Imaging Approaches and the Quantitative Analysis of Heart Development
Abstract
1. Introduction
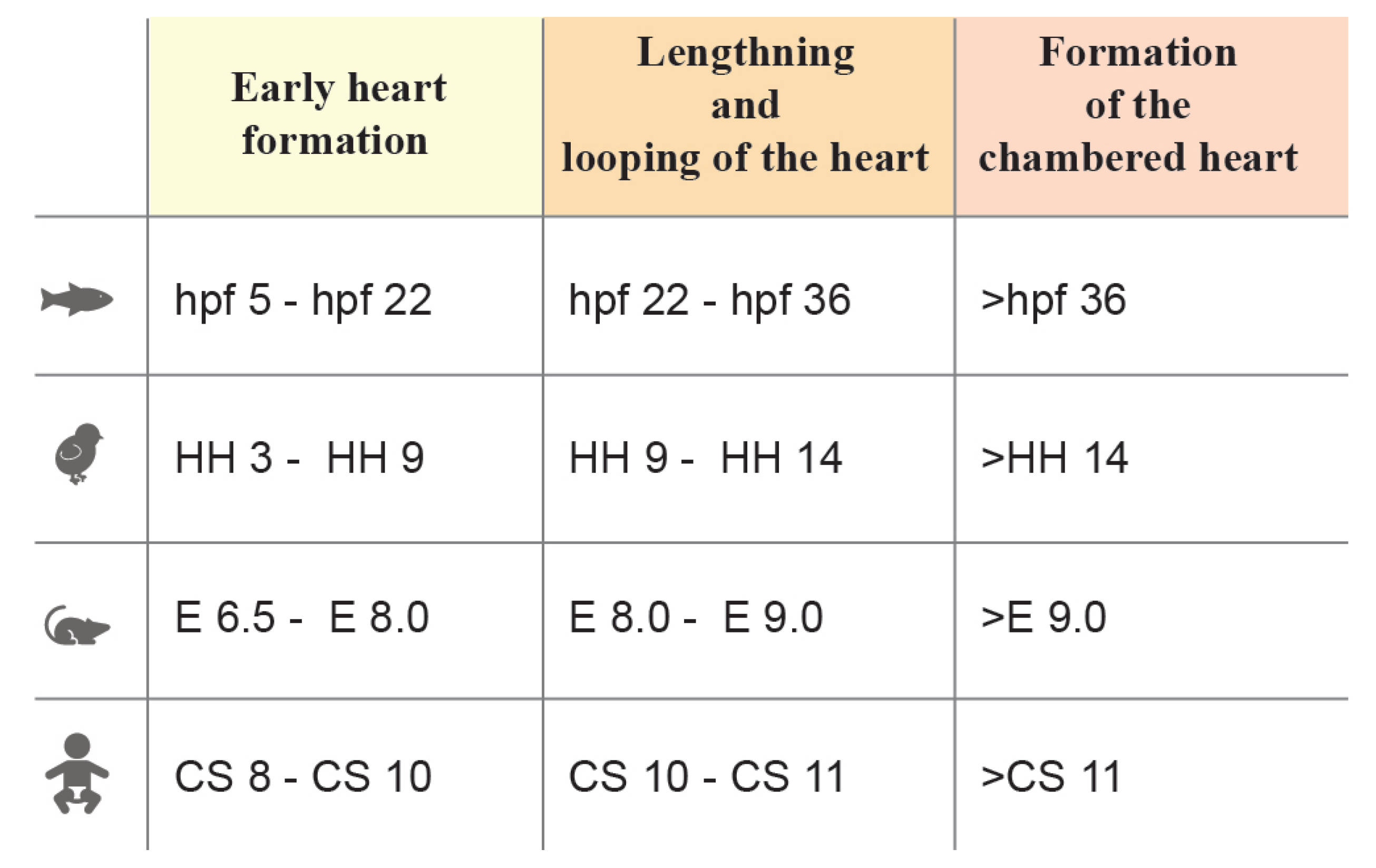
1.1. Heart Morphogenesis
1.2. Challenges in Imaging Heart Development
1.3. Quantitative Approaches towards Understanding Heart Morphogenesis
2. An Overview of Imaging Methods
2.1. Fluorescence Microscopy
| Imaging Approach | Principle | Acquisition Depth (mm) | Advantages | Limitations | Applications | Heart References | |
|---|---|---|---|---|---|---|---|
| Scanning laser | Confocal | Pinhole-restricted focal plane with high-power illumination of the whole optical path. | 0.5–0.7 | High resolution, detection of multiple fluorescent molecules. | Harsh for live sample; high photodamage; high bleaching. | Fixed samples, very high resolution, multiple fluorescent signals. Short time live imaging. Resolves cellular and sub-cellular details. | 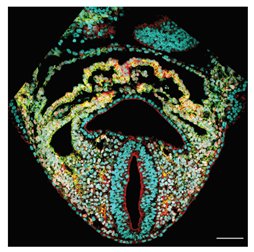 [28] a [28] a |
| 2-photon | Point-by-point selective high-power excitation by simultaneous absorption of two low-energy photons in a single event. | 1 | Deeper penetration and less photodamage than confocal. | Slow, weaker absorption, increased temperature of the sample, broad wavelength excitation bands, possible signal overlapping. Requires bright reporters. | Live imaging of small samples, cell tracking, cell shape, and filopodia analysis. Imaging of fixed scattering samples. | 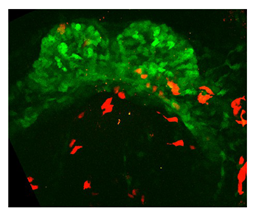 [2] b [2] b | |
| Light-sheet | Thin-sheet illumination by a laser light to illuminate a sample from the side, while a camera positioned perpendicular to the light sheet captures images of the illuminated plane at once. | 0.5 | Fast and minimal photodamage. Cell tracking, cell shape and filopodia analysis. | Needs clarification for non-translucid samples. Live imaging: less definition than two-photon in deep tissue layers due to light scattering. | Quick image of fixed and clarified large embryos. Live imaging of translucid embryos at high temporal resolution. | 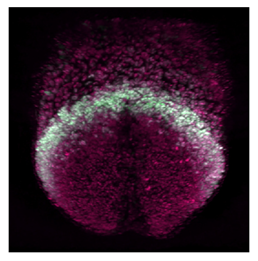 [17] c [17] c | |
| Stereology | Histological Sections | Cutting paraffin embedded or frozen tissue into thin slices. | Whole samples. | Widely available and common, optimal staining. | Variable and unpredictable distortions produced by tissue sectioning and staining. | 2D imaging, super-resolution microscopy. | 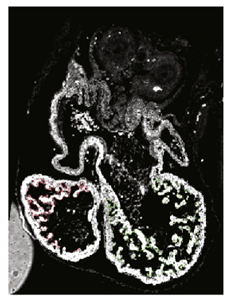 [52] d [52] d |
| HREM | White light reflection on the surface of histology blocks serially sectioned. | 12 | Histologic quality at high resolution in whole mounted samples. | Weaker contrast on big samples. Sample preparation and imaging are time-consuming. Color reactions (Xgal or BCIP/NBT) required for specific labeling of gene activity. | High-resolution images from 3D structures. 3D cell shape analysis. | 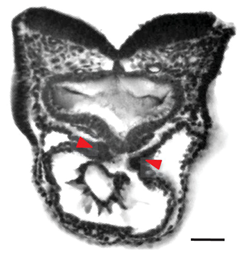 [15] e [15] e | |
