Antithrombotic Therapy in Peripheral Artery Disease: Current Evidence and Future Directions
Abstract
1. Introduction
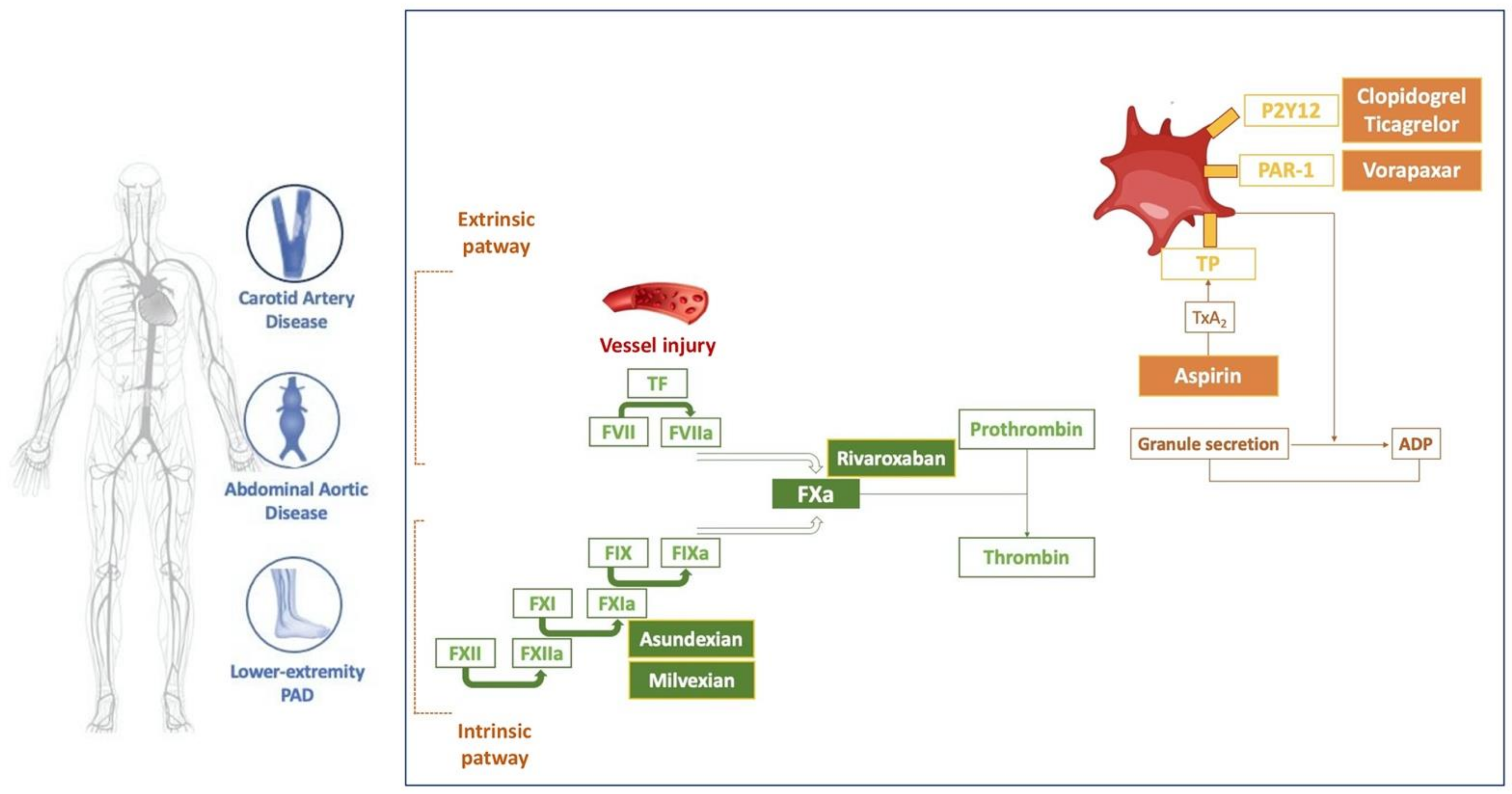
2. Role of Atherothrombosis in the Progression and Complications of Non-Coronary Artery Disease
2.1. Carotid Artery Disease
2.2. Abdominal Aortic Disease
2.3. Lower Extremity Peripheral Artery Disease
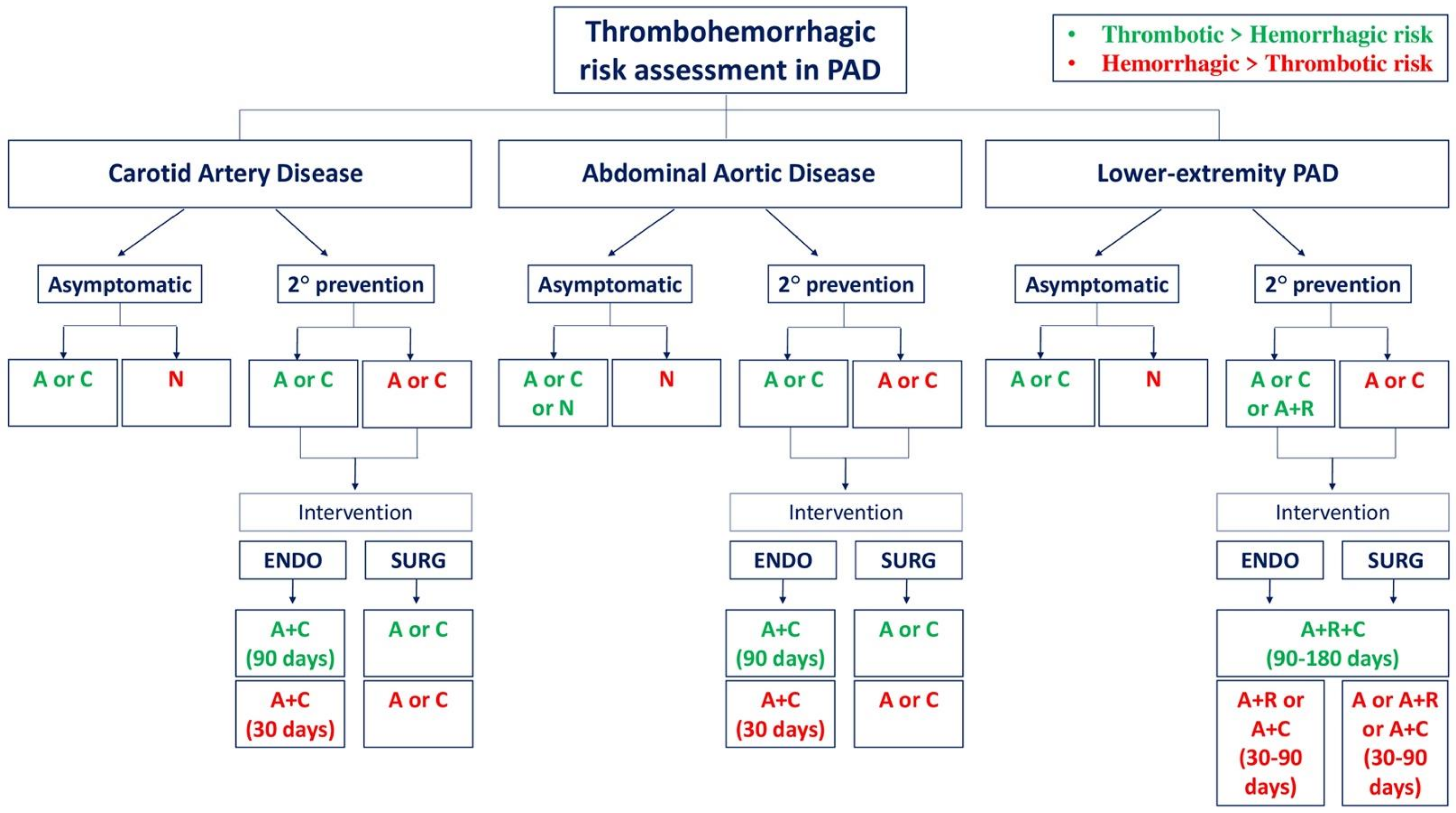
3. Approaches to Antithrombotic Therapy in Peripheral Artery Disease
3.1. Carotid Artery Disease
3.1.1. Asymptomatic Patients
3.1.2. Secondary Prevention
3.2. Abdominal Aortic Disease
3.2.1. Asymptomatic Patients
3.2.2. Secondary Prevention
3.3. Lower Extremity Peripheral Artery Disease
3.3.1. Asymptomatic Patients
3.3.2. Secondary Prevention
3.4. Antithrombotic Therapy in Peripheral Artery Disease Patients with Polyvascular Disease and Cardiovascular Comorbidities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97 (Suppl. S2), S6–S16. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; Ujueta, F. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e171–e191. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; He, Y.; Adeloye, D.; Zhu, Y.; Ye, X.; Yi, Q.; Global Health Epidemiology Research Group. The Global and Regional Prevalence of Abdominal Aortic Aneurysms: A Systematic Review and Modelling Analysis. Ann. Surg. 2022, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e686–e725. [Google Scholar] [CrossRef]
- Aboyans, V.; Björck, M.; Brodmann, M.; Collet, J.P.; Czerny, M.; De Carlo, M.; Naylor, A.R.; Roffi, M.; Tendera, M.; Vlachopoulos, C.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripherasl Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Black, I.J.H.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 80, e223–e393. [Google Scholar] [CrossRef]
- Fleischmann, D.; Afifi, R.O.; Casanegra, A.I.; Elefteriades, J.A.; Gleason, T.G.; Hanneman, K.; Roselli, E.E.; Willemink, M.J.; Fischbein, M.P. Imaging and Surveillance of Chronic Aortic Dissection: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Imaging 2022, 15, e000075. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Ding, N.; Sang, Y.; Chen, J.; Ballew, S.H.; Kalbaugh, C.A.; Salameh, M.J.; Blaha, M.J.; Allison, M.; Heiss, G.; Selvin, E.; et al. Cigarette Smoking, Smoking Cessation, and Long-Term Risk of 3 Major Atherosclerotic Diseases. J. Am. Coll. Cardiol. 2019, 74, 498–507. [Google Scholar] [CrossRef]
- Saba, L.; Brinjikji, W.; Spence, J.; Wintermark, M.; Castillo, M.; de Borst, G.; Yang, Q.; Yuan, C.; Buckler, A.; Edjlali, M.; et al. Roadmap Consensus on Carotid Artery Plaque Imaging and Impact on Therapy Strategies and Guidelines: An International, Multispecialty, Expert Review and Position Statement. Am. J. Neuroradiol. 2021, 42, 1566–1575. [Google Scholar] [CrossRef]
- Stakos, D.A.; Stamatelopoulos, K.; Bampatsias, D.; Sachse, M.; Zormpas, E.; Vlachogiannis, N.I.; Tual-Chalot, S.; Stellos, K. The Alzheimer’s Disease Amyloid-Beta Hypothesis in Cardiovascular Aging and Disease. J. Am. Coll. Cardiol. 2020, 75, 952–967. [Google Scholar] [CrossRef]
- Golledge, J.; Norman, P.E. Atherosclerosis and Abdominal Aortic Aneurysm. Arter. Thromb. Vasc. Biol. 2010, 30, 1075–1077. [Google Scholar] [CrossRef]
- Zhao, S.; Gu, H.; Chen, B.; Cheng, Z.; Yang, S.; Duan, Y.; Ghavamian, A.; Wang, X. Dynamic Imaging Features of Retrospective Cardiac Gating CT Angiography Influence Delayed Adverse Events in Acute Uncomplicated Type B Aortic Dissections. Cardiovasc. Interv. Radiol. 2020, 43, 620–629. [Google Scholar] [CrossRef]
- Burris, N.S.; Patel, H.J.; Hope, M.D. Retrograde flow in the false lumen: Marker of a false lumen under stress? J. Thorac. Cardiovasc. Surg. 2019, 157, 488–491. [Google Scholar] [CrossRef]
- Hess, C.N.; Bonaca, M.P. Contemporary Review of Antithrombotic Therapy in Peripheral Artery Disease. Circ. Cardiovasc. Interv. 2020, 13, e009584. [Google Scholar] [CrossRef]
- Narula, N.; Dannenberg, A.J.; Olin, J.W.; Bhatt, D.L.; Johnson, K.W.; Nadkarni, G.; Min, J.; Torii, S.; Poojary, P.; Anand, S.S.; et al. Pathology of Peripheral Artery Disease in Patients with Critical Limb Ischemia. J. Am. Coll. Cardiol. 2018, 72, 2152–2163. [Google Scholar] [CrossRef]
