, 1086. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, E.; Cameli, M.; Rossi, A.; Dini, F.L.; Biagioli, P.; Mengoni, A.; Jacoangeli, F.; Mandoli, G.E.; Pastore, M.C.; Maffeis, C.; et al. Left Atrial Strain in the Assessment of Diastolic Function in Heart Failure: A Machine Learning Approach. Circ. Cardiovasc. Imaging 2023, 16, e014605. [Google Scholar] [CrossRef] [PubMed]
- Chaveles, I.; Papazachou, O.; Shamari, M.A.; Delis, D.; Ntalianis, A.; Panagopoulou, N.; Nanas, S.; Karatzanos, E. Effects of exercise training on diastolic and systolic dysfunction in patients with chronic heart failure. World J. Cardiol. 2021, 13, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.B.; Skaarup, K.G.; Hauser, R.; Johansen, N.D.; Lassen, M.C.H.; Jensen, G.B.; Schnohr, P.; Møgelvang, R.; Biering-Sørensen, T. Normal values and reference ranges for left atrial strain by speckle-tracking echocardiography: The Copenhagen City Heart Study. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Al Saikhan, L.; Hughes, A.D.; Chung, W.S.; Alsharqi, M.; Nihoyannopoulos, P. Left atrial function in heart failure with mid-range ejection fraction differs from that of heart failure with preserved ejection fraction: A 2D speckle-tracking echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, K.; Motoki, H.; Popovic, Z.B.; Thomas, J.D.; Klein, A.L.; Marwick, T.H. Independent association of left atrial function with exercise capacity in patients with preserved ejection fraction. Heart 2012, 98, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Caso, P.; Romano, S.; Scarafile, R.; Cuomo, S.; Salerno, G.; Riegler, L.; Limongelli, G.; Di Salvo, G.; Romano, M.; et al. Association between left atrial myocardial function and exercise capacity in patients with either idiopathic or ischemic dilated cardiomyopathy: A two-dimensional speckle strain study. Int. J. Cardiol. 2009, 132, 354–363. [Google Scholar] [CrossRef]
- Maffeis, C.; Morris, D.A.; Belyavskiy, E.; Kropf, M.; Radhakrishnan, A.K.; Zach, V.; Rozados da Conceicao, C.; Trippel, T.D.; Pieske-Kraigher, E.; Rossi, A.; et al. Left atrial function and maximal exercise capacity in heart failure with preserved and mid-range ejection fraction. ESC Heart Fail. 2021, 8, 116–128. [Google Scholar] [CrossRef]
- Daskapan, A.; Arikan, H.; Caglar, N.; Tunali, N.; Ataman, S. Comparison of supervised exercise training and home-based exercise training in chronic heart failure. Saudi Med. J. 2005, 26, 842–847. [Google Scholar]
- Fabri, T.; Catai, A.M.; Ribeiro, F.H.O.; Junior, J.A.A.; Milan-Mattos, J.; Rossi, D.A.A.; Coneglian, R.C.; Borra, R.C.; Bazan, S.G.Z.; Hueb, J.C.; et al. Impact of a Supervised Twelve-Week Combined Physical Training Program in Heart Failure Patients: A Randomized Trial. Cardiol. Res. Pract. 2019, 2019, 1718281. [Google Scholar] [CrossRef] [PubMed]
| SCT (N = 35) | CON (N = 35) | p | |
|---|---|---|---|
| Anthropometric characteristics | |||
| Age, years | 69.4 ± 10.3 | 70.3 ± 12.5 | 0.342 |
| Males/females, n | 29/6 | 28/7 | 0.223 |
| BMI, kg/m2 | 27.6 ± 8.1 | 28.0 ± 6.9 | 0.091 |
| Waist circumference, mm | 106.8 ± 16.1 | 107.5 ± 14.2 | 0.167 |
| Previous AMI, n (%) | 28 (80) | 26 (74) | 0.098 |
| Previous PCI/CABG, n | 29/11 | 27/15 | 0.287 |
| Comorbidities | |||
| Hypertension, n (%) | 31 (88) | 32 (91) | 0.332 |
| Diabetes, n (%) | 13 (37) | 14 (40) | 0.076 |
| Hypercholesterolaemia, n (%) | 29 (82) | 27 (77) | 0.081 |
