Biventricular or Conduction System Pacing for Cardiac Resynchronization Therapy: A Strategy for Cardiac Resynchronization Based on a Hybrid Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Data
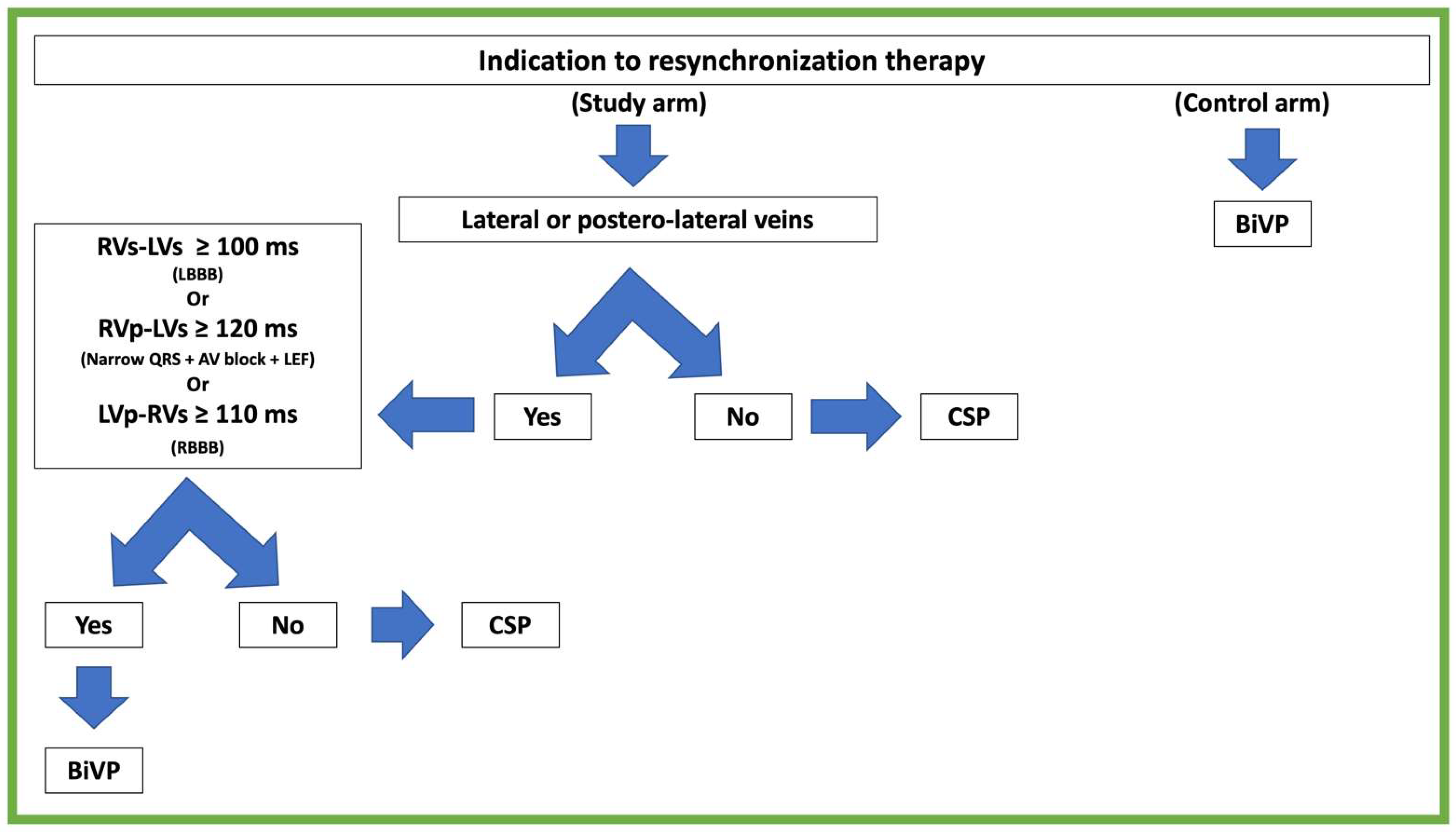
2.3. Treatment Algorithm
2.4. Conduction System Pacing
2.5. Endpoints and Clinical Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Procedural Aspects
3.3. Procedural Results
3.4. Endpoints
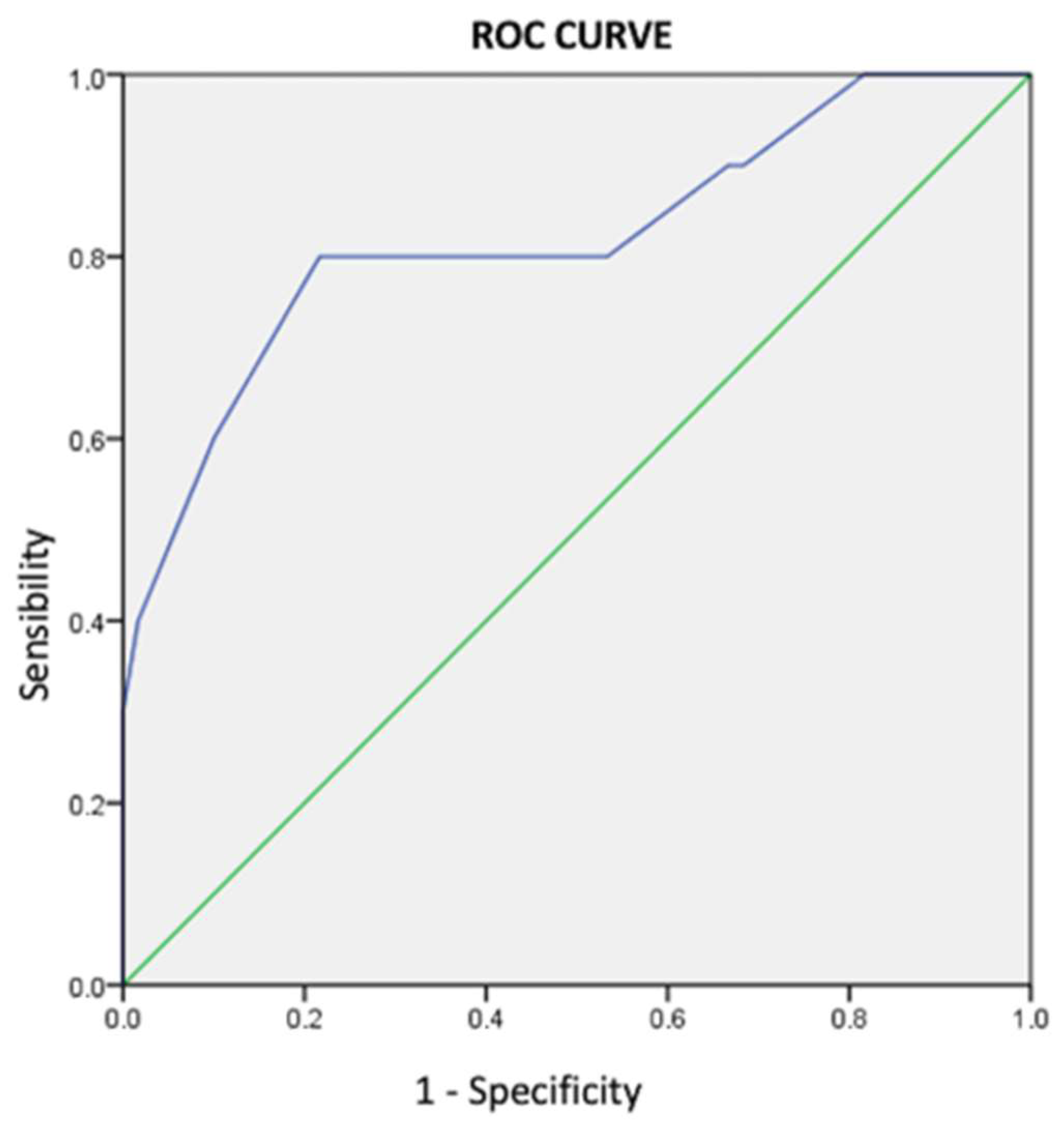
3.5. Interventricular Conduction Delays
4. Discussion
4.1. Clinical Implications
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cleland, J.G.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; DiCarlo, L.; DeMets, D.; White, B.G.; et al. Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A.M., III; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. EP Eur. 2022, 24, 71–164. [Google Scholar] [CrossRef] [PubMed]
- Linde, C.; Abraham, W.T.; Gold, M.R.; Sutton, M.S.J.; Ghio, S.; Daubert, C. REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) Study Group Randomized Trial of Cardiac Resynchronization in Mildly Symptomatic Heart Failure Patients and in Asymptomatic Patients with Left Ventricular Dysfunction and Previous Heart Failure Symptoms. J. Am. Coll. Cardiol. 2008, 52, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.L.; van Stipdonk, A.; Illner, A.; D’Ambrosio, G.; Regoli, F.; Conte, G.; Moccetti, T.; Klersy, C.; Prinzen, F.W.; Vernooy, K.; et al. The definition of left bundle branch block influences the response to cardiac resynchronization therapy. Int. J. Cardiol. 