- Narula, N.; Olin, J.W.; Narula, N. Pathologic Disparities Between Peripheral Artery Disease and Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1982–1989. [Google Scholar] [CrossRef]
- King, R.W.; Canonico, M.E.; Bonaca, M.P.; Hess, C.N. Management of Peripheral Arterial Disease: Lifestyle Modifications and Medical Therapies. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100513. [Google Scholar] [CrossRef]
- Aboyans, V.; Bauersachs, R.; Mazzolai, L.; Brodmann, M.; Palomares, J.F.R.; Debus, S.; Collet, J.-P.; Drexel, H.; Espinola-Klein, C.; Lewis, B.S.; et al. Antithrombotic therapies in aortic and peripheral arterial diseases in 2021: A consensus document from the ESC working group on aorta and peripheral vascular diseases, the ESC working group on thrombosis, and the ESC working group on cardiovascular pharmacotherapy. Eur. Heart J. 2021, 42, 4013–4024. [Google Scholar]
- Kansal, A.; Huang, Z.; Rockhold, F.W.; Baumgartner, I.; Berger, J.; Blomster, J.I.; Fowkes, F.G.; Katona, B.; Mahaffey, K.W.; Norgren, L.; et al. Impact of Procedural Bleeding in Peripheral Artery Disease. Circ. Cardiovasc. Interv. 2019, 12, e008069. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; Meade, T. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar] [PubMed]
- Sharma, M.; Hart, R.G.; Connolly, S.J.; Bosch, J.; Shestakovska, O.; Ng, K.K.H.; Catanese, L.; Keltai, K.; Aboyans, V.; Alings, M.; et al. Stroke Outcomes in the COMPASS Trial. Circulation 2019, 139, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- ESPRIT Study Group; Halkes, P.H.; van Gijn, J.; Kappelle, L.J.; Koudstaal, P.J.; Algra, A. Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): A randomised controlled trial. Lancet Neurol. 2007, 6, 115–124. [Google Scholar] [PubMed]
- Warfarin Antiplatelet Vascular Evaluation Trial Investigators; Anand, S.S.; Yusuf, S.; Xie, C.; Pogue, J.; Eikelboom, J.; Budaj, A.; Sussex, B.; Liu, L.; Guzman, R.; et al. Oral Anticoagulant and Antiplatelet Therapy and Peripheral Arterial Disease. N. Engl. J. Med. 2007, 357, 217–227. [Google Scholar]
- Markus, H.S.; Droste, D.W.; Kaps, M.; Larrue, V.; Lees, K.R.; Siebler, M.; Ringelstein, E.B. Dual Antiplatelet Therapy with Clopidogrel and Aspirin in Symptomatic Carotid Stenosis Evaluated Using Doppler Embolic Signal Detection. Circulation 2005, 111, 2233–2240. [Google Scholar] [CrossRef]
- Wong, K.S.L.; Chen, C.; Fu, J.; Chang, H.M.; Suwanwela, N.C.; Huang, Y.N.; Han, Z.; Tan, K.S.; Ratanakorn, D.; Chollate, P.; et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): A randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010, 9, 489–497. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Zhao, X.; Li, H.; Wang, D.; Johnston, S.C.; Liu, L.; Meng, X.; Wang, A.; Wang, C.; et al. Clopidogrel with Aspirin in Acute Minor Stroke or Transient Ischemic Attack. N. Engl. J. Med. 2013, 369, 11–19. [Google Scholar] [CrossRef]
- Johnston, S.C.; Easton, J.D.; Farrant, M.; Barsan, W.; Conwit, R.A.; Elm, J.J.; Kim, A.S.; Lindblad, A.S.; Palesch, Y.Y.; Neurological Emergencies Treatment Trials Network; et al. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. N. Engl. J. Med. 2018, 379, 215–225. [Google Scholar] [CrossRef]
- Pan, Y.; Elm, J.J.; Li, H.; Easton, J.D.; Wang, Y.; Farrant, M.; Meng, X.; Kim, A.S.; Zhao, X.; Meurer, W.J.; et al. Outcomes Associated with Clopidogrel-Aspirin Use in Minor Stroke or Transient Ischemic Attack. JAMA Neurol. 2019, 76, 1466. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Lin, J.; Li, H.; Johnston, S.C.; Lin, Y.; Pan, Y.; Liu, L.; Wang, D.; Wang, C.; et al. Association Between CYP2C19 Loss-of-Function Allele Status and Efficacy of Clopidogrel for Risk Reduction Among Patients with Minor Stroke or Transient Ischemic Attack. JAMA 2016, 316, 70–78. [Google Scholar] [CrossRef]