| Tomography | Micro-CT | Differential X-ray attenuation depending on tissue density. | 500 | Resolves thick and opaque tissues. | High radiation dose. Soft tissues need additives for contrast. Not suitable to detect gene/marker expression patterns. Does not reach a cellular resolution. | Big samples and advanced embryos. Isotropic 3D tissue reconstruction. | 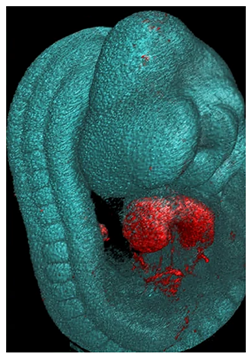 [53] f [53] f |
| Tomography | OCT | Optical scattering based on changes in its refractive index. | 2 | Fast and non-invasive. Label-free. | Limited molecular information. Not suitable to detect gene/marker expression patterns or detect individual cells. | Live imaging in utero and ex utero. Isotropic 3D tissue reconstruction. | 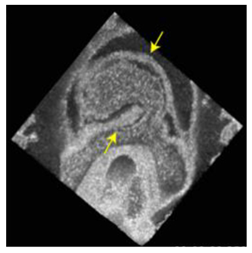 [54] g [54] g |
| OPT | Optical equivalent of micro-CT. It uses ultraviolet, visible, and near-infrared photons instead. | 10 | Detects specific fluorescent signals, reporters and stains. | Requires sample clarification with an organic solvent that may disrupt antibody stainings. | Resolving specific fluorescent and histochemical reporters. Isotropic 3D tissue reconstruction. | 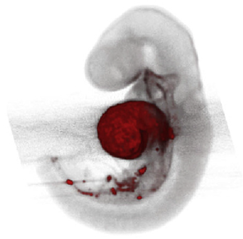 [55] h [55] h |
2.2. Stereology
2.3. Tomography
3. Quantitative Approaches for Heart Morphogenesis
3.1. Static Imaging
3.1.1. Tissue-Level Analysis
3.1.2. Cellular-Level Analysis
3.2. Live Imaging
3.2.1. Tissue-Level Analysis
3.2.2. Cellular-Level Analysis
3.3. Computational Modeling
| Reference | Biological Insight | Image Approach | Quantitative Approach | Measurement | Stage | |
|---|---|---|---|---|---|---|
| Tissue-Level | Esteban et al. (2022) [28] | A continuous 4D digital Atlas of heart development. First of L-R asymmetry in mouse heart detected in the IFTs directions. | 3D confocal images. | 3D mesh of the heart tissues. Morphometric staging system. | IFT directions defined as the angles between the IFT axis and craniocaudal embryo plane at different stages. |  a a |
| Ivanovitch et al. (2017) [2] | Different growth rates and splanchnic mesoderm migration shape the mouse heart: 1.earlyCC-to-lateCC growth rate ↑; 2.CC-to-openHT growth rate ↓; 3.openHT-to-linearHT growth rate ↑. | 3D confocal images. | 3D tissue segmentation. | Volume estimation at different stages. |  a a | |
| Le Garrec et al. (2017) [15] | Sequence of the main mechanical events driving looping in mouse heart: 1 HT elongation; 2 progressive breakdown of the DM; 3 L-R asymmetry at the poles. | HREM images; 2-photons 3D+t images. | Mesh reconstruction of planar section. | 1 Axis length intersecting the centroids of each 2D reconstruction; 2 DM thikness in different planar section over time; 3 L-R angles between HT and DM. |  b b | |
| Kawahira et al. (2020) [78] | 1 Tissue motion map of the chick heart during looping C shows an L-R asymmetry in the 2 direction of deformation. Left side with circumferential stretching, right side with longitudinal elongation. Comparable 3 growth rate between L-R. | 2-photons 3D+t images. | 1 Cellular tracking + SHE + Bayesian method resulted in 3D+t tissue mesh. 2–3 Continuum Mechanics laws on the tissue mesh. | 2 Anisotropy deformation and deformation direction as the main eigenvalue. 3 Growth rate. |  b b | |