2018, 269, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.R.; Thébault, C.; Linde, C.; Abraham, W.T.; Gerritse, B.; Ghio, S.; Sutton, M.S.J.; Daubert, J.-C. Effect of QRS Duration and Morphology on Cardiac Resynchronization Therapy Outcomes in Mild Heart Failure: Results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Circulation 2012, 126, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.J.; Emerek, K.; Sørensen, P.L.; Zeitler, E.P.; Goldstein, S.A.; Al-Khatib, S.M.; Søgaard, P.; Graff, C.; Atwater, B.D. Sex differences in left ventricular electrical dyssynchrony and outcomes with cardiac resynchronization therapy. Heart Rhythm O2 2020, 1, 243–249. [Google Scholar] [CrossRef]
- Goldenberg, I.; Moss, A.J.; Hall, W.J.; Foster, E.; Goldberger, J.J.; Santucci, P.; Shinn, T.; Solomon, S.; Steinberg, J.S.; Wilber, D.; et al. Predictors of Response to Cardiac Resynchronization Therapy in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011, 124, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Hua, W.; Ding, L.-G.; Wang, J.; Chen, K.-P.; Yang, X.-W.; Liu, Z.-M.; Zhang, S. Association of Body Mass Index with Cardiac Reverse Remodeling and Long-Term Outcome in Advanced Heart Failure Patients With Cardiac Resynchronization Therapy. Circ. J. 2014, 78, 2899–2907. [Google Scholar] [CrossRef]