- Toyoda, K.; Uchiyama, S.; Yamaguchi, T.; Easton, J.D.; Kimura, K.; Hoshino, H.; Sakai, N.; Okada, Y.; Tanaka, K.; Origasa, H.; et al. Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: A multicentre, open-label, randomised controlled trial. Lancet Neurol. 2019, 18, 539–548. [Google Scholar] [CrossRef]
- Johnston, S.C.; Amarenco, P.; Albers, G.W.; Denison, H.; Easton, J.D.; Evans, S.R.; Held, P.; Jonasson, J.; Minematsu, K.; Molina, C.A.; et al. Ticagrelor versus Aspirin in Acute Stroke or Transient Ischemic Attack. N. Engl. J. Med. 2016, 375, 35–43. [Google Scholar] [CrossRef]
- Wong, K.L.; Amarenco, P.; Albers, G.W.; Denison, H.; Easton, J.D.; Evans, S.R.; Held, P.; Himmelmann, A.; Kasner, S.E.; Knutsson, M.; et al. Efficacy and Safety of Ticagrelor in Relation to Aspirin Use within the Week before Randomization in the SOCRATES Trial. Stroke 2018, 49, 1678–1685. [Google Scholar] [CrossRef]
- Johnston, S.C.; Amarenco, P.; Denison, H.; Evans, S.R.; Himmelmann, A.; James, S.; Knutsson, M.; Ladenvall, P.; Molina, C.A.; Wang, Y. Ticagrelor and Aspirin or Aspirin Alone in Acute Ischemic Stroke or TIA. N. Engl. J. Med. 2020, 383, 207–217. [Google Scholar] [CrossRef]
- McKevitt, F.M.; Randall, M.S.; Cleveland, T.J.; Gaines, P.A.; Tan, K.T.; Venables, G.S. The Benefits of Combined Anti-platelet Treatment in Carotid Artery Stenting. Eur. J. Vasc. Endovasc. Surg. 2005, 29, 522–527. [Google Scholar] [CrossRef]
- Dalainas, I.; Nano, G.; Bianchi, P.; Stegher, S.; Malacrida, G.; Tealdi, D.G. Dual Antiplatelet Regime Versus Acetyl-acetic Acid for Carotid Artery Stenting. Cardiovasc. Interv. Radiol. 2006, 29, 519–521. [Google Scholar] [CrossRef]
- Taylor, D.W.; Barnett, H.J.; Haynes, R.B.; Ferguson, G.G.; Sackett, D.L.; E Thorpe, K.; Simard, D.; Silver, F.L.; Hachinski, V.; Clagett, G.P.; et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: A randomised controlled trial. Lancet 1999, 353, 2179–2184. [Google Scholar] [CrossRef]
- Barkat, M.; Hajibandeh, S.; Hajibandeh, S.; Torella, F.; Antoniou, G.A. Systematic Review and Meta-analysis of Dual Versus Single Antiplatelet Therapy in Carotid Interventions. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 53–67. [Google Scholar] [CrossRef]
- De Caterina, R.; Prisco, D.; Eikelboom, J.W. Factor XI inhibitors: Cardiovascular perspectives. Eur. Heart J. 2023, 44, 280–292. [Google Scholar] [CrossRef]
- Shoamanesh, A.; Mundl, H.; Smith, E.E.; Masjuan, J.; Milanov, I.; Hirano, T.; Agafina, A.; Campbell, B.; Caso, V.; Mas, J.-L.; et al. Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): An international, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet 2022, 400, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Molina, C.A.; Toyoda, K.; Bereczki, D.; Kasner, S.E.; Lutsep, H.L.; Tsivgoulis, G.; Ntaios, G.; Czlonkowska, A.; Shuaib, A.; et al. Rationale and design of the AXIOMATIC-SSP phase II trial: Antithrombotic treatment with factor XIa inhibition to Optimize Management of Acute Thromboembolic events for Secondary Stroke Prevention. J. Stroke Cerebrovasc. Dis. 2022, 31, 106742. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Mani, K.; Kullberg, J.; Svensjö, S.; Bersztel, A.; Karlsson, L.; Holst, J.; Gottsäter, A.; Linné, A.; Gillgren, P.; et al. The effect of ticagrelor on growth of small abdominal aortic aneurysms—A randomized controlled trial. Cardiovasc. Res. 2019, 116, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Belch, J.; MacCuish, A.; Campbell, I.; Cobbe, S.; Taylor, R.; Prescott, R.; Lee, R.; Bancroft, J.; MacEwan, S.; Shepherd, J.; et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: Factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008, 337, a1840. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.R.; Price, J.F.; Stewart, M.C.W.; Butcher, I.; Leng, G.C.; Pell, A.C.H.; Sandercock, P.; Fox, K.; Lowe, G.D.O.; Murray, G.; et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: A randomized controlled trial. JAMA 2010, 303, 841–848. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Fowkes, F.G.R.; Heizer, G.; Berger, J.S.; Baumgartner, I.; Held, P.; Katona, B.G.; Mahaffey, K.W.; Norgren, L.; Jones, W.S. Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease. N. Engl. J. Med. 2017, 376, 32–40. [Google Scholar] [CrossRef]