| Yue et al. (2020) [27] | Cell intercalation and directional proliferation are driving forces in ballooning and trabeculation process in mouse hearts. Cellular intercalation(+) and horizontal division(++) drive ballooning process. Early cell fate(+), oriented cell division and directional migration(++) drive trabeculation process. | 3D+t vLSFM images. | 3D nuclei segmentation, nuclei tracking. | Angle between the parent-to-daughter line and the line connecting the centroid of the left ventricle and the parent cell. |  c c | |
| Tissue-Level | Le Garrec et al. (2013) [71] | 1 Cell polarity and 2 oriented cell division drive planar expansion of the mouse ventricle with 3 local coordination. | 3D confocal images. | 3D cellular segmentation, statistical test, clustering algorithm. | 1 Axis nucleus-the centrosome. 2 Orientation of cell division as the angle between the two daughter nucleus. 3 spatial correlation function. |  c c |
| Reference | Biological Insight | Image Approach | Quantitative Approach | Measurement | Stage | |
|---|---|---|---|---|---|---|
| Cellular-Level | Dominguez et al. (2023) [17] | Spatiotemporal organization of cardiac cells in mouse model. | 3D+t LSFM images. | Nuclei tracking. | Nuclei orientation, spatio temporal displacement of cells in FHF, SHF and juxta-cardiac field. |  a a |
| Ivanovitch et al. (2017) [2] | 1 Differentiation schedule tracing showed alternating phases of differentiation and morphogenesis during heart tube formation; 2 Timing early morphogenesis events in a mouse heart. CC-to-openHT in aprox 5–7 h. | 1 3D confocal images. 2 2-photons 3D+t images. | 1 Cellular segmentation in 2D section. 2 Cellular tracking. | 1 Roundness index. 2 Cell speed at the border to reach the embryo midline. |  a a | |
| de Boer et al. (2012) [52] | Proliferation pattern in mouse HT: Asymmetric L-R ventral myocardium growth. High proliferation in SPL. | Fluorescence images. | 3D reconstruction+ design-based stereological method. | Proliferation rate (fraction of proliferating cells). |  a a | |
| Ebrahimi et al. (2022) [73] | Cellular changes during C-looping reveal spatiotemporal patterns of differentiated growth in chick hearts, highlighting the inter-cellular space’s relevance. | 3D confocal images; mCT. | 3D cell segmentation + 3D tissue mesh. | Geometric parameters (cell volume, cell density, cell anisotropy). |  b’ b’ | |
| Cellular-Level | Kawahira et al. (2020) [78] | Right-specific directional cell rearrangement during looping in chick heart. No significant differential growth between L-R sides. | 2-photons 3D+t patch images. | 1 Nuclei tracking; 2 tectonic theory from Blanchard et al.; 3 3D cell segmentation and ellipsoid approximation. | 2 Cell rearrangement is defined as the difference of tissue and 1 velocity gradient tensor and the 3 cells shape strain rate tensor; 3 geometric parameters (cell volume, cell orientation, cell anisotropy, cell section area). |  b’ b’ |
| Francou et al. (2017) [79] | Epithelia tension promotes mouse HT elongation. | 2D confocal images. | Cellular segmentation. | Geometric parameters(cell elongation, cell orientation). Orientation of cell division as the angle between the two daughter nuclei. |  b b | |