- Sharma, P.S.; Dandamudi, G.; Herweg, B.; Wilson, D.; Singh, R.; Naperkowski, A.; Koneru, J.N.; Ellenbogen, K.A.; Vijayaraman, P. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: A multicenter experience. Heart Rhythm 2018, 15, 413–420. [Google Scholar] [CrossRef]
- Moubarak, G.; Sebag, F.A.; Socie, P.; Villejoubert, O.; Louembe, J.; Ferchaud, V. Interrelationships between interventricular electrical delays in cardiac resynchronization therapy. J. Cardiovasc. Electrophysiol. 2020, 31, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Dandamudi, G.; Zanon, F.; Sharma, P.S.; Tung, R.; Huang, W.; Koneru, J.; Tada, H.; Ellenbogen, K.A.; Lustgarten, D.L. Permanent His bundle pacing: Recommendations from a Multicenter His Bundle Pacing Collaborative Working Group for standardization of definitions, implant measurements, and follow-up. Heart Rhythm 2018, 15, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Su, L.; Wu, S.; Xu, L.; Xiao, F.; Zhou, X.; Ellenbogen, K.A. A Novel Pacing Strategy with Low and Stable Output: Pacing the Left Bundle Branch Immediately Beyond the Conduction Block. Can. J. Cardiol. 2017, 33, 1736.e1–1736.e3. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, X.; Su, L.; Wu, S.; Xia, X.; Vijayaraman, P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm 2019, 16, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Lecoq, G.; Leclercq, C.; Leray, E.; Crocq, C.; Alonso, C.; de Place, C.; Mabo, P.; Daubert, C. Clinical and electrocardiographic predictors of a positive