- Dutch Bypass Oral Anticoagulants or Aspirin (BOA) Study Group. Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral Anticoagulants or Aspirin study): A randomised trial. Lancet 2000, 355, 346–351. [Google Scholar] [CrossRef]
- Belch, J.J.; Dormandy, J. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J. Vasc. Surg. 2010, 52, 825–833.e2. [Google Scholar] [CrossRef]
- Iida, O.; Nanto, S.; Uematsu, M.; Morozumi, T.; Kitakaze, M.; Nagata, S. Cilostazol reduces restenosis after endovascular therapy in patients with femoropopliteal lesions. J. Vasc. Surg. 2008, 48, 144–149. [Google Scholar] [CrossRef]
- Tepe, G.; Bantleon, R.; Brechtel, K.F.M.; Schmehl, J.-M.; Zeller, T.; Claussen, C.D.; Strobl, F.F.X. Management of peripheral arterial interventions with mono or dual antiplatelet therapy—The MIRROR study: A randomised and double-blinded clinical trial. Eur. Radiol. 2012, 22, 1998–2006. [Google Scholar] [CrossRef]
- Moll, F.; Baumgartner, I.; Jaff, M.; Nwachuku, C.; Tangelder, M.; Ansel, G.; Adams, G.; Zeller, T.; Rundback, J.; Grosso, M.; et al. Edoxaban Plus Aspirin vs. Dual Antiplatelet Therapy in Endovascular Treatment of Patients With Peripheral Artery Disease: Results of the ePAD Trial. J. Endovasc. Ther. 2018, 25, 158–168. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bauersachs, R.M.; Anand, S.S.; Debus, E.S.; Nehler, M.R.; Patel, M.R.; Hiatt, W.R. Rivaroxaban in Peripheral Artery Disease after Revascularization. N. Engl. J. Med. 2020, 382, 1994–2004. [Google Scholar] [CrossRef]
- Wong, K.H.F.; Zlatanovic, P.; Bosanquet, D.C.; Saratzis, A.; Kakkos, S.K.; Aboyans, V.; Twine, C.P. Antithrombotic Therapy for Aortic Aneurysms: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2022, 64, 544–556. [Google Scholar] [CrossRef]
- Moran, C.S.; Seto, S.-W.; Krishna, S.M.; Sharma, S.; Jose, R.J.; Biros, E.; Wang, Y.; Morton, S.K.; Golledge, J. Parenteral administration of factor Xa/IIa inhibitors limits experimental aortic aneurysm and atherosclerosis. Sci. Rep. 2017, 7, 43079. [Google Scholar] [CrossRef]
- De Bruin, J.L.; Brownrigg, J.R.; Patterson, B.O.; Karthikesalingam, A.; Holt, P.J.; Hinchliffe, R.J.; Loftus, I.M.; Thompson, M.M. The Endovascular Sealing Device in Combination with Parallel Grafts for Treatment of Juxta/Suprarenal Abdominal Aortic Aneurysms: Short-term Results of a Novel Alternative. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 458–465. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- He, R.-X.; Zhang, L.; Zhou, T.-N.; Yuan, W.-J.; Liu, Y.-J.; Fu, W.-X.; Jing, Q.-M.; Liu, H.-W.; Wang, X.-Z. Safety and Necessity of Antiplatelet Therapy on Patients Underwent Endovascular Aortic Repair with Both Stanford Type B Aortic Dissection and Coronary Heart Disease. Chin. Med. J. 2017, 130, 2321–2325. [Google Scholar]
- De Rango, P.; Verzini, F.; Parlani, G.; Cieri, E.; Simonte, G.; Farchioni, L.; Isernia, G.; Cao, P. Safety of Chronic Anticoagulation Therapy after Endovascular Abdominal Aneurysm Repair (EVAR). Eur. J. Vasc. Endovasc. Surg. 2014, 47, 296–303. [Google Scholar] [CrossRef]
- Canonico, M.E.; Hsia, J.; Hess, C.N.; Bonaca, M.P. Sex differences in guideline-directed medical therapy in 2021–22 among patients with peripheral artery disease. Vasc. Med. 2023. [Google Scholar] [CrossRef]
- Franzone, A.; Piccolo, R.; Gargiulo, G.; Ariotti, S.; Marino, M.; Santucci, A.; Baldo, A.; Magnani, G.; Moschovitis, A.; Windecker, S.; et al. Prolonged vs. Short Duration of Dual Antiplatelet Therapy after Percutaneous Coronary Intervention in Patients with or without Peripheral Arterial Disease. JAMA Cardiol. 2016, 1, 795. [Google Scholar] [CrossRef]
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996, 348, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.P.; Bhatt, D.L.; Steg, P.G.; Topol, E.J.; Creager, M.A. Patients with peripheral arterial disease in the CHARISMA trial. Eur. Heart J. 2008, 30, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Bhatt, D.L.; Storey, R.F.; Steg, P.h.G.; Cohen, M.; Kuder, J.; Goodrich, E.; Nicolau, J.C.; Parkhomenko, A.; López-Sendón, J.; et al. Ticagrelor for Prevention of Ischemic Events after Myocardial Infarction in Patients with Peripheral Artery Disease. J. Am. Coll. Cardiol. 2016, 67, 2719–2728. [Google Scholar] [CrossRef] [PubMed]