| Francou et al. (2014) [72] | 1 Cell polarity and 2 filopodia activity in SHF play an important role in mouse HT elongation and OFT morphogenesis. | 1 2D confocal images; 2 2-photons of thick-slice tissue. | Cellular segmentation. | 1 Circularity index, apical/basolateral membrane ratio. 2 Filopodia max length and filopodia lifetime. |  b b | |
| Cellular-Level | de Boer et al. (2012) [52] | Proliferation pattern in mouse HT: asymmetric ventrodorsal myocardium growth. High proliferation in the outer curvature. Low proliferation in the inner curvature. High proliferation in SPL. | Florescence images. | Design-based stereological method; 3D reconstruction. | Proliferation rate (fraction of proliferating cells). |  b b |
| Paun et al. (2017) [70] | Spatiotemporal characterization of the complexity of myocardium tissue during mouse heart trabeculation. The complexity of trabeculations increases longitudinally from the base to the apex during gestational age. | HREM images. | 3D reconstruction; geometry independent representation to establish correspondence between different objects. | 3D fractal dimension, myocardia volume, myocardial surface area and the ratio between the two. |  c c | |
| Lee et al. (2016) [76] | Close link between 1 trabeculation and 2 contractile ability of the ventricle in zebrafish model. | 3D+t SPIM images. | Synchronization algorithm for heart beating. 3D segmentation + 3D tissue mesh. | 1 Trabecular structure(volume changes of the trabecular myocardial ridges). 2 Ventricular strain (changes in circumferential displacement), fractional shortening(difference between the end -diastolic displacement and end-systolic) and ejection fraction(end-diastolic on end-systolic volumes of the ventricle). |  c’’’ c’’’ | |
| Cellular-Level | de Boer et al. (2012) [52] | Proliferation pattern in mouse heart: low proliferation in the OFT. High chambers proliferation. | Florescence images. | Design-based stereological method; 3D reconstruction. | Proliferation rate (fraction of proliferating cells). |  c c |
| Reference | Biological Insight | Acting Force | Result | Stage | |
|---|---|---|---|---|---|
| Computational Modeling | Honda et al. (2021) [89] | Cell-based modeling combines ventral bending and rightward displacement of the mouse HT. | 1 Differential proliferation and directional division. 2 Anisotropic contractile force of cell edges. | 1 HT extension and HT bending. 2Convergent extension of collective cells |  b b |
| Le Garrec et al. (2017) [15] | Tissue-based modeling to show that asymmetries at the fixed heart poles, generating opposite deformations, associated with the progressive release of the heart tube dorsally, are sufficient to generate looping of a tube growing between fixed poles. | Boundary constraints, anterior-rightward-biased contraction force. | HT extension, HT bending and left-right asymmetries at the poles |  b b | |