response to cardiac resynchronization therapy in advanced heart failure. Eur. Heart J. 2005, 26, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Daubert, C.; Behar, N.; Martins, R.P.; Mabo, P.; Leclercq, C. Avoiding non-responders to cardiac resynchronization therapy: A practical guide. Eur. Heart J. 2017, 38, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, A.; Prinzen, F.W. Non-Responders to Cardiac Resynchronization Therapy—The Magnitude of the Problem and the Issues. Circ. J. 2011, 75, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Soejima, K.; Kondo, Y.; Sasaki, S.; Adachi, K.; Kato, R.; Hagiwara, N.; Harada, T.; Kusano, K.; Miura, F.; Morishima, I.; et al. Intracardiac conduction time as a predictor of cardiac resynchronization therapy response: Results of the BIO|SELECT pilot study. Heart Rhythm O2 2021, 2, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Field, M.E.; Yu, N.; Wold, N.; Gold, M.R. Comparison of measures of ventricular delay on cardiac resynchronization therapy response. Heart Rhythm 2020, 17, 615–620. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | DRG (n = 160) | SRG (n = 132) | p-Values |
|---|---|---|---|
| Demographic, Anthropomorphic, Clinical | |||
| Age (years) | 69.9 ± 10.6 | 70.6 ± 12.7 | 0.80 |
| Female (n/%) | 44/27.5 | 40/30.0 | 0.30 |
| BSA (m2) | 1.82 ± 0.20 | 1.79 ± 1.20 | 0.08 |
| Ischemic etiology (n/%) | 75/46.8 | 49/37.1 | 0.20 |
| History of AF (n/%) | 30/18.7 | 28/21.2 | 0.10 |