| Previous smoking habit, n (%) | 24 (68) | 22 (63) | 0.165 |
| Obstructive sleep apnea, n (%) | 11 (31) | 10 (28) | 0.067 |
| Treatment | |||
| Antiplatelet therapy, n (%) | 35 (100) | 35 (100) | - |
| ACE-Is/ARBs, n (%) | 32 (91) | 30 (86) | 0.079 |
| Betablockers, n (%) | 31 (88) | 33 (94) | 0.123 |
| MRAs, n (%) | 29 (83) | 30 (86) | 0.085 |
| Nitrates, n (%) | 10 (28) | 8 (23) | 0.321 |
| Diuretics n (%) | 12 (34) | 13(37) | 0.184 |
| Statins, n (%) | 32 (91) | 35 (100) | 0.065 |
| SCT (34) | CON (31) | |||
|---|---|---|---|---|
| Baseline | 12 Weeks | Baseline | 12 Weeks | |
| PALS, % | 21.4 ± 4.3 | 27.6 ± 5.1 *° | 21.8 ± 5.6 | 22.7 ± 6.0 |
| Conduit, % | 14.6 ± 4.3 | 19.6 ± 5.2 *° | 15.2.6 ± 4.7 | 14.9 ± 3.8 |
| PACS, % | 16.8 ± 7.3 | 20.4 ± 8.1 *° | 16.3.6 ± 6.4 | 18.7 ± 6.9 |
| LAVI, mLm2 | 26.2 ± 4.5 | 25.8 ± 5.8 | 26.0 ± 6.8 | 26.3 ± 5.0 |
| E wave, cm/s | 57.7 ± 17.8 | 58.1 ± 14.4 | 57.3 ± 15.8 | 58.7 ± 19.4 |
| A wave, cm/s | 59.3 ± 20.0 | 60.2 ± 22.8 | 61.4 ± 17.4 | 60.2 ± 22.3 |
| E/A ratio | 0.97 ± 0.5 | 0.96 ± 0.8 | 0.93 ± 0.7 | 0.97 ± 0.3 |
| LVEDV, mL | 124.2 ± 39.5 | 127.1 ±39.5 | 125.7 ±39.5 | 124.1 ± 39.5 |
| LVESV, mL | 69.5 ± 26 | 65.8± 22.6 | 67.1 ± 26 | 66.3± 22.6 |
| E/e’ ratio | 8.3 ± 1.8 | 7.8 ± 1.5 | 8.8 ± 2.2 | 8.4 ± 1.9 |
| LVEF, % | 45.2 ± 3.9 | 46.9 ± 4.7 | 46.2 ± 6.3 | 46.6.6 ± 5.8 |
| GLS, % | −16.5 ± 6.3 | −21.4 ± 7.5 *° | −16.1 ± 5.8 | −17.5 ± 8.7 |
| SV, mL | 56.5 ± 17.0 | 58.2 ± 15.1 | 58.4 ± 17.3 | 57.8 ± 14.6 |
| HR, bpm | 65.4 ± 12.2 | 63.5 ± 16.7 | 64.6 ± 14.6 | 65.0 ± 15.9 |
| CO, l/min | 3.6 ± 1.3 | 3.7 ± 1.8 | 3.7 ± 1.5 | 3.7 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caminiti, G.; Perrone, M.A.; D’Antoni, V.; Marazzi, G.; Gismondi, A.; Vadalà, S.; Di Biasio, D.; Manzi, V.; Iellamo, F.; Volterrani, M. The Improvement of Left Atrial Function after Twelve Weeks of Supervised Concurrent Training in Patients with Heart Failure with Mid-Range Ejection Fraction: A Pilot Study. J. Cardiovasc. Dev. Dis. 2023, 10, 276. https://doi.org/10.3390/jcdd10070276
Caminiti G, Perrone MA, D’Antoni V, Marazzi G, Gismondi A, Vadalà S, Di Biasio D, Manzi V, Iellamo F, Volterrani M. The Improvement of Left Atrial Function after Twelve Weeks of Supervised Concurrent Training in Patients with Heart Failure with Mid-Range Ejection Fraction: A Pilot Study. Journal of Cardiovascular Development and Disease. 2023; 10(7):276. https://doi.org/10.3390/jcdd10070276
Chicago/Turabian StyleCaminiti, Giuseppe, Marco Alfonso Perrone, Valentino D’Antoni, Giuseppe Marazzi, Alessandro Gismondi, Sara Vadalà, Deborah Di Biasio, Vincenzo Manzi, Ferdinando Iellamo, and Maurizio Volterrani. 2023. "The Improvement of Left Atrial Function after Twelve Weeks of Supervised Concurrent Training in Patients with Heart Failure with Mid-Range Ejection Fraction: A Pilot Study" Journal of Cardiovascular Development and Disease 10, no. 7: 276. https://doi.org/10.3390/jcdd10070276
APA StyleCaminiti, G., Perrone, M. A., D’Antoni, V., Marazzi, G., Gismondi, A., Vadalà, S., Di Biasio, D., Manzi, V., Iellamo, F., & Volterrani, M. (2023). The Improvement of Left Atrial Function after Twelve Weeks of Supervised Concurrent Training in Patients with Heart Failure with Mid-Range Ejection Fraction: A Pilot Study. Journal of Cardiovascular Development and Disease, 10(7), 276. https://doi.org/10.3390/jcdd10070276