| Vedula et al. (2017) [97] | Computational fluid dynamics modeling to quantify the biomechanical forces involved in cardiac trabeculation in zebrafish model. | Hemodynamic from the blood flow. | Oscillatory forces as a potential mechanism for regulating the development of cardiac trabeculation. |  c’’’ c’’’ |
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CC | cardiac crescent |
| CFD | computational fluid dynamics |
| CS | Carnegie stages |
| DM | dorsal mesoderm |
| DPW | dorsal pericardial wall |
| FHF | first heart field |
| HH | Hamburger-Hamilton |
| HPF | hours post fertilization |
| HREM | high-resolution episcopic microscopy |
| HT | heart tube |
| IFT | inflow tract |
| LSFM | light-sheet fluorescence microscopy |
| micro-CT | micro-computed tomography |
| OCT | optical coherence tomography |
| OFT | outflow tract |
| OPT | optical projection tomography |
| OSI | oscillatory shear index |
| SHE | spherical harmonics expansion |
| SHF | second heart field |
| SPIM | selective-plane illumination microscopy |
| SPL | splanchnic mesoderm |
| WSS | wall shear stress |
References
- Meilhac, S.M.; Buckingham, M.E. The deployment of cell lineages that form the mammalian heart. Nat. Rev. Cardiol. 2018, 15, 705–724. [Google Scholar] [CrossRef]
- Ivanovitch, K.; Temiño, S.; Torres, M. Live imaging of heart tube development in mouse reveals alternating phases of cardiac differentiation and morphogenesis. eLife 2017, 6, 30668. [Google Scholar] [CrossRef]
- Kelly, R.G.; Buckingham, M.E.; Moorman, A.F. Heart fields and cardiac morphogenesis. Cold Spring Harb. Perspect. Med. 2014, 4, a015750. [Google Scholar] [CrossRef] [PubMed]
- Meilhac, S.M.; Esner, M.; Kerszberg, M.; Moss, J.E.; Buckingham, M.E. Oriented clonal cell growth in the developing mouse myocardium underlies cardiac morphogenesis. J. Cell Biol. 2004, 164, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, C.L.; Riemslagh, F.W.; Moran, H.R.; Mosimann, C. From stripes to a beating heart: Early cardiac development in zebrafish. J. Cardiovasc. Dev. Dis. 2021, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Sendra, M.; Domínguez, J.N.; Torres, M.; Ocaña, O.H. Dissecting the complexity of early heart progenitor cells. J. Cardiovasc. Dev. Dis. 2022, 9, 5. [Google Scholar] [CrossRef]
- Shewale, B.; Dubois, N. Of form and function: Early cardiac morphogenesis across classical and emerging model systems. Semin. Cell Dev. Biol. 2021, 118, 107–118. [Google Scholar] [CrossRef]
- Tam, P.P.; Parameswaran, M.; Kinder, S.J.; Weinberger, R.P. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: The role of ingression and tissue movement during gastrulation. Development 1997, 124, 1631–1642. [Google Scholar] [CrossRef]
- Ivanovitch, K.; Esteban, I.; Torres, M. Growth and morphogenesis during early heart development in amniotes. J. Cardiovasc. Dev. Dis. 2017, 4, 20. [Google Scholar] [CrossRef]
- Sylva, M.; den Hoff, M.J.V.; Moorman, A.F. Development of the human heart. Am. J. Med. Genet. Part A 2014, 164, 1347–1371. [Google Scholar] [CrossRef]