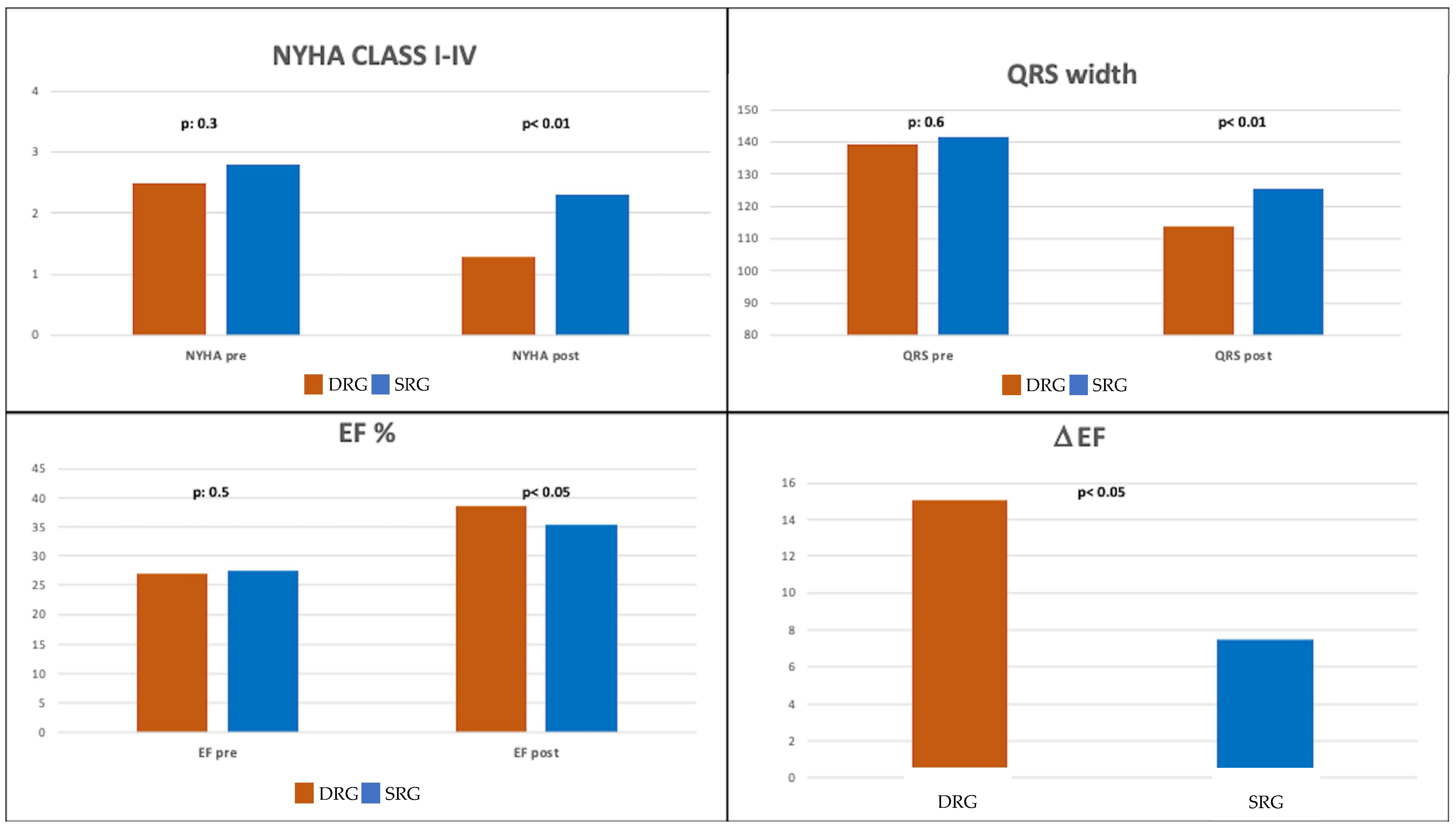
| NYHA class | 2.5 ± 1.9 | 2.8 ± 1.3 | 0.30 |
| Electrocardiogram | |||
| QRS width (ms) | 139 ± 22 | 141 ± 30 | 0.60 |
| LBBB (n/%) | 120/75.1 | 111/84.1 | 0.10 |
| AVB (with rEF) (n/%) | 14/8.7 | 16/12.1 | 0.09 |
| RBBB (n/%) | 11/6.8 | 5/3.8 | 0.20 |
| Echocardiogram | |||
| EF (%) | 26.9 ± 9.1 | 27.5 ± 6.9 | 0.50 |
| EDD (mm) | 67.1 ± 10.5 | 70.0 ± 3.5 | 0.07 |
| ESD (mm) | 48.6 ± 9.3 | 50.0 ± 5.3 | 0.10 |
| EDD index (mm/m2) | 36.8 ± 3.2 | 39.1 ± 2.7 | 0.09 |
| ESD index (mm/m2) | 26.7 ± 4.1 | 27.8 ± 1.1 | 0.30 |
| EDV (mL) | 172.4 ± 49.1 | 174.4 ± 34.2 | 0.20 |
| ESV (mL) | 120.6 ± 46.2 | 123.2 ± 41.0 | 0.09 |
| EDV index (mL/m2) | 94.5 ± 26.9 | 97.2 ± 18.9 | 0.07 |
| ESV index (mL/m2) | 65.9 ± 25.3 | 68.7 ± 22.9 | 0.09 |
| Medical therapy | |||
| Beta-blockers (n/%) | 143/89.3 | 111/84.1 | 0.30 |
| ACEI-ATIIra-Sa/Va (n/%) | 149/ 93.1 | 123/93.2 | 0.90 |
| Diuretics (n/%) | 115/71.8 | 111/84.1 | 0.09 |
| Aldosterone antagonist (n/%) | 75/46.8 | 53/40.2 | 0.40 |
| Procedural Results | DRG (n = 160) | SRG (n = 132) | p-Values |
|---|---|---|---|
| Clinical | |||
| NYHA class | 1.3 ± 0.5 | 2.3 ± 0.6 | <0.05 |
| Electrocardiogram | |||
| QRS width (ms) | 113 ± 13 | 125 ± 25 | <0.01 |
| BiVP or CSP stimulation (%) | 98.2 ± 10.1 | 97.7 ± 1.8 | 0.80 |
| CSP stimulation (n/%) | 41/25.6 | 0/0 | - |
| HBP (n%) | 28/68.3 | 0/0 | |
| sHBP (n.) | 23 | 0 | |
| nsHBP (n.) | 5 | 0 | |
| LBBP (n/%) | 13/31.7 | 0/0 | |