- Steg, P.G.; Bhatt, D.L.; Simon, T.; Fox, K.; Mehta, S.R.; Harrington, R.A.; Held, C.; Andersson, M.; Himmelmann, A.; Ridderstråle, W.; et al. Ticagrelor in Patients with Stable Coronary Disease and Diabetes. N. Engl. J. Med. 2019, 381, 1309–1320. [Google Scholar] [CrossRef]
- Morrow, D.A.; Braunwald, E.; Bonaca, M.P.; Ameriso, S.F.; Dalby, A.J.; Fish, M.P.; Fox, K.A.A.; Lipka, L.J.; Liu, X.; Nicolau, J.C.; et al. Vorapaxar in the Secondary Prevention of Atherothrombotic Events. N. Engl. J. Med. 2012, 366, 1404–1413. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Scirica, B.M.; Creager, M.A.; Olin, J.; Bounameaux, H.; Dellborg, M.; Lamp, J.M.; Murphy, S.A.; Braunwald, E.; Morrow, D.A. Vorapaxar in Patients with Peripheral Artery Disease. Circulation 2013, 127, 1522–1529. [Google Scholar] [CrossRef]
- Qamar, A.; Morrow, D.A.; Creager, M.A.; Scirica, B.M.; Olin, J.W.; Beckman, J.A.; Murphy, S.A.; Bonaca, M.P. Effect of vorapaxar on cardiovascular and limb outcomes in patients with peripheral artery disease with and without coronary artery disease: Analysis from the TRA 2°P-TIMI 50 trial. Vasc. Med. 2020, 25, 124–132. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Kaplovitch, E.; Eikelboom, J.W.; Dyal, L.; Aboyans, V.; Abola, M.T.; Verhamme, P.; Avezum, A.; Fox, K.A.A.; Berkowitz, S.D.; Bangdiwala, S.I.; et al. Rivaroxaban and Aspirin in Patients with Symptomatic Lower Extremity Peripheral Artery Disease. JAMA Cardiol. 2020, 6, 21–29. [Google Scholar] [CrossRef]
- Dawson, D.L.; Cutler, B.S.; Hiatt, W.R.; Hobson, R.W.; Martin, J.D.; Bortey, E.B.; Forbes, W.P.; Strandness, D. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am. J. Med. 2000, 109, 523–530. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Hamburg, N.M.; Creager, M.A. Contemporary Medical Management of Peripheral Artery Disease. Circ. Res. 2021, 128, 1868–1884. [Google Scholar] [CrossRef]
- Bauersachs, R.M.; Szarek, M.; Brodmann, M.; Gudz, I.; Debus, E.S.; Nehler, M.R.; VOYAGER PAD Committees and Investigators. Total Ischemic Event Reduction with Rivaroxaban after Peripheral Arterial Revascularization in the VOYAGER PAD Trial. J. Am. Coll. Cardiol. 2021, 78, 317–326. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Bonaca, M.P.; Patel, M.R.; Nehler, M.R.; Debus, E.S.; Anand, S.S.; Capell, W.H.; Brackin, T.; Jaeger, N.; Hess, C.N.; et al. Rivaroxaban and Aspirin in Peripheral Artery Disease Lower Extremity Revascularization. Circulation 2020, 142, 2219–2230. [Google Scholar] [CrossRef]
- Hess, C.N.; Debus, E.S.; Nehler, M.R.; Anand, S.S.; Patel, M.R.; Szarek, M.; Capell, W.H.; Hsia, J.; Beckman, J.A.; Brodmann, M.; et al. Reduction in Acute Limb Ischemia with Rivaroxaban Versus Placebo in Peripheral Artery Disease after Lower Extremity Revascularization: Insights from VOYAGER PAD. Circulation 2021, 144, 1831–1841. [Google Scholar] [CrossRef]
- Anand, S.S.; Hiatt, W.; Dyal, L.; Bauersachs, R.; Berkowitz, S.D.; Branch, K.R.H.; Debus, S.; Fox, K.A.A.; Liang, Y.; Muehlhofer, E.; et al. Low-dose rivaroxaban and aspirin among patients with peripheral artery disease: A meta-analysis of the COMPASS and VOYAGER trials. Eur. J. Prev. Cardiol. 2022, 29, e181–e189. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Szarek, M.; Debus, E.S.; Nehler, M.R.; Patel, M.R.; Anand, S.S.; Muehlhofer, E.; Berkowitz, S.D.; Haskell, L.P.; Bauersachs, R.M. Efficacy and safety of rivaroxaban versus placebo after lower extremity bypass surgery: A post hoc analysis of a “CASPAR like” outcome from VOYAGER PAD. Clin. Cardiol. 2022, 45, 1143–1146. [Google Scholar] [CrossRef]
- Secemsky, E.A.; Yeh, R.W.; Kereiakes, D.J.; Cutlip, D.E.; Steg, P.G.; Massaro, J.M.; Apruzzese, P.K.; Mauri, L. Extended Duration Dual Antiplatelet Therapy after Coronary Stenting Among Patients with Peripheral Arterial Disease. JACC Cardiovasc. Interv. 2017, 10, 942–954. [Google Scholar] [CrossRef]