- Lindsey, S.E.; Butcher, J.T.; Yalcin, H.C. Mechanical regulation of cardiac development. Front. Physiol. 2014, 5, 318. [Google Scholar] [CrossRef]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef]
- Lescroart, F.; Chabab, S.; Lin, X.; Rulands, S.; Paulissen, C.; Rodolosse, A.; Auer, H.; Achouri, Y.; Dubois, C.; Bondue, A.; et al. Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nat. Cell Biol. 2014, 16, 829–840. [Google Scholar] [CrossRef]
- Buckingham, M.; Meilhac, S.; Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005, 6, 826–835. [Google Scholar] [CrossRef]
- Garrec, J.F.L.; Domínguez, J.N.; Desgrange, A.; Ivanovitch, K.D.; Raphaël, E.; Bangham, J.A.; Torres, M.; Coen, E.; Mohun, T.J.; Meilhac, S.M. A predictive model of asymmetric morphogenesis from 3D reconstructions of mouse heart looping dynamics. eLife 2017, 6, e28951. [Google Scholar] [CrossRef]
- Moorman, A.F.; Christoffels, V.M. Cardiac chamber formation: Development, genes, and evolution. Physiol. Rev. 2003, 83, 1223–1267. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.H.; Krup, A.L.; Muncie, J.M.; Bruneau, B.G. Graded mesoderm assembly governs cell fate and morphogenesis of the early mammalian heart. Cell 2023, 186, 479–496.e23. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.W.; Watanabe, M.; Rollins, A.M. Longitudinal imaging of heart development with optical coherence tomography. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1166–1175. [Google Scholar] [CrossRef]
- Mohun, T.J.; Weninger, W.J. Imaging heart development using high-resolution episcopic microscopy. Curr. Opin. Genet. Dev. 2011, 21, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, K.; Wright, A.C.; Horng, D.; Padmanabhan, A.; Epstein, J.A. Rapid 3D phenotyping of cardiovascular development in mouse embryos by micro-CT with iodine staining. Circ. Cardiovasc. Imaging 2010, 3, 314–322. [Google Scholar] [CrossRef]
- Kidokoro, H.; Yonei-Tamura, S.; Tamura, K.; Schoenwolf, G.C.; Saijoh, Y. The heart tube forms and elongates through dynamic cell rearrangement coordinated with foregut extension. Development 2018, 145, dev152488. [Google Scholar] [CrossRef] [PubMed]
- Zamir, L.; Singh, R.; Nathan, E.; Patrick, R.; Yifa, O.; Yahalom-Ronen, Y.; Arraf, A.A.; Schultheiss, T.M.; Suo, S.; Han, J.D.J.; et al. Nkx2.5 marks angioblasts that contribute to hemogenic endothelium of the endocardium and dorsal aorta. eLife 2017, 6, e20994. [Google Scholar] [CrossRef]
- Goudy, J.; Henley, T.; Méndez, H.G.; Bressan, M. Simplified platform for mosaic in vivo analysis of cellular maturation in the developing heart. Sci. Rep. 2019, 9, 10716. [Google Scholar] [CrossRef] [PubMed]
- Sendra, M.; Mañes, J.; Domínguez, J.N.; Torres, M. Live Imaging of Early Cardiac Progenitors in the Mouse Embryo. J. Vis. Exp. 2022, 2022, e64273. [Google Scholar] [CrossRef]
- Aguilera-Castrejon, A.; Oldak, B.; Shani, T.; Ghanem, N.; Itzkovich, C.; Slomovich, S.; Tarazi, S.; Bayerl, J.; Chugaeva, V.; Ayyash, M.; et al. Ex utero mouse embryogenesis from pre-gastrulation to late organogenesis. Nature 2021, 593, 119–124. [Google Scholar] [CrossRef]