| Echocardiogram | |||
| EF (%) | 38.8 ± 10.1 | 35.4 ± 12.3 | <0.05 |
| EDD (mm) | 57.4 ± 9.8 | 62.3 ± 8.3 | <0.05 |
| ESD (mm) | 45.7 ± 11.7 | 49.1 ± 10.3 | <0.05 |
| EDD index (mm/m2) | 31.5 ± 5.4 | 34.8 ± 4.6 | <0.05 |
| ESD index (mm/m2) | 25.1 ± 5.3 | 28.4 ± 5.8 | 0.10 |
| EDV (mL) | 160.1 ± 59.6 | 165.4 ± 8.9 | 0.09 |
| ESV (mL) | 90.1 ± 53.1 | 99.7 ± 52.2 | <0.05 |
| EDV index (mL/m2) | 87.9 ± 32.7 | 92.4 ± 5.7 | <0.05 |
| ESV index (mL/m2) | 50.5 ± 29.2 | 56.1 ± 31.4 | <0.05 |
| ΔEF (%) | 11.3 ± 10.5 | 7.5 ± 13.7 | <0.01 |
| Endpoints | DRG (n = 160) | SRG (n = 132) | p-Values |
|---|---|---|---|
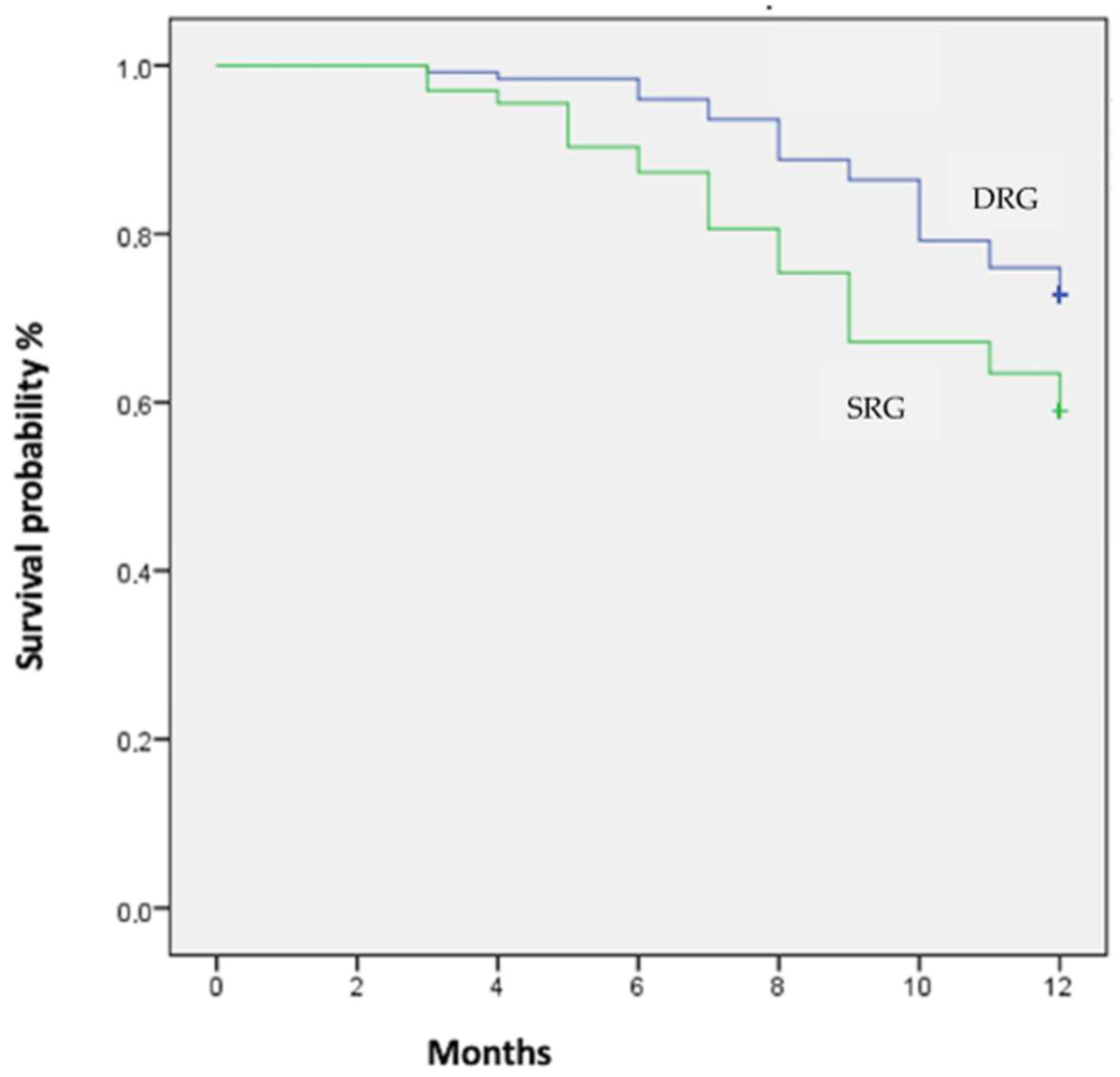
| Composite outcome | 52 (32.5) | 74 (56.0) | <0.01 |
| Cardiovascular death (n/%) | 11 (6.9) | 16 (12.1) | 0.09 |
| HF hospitalization (n/%) | 15 (9.4) | 43 (32.6) | <0.01 |
| Urgent clinic visit (n/%) | 29 (18.1) | 50 (37.9) | <0.01 |
| Echocardiographic responders (n/%) | 124 (77.5) | 84 (63.6) | <0.01 |
| NYHA class | 1.3 ± 0.5 | 2.3 ± 0.6 | <0.05 |
| Determinants of NYHA Class Improvement | |||
|---|---|---|---|
| Parameter | B | HR (95% CI) | p-Values |
| DRG | 0.3 | 0.6 (0.3–0.9) | 0.001 |
| Determinants ofΔEF | |||
| Parameter | B | HR (95% CI) | p-Values |
| DRG | 0.2 | 0.5 (0.3–0.9) | 0.010 |
| HF etiology | 0.2 | 0.5 (0.2–0.9) | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, A.; Landra, F.; Marallo, C.; Taddeucci, S.; Sisti, N.; Pica, A.; Stefanini, A.; Tavera, M.C.; Pagliaro, A.; Baiocchi, C.; et