- Capodanno, D.; Angiolillo, D.J. Timing, Selection, Modulation, and Duration of P2Y12 Inhibitors for Patients with Acute Coronary Syndromes Undergoing PCI. JACC Cardiovasc. Interv. 2023, 16, 1–18. [Google Scholar] [CrossRef]
- Jones, W.S.; Hellkamp, A.S.; Halperin, J.; Piccini, J.P.; Breithardt, G.; Singer, D.E.; Patel, M.R. Efficacy and safety of rivaroxaban compared with warfarin in patients with peripheral artery disease and non-valvular atrial fibrillation: Insights from ROCKET AF. Eur. Heart J. 2014, 35, 242–249. [Google Scholar] [CrossRef]
- Coleman, C.I.; Baker, W.L.; Meinecke, A.K.; Eriksson, D.; Martinez, B.K.; Bunz, T.J.; Alberts, M.J. Effectiveness and safety of rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation and coronary or peripheral artery disease. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 159–166. [Google Scholar] [CrossRef]
- Hu, P.T.; Lopes, R.D.; Stevens, S.R.; Wallentin, L.; Thomas, L.; Alexander, J.H.; Jones, W.S. Efficacy and Safety of Apixaban Compared with Warfarin in Patients with Atrial Fibrillation and Peripheral Artery Disease: Insights From the ARISTOTLE Trial. J. Am. Heart Assoc. 2017, 6, e004699. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Antman, E.M.; Cunningham, J.W.; Wiviott, S.D.; Murphy, S.A.; Halperin, J.L.; Weitz, J.I.; Grosso, M.A.; Lanz, H.J.; Braunwald, E.; et al. Ischaemic and bleeding risk in atrial fibrillation with and without peripheral artery disease and efficacy and safety of full- and half-dose edoxaban vs. warfarin: Insights from ENGAGE AF-TIMI 48. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Anantha-Narayanan, M.; Sheikh, A.B.; Nagpal, S.; Smolderen, K.G.; Regan, C.; Ionescu, C.; Mena-Hurtado, C. Systematic review and meta-analysis of outcomes of lower extremity peripheral arterial interventions in patients with and without chronic kidney disease or end-stage renal disease. J. Vasc. Surg. 2021, 73, 331–340.e4. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, A.S.; Caluwé, R.; Van Der Meersch, H.; De Boeck, K.; De Bacquer, D. Safety and Efficacy of Vitamin K Antagonists versus Rivaroxaban in Hemodialysis Patients with Atrial Fibrillation: A Multicenter Randomized Controlled Trial. J. Am. Soc. Nephrol. 2021, 32, 1474–1483. [Google Scholar] [CrossRef]
- Greco, A.; Laudani, C.; Spagnolo, M.; Agnello, F.; Faro, D.C.; Finocchiaro, S.; Legnazzi, M.; Mauro, M.S.; Mazzone, P.M.; Occhipinti, G.; et al. Pharmacology and Clinical Development of Factor XI Inhibitors. Circulation 2023, 147, 897–913. [Google Scholar] [CrossRef]
- Rossing, P.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; de Boer, I.H. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022, 102, S1–S127. [Google Scholar] [CrossRef]
| Study | Enrolled Patients | Population | Treatment | Follow-Up | Primary Endpoint | |
|---|---|---|---|---|---|---|
| Efficacy | Safety | |||||
| Carotid Artery Disease | ||||||
| ESPRIT Trial [24] | 1068 | Recent TIA or minor stroke (within 6 months) | Oral anticoagulation (phenprocoumon, warfarin or acenocoumarol) vs. Aspirin | 4.6 years | No difference in the composite outcome of all-cause death, non-fatal stroke, non-fatal MI (HR 1.02; 95% CI 0.77–1.35) | Increased major bleeding (HR 2.56; 95% CI 1.48–4.43) |
| CARESS Trial [26] | 107 | Symptomatic ≥50% carotid stenosis | DAPT (Aspirin + Clopidogrel) vs. Aspirin | 7 days | Reduction in the risk of asymptomatic embolization (RR 39.8%; 95% CI 13.8–58.0) | Bleeding adverse events 3.9% vs. 1.8%, p = NS |
| CLAIR Trial [27] | 100 | Acute IS or TIA | DAPT (Aspirin + Clopidogrel) vs. Aspirin | 7 days | Reduction of microembolic signals (RR 42.4%; 95% CI 4.6–65.2) | No difference in any bleeding complications |
| CHANCE Trial [28] | 5170 | Minor IS or high-risk TIA | DAPT (Aspirin + Clopidogrel) vs. Aspirin + Placebo | 90 days | Reduction of stroke rate in the DAPT group (HR 0.68; 95% CI 0.57–0.81) | No difference in bleeding complications (HR, 1.41; 95% CI 0.95–2.10) |
| POINT Trial [29] | 4881 | Minor IS or high-risk TIA | DAPT (Aspirin + Clopidogrel) vs. Aspirin | 90 days | Reduction of major ischemic events (IS, MI and death due to ischemic vascular events) (HR 0.75; 95% CI 0.59–0.95) | Increased hemorrhagic complications (HR 2.32; 95% CI 1.10–4.87) |
| Dual Antiplatelet therapy using Cilostazol for Secondary Prevention in Patients with high-risk ischemic stroke in Japan [32] | 1884 | High-risk non-cardioembolic IS | Aspirin/Clopidogrel + Cilostazol vs. Aspirin or Clopidogrel | 1.4 years | Reduction of IS in the DAPT group (HR 0.49; 95% CI 0.31–0.76) | No difference in severe or life-threatening bleeding (HR 0.66; 95% CI 0.27–1.60) |
| SOCRATES Trial [33] | 13,199 | Non-severe IS or high-risk TIA | Ticagrelor vs. Aspirin | 90 days | No difference in the composite outcome of stroke, MI or death (HR 0.89; 95% CI 0.87–1.01) | No difference in major bleeding complications (HR 0.83; 95% CI 0.52–1.34) |
| THALES Trial [35] | 11,016 | Mild-to-moderate acute noncardioembolic IS or TIA | DAPT (Aspirin + Ticagrelor) vs. Aspirin | 30 days | Reduction of composite of stroke or death in the DAPT group (HR 0.83; 95% CI 0.71–0.96) | Increased severe bleeding (HR 3.99; 95% CI 1.74–9.14) |
| The Benefits of Combined Antiplatelet Treatment in Carotid Artery Stenting [36] | 47 | Patients undergoing carotid artery stenting | DAPT (Aspirin + Clopidogrel) vs. Aspirin + 24-h heparin | 30 days | Neurological events (amaurosis fugax, TIA and all stroke) 0% vs. 25% | No difference in major bleeding 9% vs. 17%, p = NS |
| Dual Antiplatelet Regime Versus Acetyl-acetic Acid for Carotid Artery Stenting [37] | 100 | Patients undergoing carotid artery stenting | DAPT (Aspirin 325 mg + Ticlopidine) vs. Aspirin 325 mg + 24-h heparin | 30 days | Reduction of minor IS/TIA in the DAPT group, 2% vs. 16%, p < 0.05 | No difference in major bleeding |
| ACE RCT [38] | 2849 | Patients undergoing carotid endarterectomy | Aspirin 81–325 mg vs. Aspirin 650–1300 mg | 90 days | Lower rate of IS, MI and death in low-dose group 6.2% vs. 8.4%, p = 0.03 | Increased hemorrhagic stroke in high dose group (RR, 1.68; 95% CI 0.77–3.68) |
| PACIFIC Stroke Trial [41] | 1808 | Acute non-cardioembolic IS | Asundexian 10 mg vs. Asundexian 20 mg vs. Asundexian 50 mg vs. Placebo | 26 weeks | No differences in IS and incident covert brain infarct detected by MRI (HR 0.99; 95% CI, 0.79–1.24) 10 mg (HR 1.15; 95% CI 0.93–1.43) 20 mg (HR 1.06; 95% CI 0.85–1.32) 50 mg | No difference in major or clinically relevant non-major bleeding (HR 1.57; 95% CI 0.91–2.71) |
| AXIOMATIC-SPP [42] | 2366 | Acute non-lacunar IS | Milvexian 25 mg vs. Milvexian 50 mg vs. Milvexian 100 mg vs. Milvexian 200 mg vs. Placebo | 90 days | No differences in IS and incident covert brain infarct detected by MRI 25 mg, 16.2% and 18.5% 50 mg, 14.1% 100 mg, 14.7% 200 mg, 16.4% Placebo, 16.6% | No differences in rates of BARC 3 or 5 25 mg, 0.6% 50 mg, 1.5% 100 mg, 1.6% 200 mg, 1.5% Placebo, 0.6% |
| Abdominal Aortic Disease | ||||||
| TicAAA Trial [43] | 144 | Patients with AAA and with a maximum aortic diameter 35–49 mm | Ticagrelor vs. Placebo | 12 months | No differences were found in AAA volume increase assessed by MRI (HR 1.013; 95% CI 0.993–1.034) | Increased bleeding events rate in ticagrelor group (33% vs. 11%, p = 0.002) |
| Lower-extremity PAD | ||||||
| POPADAD Trial [44] | 1276 | Patients with diabetes with ABI < 0.99 | Aspirin vs. Placebo | 6.7 years | No difference in the composite of death due to CAD or stroke, non-fatal MI or stroke, or amputation (HR 0.98; 95% CI 0.76–1.26) | No difference in gastrointestinal bleeding (HR 0.90; 95% CI 0.53–1.52) |
| AAA Trial [45] | 3350 | General population with ABI ≤ 0.95 | Aspirin vs. Placebo | 8.2 years | No difference in the composite of fatal or non-fatal coronary event, stroke, or revascularization (HR 1.03; 95% CI 0.84–1.27) | Increased major bleeding (HR 1.71;95% CI 0.99–2.97) |