al. Biventricular or Conduction System Pacing for Cardiac Resynchronization Therapy: A Strategy for Cardiac Resynchronization Based on a Hybrid Approach. J. Cardiovasc. Dev. Dis. 2023, 10, 169. https://doi.org/10.3390/jcdd10040169
Santoro A, Landra F, Marallo C, Taddeucci S, Sisti N, Pica A, Stefanini A, Tavera MC, Pagliaro A, Baiocchi C, et al. Biventricular or Conduction System Pacing for Cardiac Resynchronization Therapy: A Strategy for Cardiac Resynchronization Based on a Hybrid Approach. Journal of Cardiovascular Development and Disease. 2023; 10(4):169. https://doi.org/10.3390/jcdd10040169
Chicago/Turabian StyleSantoro, Amato, Federico Landra, Carmine Marallo, Simone Taddeucci, Nicolò Sisti, Andrea Pica, Andrea Stefanini, Maria Cristina Tavera, Antonio Pagliaro, Claudia Baiocchi, and et al. 2023. "Biventricular or Conduction System Pacing for Cardiac Resynchronization Therapy: A Strategy for Cardiac Resynchronization Based on a Hybrid Approach" Journal of Cardiovascular Development and Disease 10, no. 4: 169. https://doi.org/10.3390/jcdd10040169
APA StyleSantoro, A., Landra, F., Marallo, C., Taddeucci, S., Sisti, N., Pica, A., Stefanini, A., Tavera, M. C., Pagliaro, A., Baiocchi, C., & Cameli, M. (2023). Biventricular or Conduction System Pacing for Cardiac Resynchronization Therapy: A Strategy for Cardiac Resynchronization Based on a Hybrid Approach. Journal of Cardiovascular Development and Disease, 10(4), 169. https://doi.org/10.3390/jcdd10040169