| WAVE Trial [25] | 2161 | PAD | Antiplatelet therapy (aspirin, ticlopidine or clopidogrel) + Oral anticoagulation (warfarin or acenocoumarol) vs. Antiplatelet therapy alone | 35 months | No difference in the composite outcome of all-cause death, stroke and MI (RR 0.92; 95% CI 0.73–1.16) | Increased life-threatening or moderate bleeding (RR 3.21; 95% CI 2.02–5.08) |
| EUCLID Trial [46] | 13,885 | Symptomatic LEPAD | Ticagrelor vs. Clopidogrel | 30 months | Ticagrelor not superior to clopidogrel for MACE reduction (HR 1.02; 95% CI 0.92–1.13) | No increase in bleeding (HR 1.1; 95% CI 0.84–1.43) |
| Dutch BOA [47] | 2690 | Patients with LEPAD after infrainguinal arterial grafting | Oral anticoagulant (phenprocoumon or acenocoumarol; coumarin derivatives) vs. aspirin equivalent | 21 months | No difference in graft occlusion (HR 0.95; 95% CI, 0.82–1.11) No difference in the composite of vascular mortality, MI, stroke, or amputation (HR 0.89; 95% CI 0.75–1.06) | Increase in severe bleeding (HR 1.96; 95% CI 1.42–2.71) |
| CASPAR Trial [48] | 851 | Patients with LEPAD undergoing below-knee bypass grafting | Aspirin + Clopidogrel vs. Aspirin + Placebo | 24 months | No reduction in the composite of graft occlusion, revascularization, major amputation, or death (HR 0.98; 95% CI 0.78–1.23) | No difference in severe bleeding (2.1% vs. 1.2%) |
| Cilostazol reduces restenosis after endovascular therapy in patients with femoropopliteal lesions [49] | 127 | Patients with LEPAD after endovascular LER | Aspirin + Cilostazol vs. Aspirin + Ticlopidine | 36 months | Higher patency lesion rates at 12, 24, 36 months in Cilostazol group (87%, 82%, 73%) vs. Ticlopidine group (65%, 60%, 51%), p = 0.013 | No difference in bleeding p = 0.72 |
| MIRROR Study [50] | 80 | Patients with LEPAD undergoing endovascular LER | Clopidogrel + Aspirin vs. Aspirin + Placebo | 6 months | Decreased risk of target lesion revascularization (5% vs. 8%, p = 0.04) at 6 months but no difference at 1 year (25% vs. 32%, p = 0.35) | No increase in bleeding (2.5% vs. 5%, p = 0.56) |
| ePAD Trial [51] | 203 | Patients with LEPAD after endovascular LER | Edoxaban + Aspirin vs. Aspirin + Clopidogrel | 3 months | No difference in restenosis or reocclusion of femoropopliteal targets (HR 0.89; 95% CI 0.59–1.34) | No difference in bleeding (RR 0.56; 95% CI 0.19–1.62) |
| VOYAGER-PAD Trial [52] | 6564 | Patients with LEPAD after LER | Aspirin + Rivaroxaban vs. Aspirin + Placebo | 3 years | Composite of reduction of MACE and MALE (HR 0.85; 95% CI 0.76–0.96) | No difference in TIMI major bleeding (HR 1.43; 95% CI 0.97–2.10) increase in ISTH major bleeding (HR 1.42; 95% CI 1.10–1.84) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canonico, M.E.; Piccolo, R.; Avvedimento, M.; Leone, A.; Esposito, S.; Franzone, A.; Giugliano, G.; Gargiulo, G.; Hess, C.N.; Berkowitz, S.D.; et al. Antithrombotic Therapy in Peripheral Artery Disease: Current Evidence and Future Directions. J. Cardiovasc. Dev. Dis. 2023, 10, 164. https://doi.org/10.3390/jcdd10040164
Canonico ME, Piccolo R, Avvedimento M, Leone A, Esposito S, Franzone A, Giugliano G, Gargiulo G, Hess CN, Berkowitz SD, et al. Antithrombotic Therapy in Peripheral Artery Disease: Current Evidence and Future Directions. Journal of Cardiovascular Development and Disease. 2023; 10(4):164. https://doi.org/10.3390/jcdd10040164
Chicago/Turabian StyleCanonico, Mario Enrico, Raffaele Piccolo, Marisa Avvedimento, Attilio Leone, Salvatore Esposito, Anna Franzone, Giuseppe Giugliano, Giuseppe Gargiulo, Connie N. Hess, Scott D. Berkowitz, and et al. 2023. "Antithrombotic Therapy in Peripheral Artery Disease: Current Evidence and Future Directions" Journal of Cardiovascular Development and Disease 10, no. 4: 164. https://doi.org/10.3390/jcdd10040164
APA StyleCanonico, M. E., Piccolo, R., Avvedimento, M., Leone, A., Esposito, S., Franzone, A., Giugliano, G., Gargiulo, G., Hess, C. N., Berkowitz, S. D., Hsia, J., Cirillo, P., Esposito, G., & Bonaca, M. P. (2023). Antithrombotic Therapy in Peripheral Artery Disease: Current Evidence and Future Directions. Journal of Cardiovascular Development and Disease, 10(4), 164. https://doi.org/10.3390/jcdd10040164