- McDole, K.; Guignard, L.; Amat, F.; Berger, A.; Malandain, G.; Royer, L.A.; Turaga, S.C.; Branson, K.; Keller, P.J. In Toto Imaging and Reconstruction of Post-Implantation Mouse Development at the Single-Cell Level. Cell 2018, 175, 859–876.e33. [Google Scholar] [CrossRef]
- Yue, Y.; Zong, W.; Li, X.; Li, J.; Zhang, Y.; Wu, R.; Liu, Y.; Cui, J.; Wang, Q.; Bian, Y.; et al. Long-term, in toto live imaging of cardiomyocyte behaviour during mouse ventricle chamber formation at single-cell resolution. Nat. Cell Biol. 2020, 22, 332–340. [Google Scholar] [CrossRef]
- Esteban, I.; Schmidt, P.; Desgrange, A.; Raiola, M.; Temiño, S.; Meilhac, S.M.; Kobbelt, L.; Torres, M. Pseudodynamic analysis of heart tube formation in the mouse reveals strong regional variability and early left–right asymmetry. Nat. Cardiovasc. Res. 2022, 1, 504–517. [Google Scholar] [CrossRef]
- Tyser, R.C.; Ibarra-Soria, X.; McDole, K.; Jayaram, S.A.; Godwin, J.; Brand, T.A.D.; Miranda, A.M.; Scialdone, A.; Keller, P.J.; Marioni, J.C.; et al. Characterization of a common progenitor pool of the epicardium and myocardium. Science 2021, 371, eabb2986. [Google Scholar] [CrossRef]
- Zhang, Q.; Carlin, D.; Zhu, F.; Cattaneo, P.; Ideker, T.; Evans, S.M.; Bloomekatz, J.; Chi, N.C. Unveiling Complexity and Multipotentiality of Early Heart Fields. Circ. Res. 2021, 129, 474–487. [Google Scholar] [CrossRef]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. Ilastik: Interactive Machine Learning for (Bio)Image Analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef]
- Frank, E.; Hall, M.A.; Witten, I.H. The WEKA workbench. Data Mining 2017, 553–571. [Google Scholar] [CrossRef]
- Stringer, C.; Wang, T.; Michaelos, M.; Pachitariu, M. Cellpose: A generalist algorithm for cellular segmentation. Nat. Methods 2021, 18, 100–106. [Google Scholar] [CrossRef]
- Legland, D.; Arganda-Carreras, I.; Andrey, P. MorphoLibJ: Integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics 2016, 32, 3532–3534. [Google Scholar] [CrossRef]
- Weigert, M.; Schmidt, U.; Haase, R.; Sugawara, K.; Myers, G. Star-convex polyhedra for 3D object detection and segmentation in microscopy. In Proceedings of the 2020 IEEE Winter Conference on Applications of Computer Vision, WACV 2020, Snowmass Village, CO, USA, 1–5 March 2020; pp. 3655–3662. [Google Scholar] [CrossRef]
- Cignoni, P.; Callieri, M.; Corsini, M.; Dellepiane, M.; Ganovelli, F.; Ranzuglia, G. MeshLab: An open-source mesh processing tool. In Proceedings of the 6th Eurographics Italian Chapter Conference 2008, Salerno, Italy, 2–4 July 2008; pp. 129–136. [Google Scholar]
- Gussakovsky, E.; Kuzio, B.; Yang, Y.; Kupriyanov, V. Fluorescence imaging to quantify the fluorescent microspheres in cardiac tissue. J. Biophotonics 2011, 4, 277–287. [Google Scholar] [CrossRef]
- Mejía-Alvarez, R.; Manno, C.; Villalba-Galea, C.A.; Fernández, L.D.V.; Costa, R.R.; Fill, M.; Gharbi, T.; Escobar, A.L. Pulsed local-field fluorescence microscopy: A new approach for measuring cellular signals in the beating heart. Pflugers Arch. Eur. J. Physiol. 2003, 445, 747–758. [Google Scholar] [CrossRef]
- Minsky, M. Microscopy Apparatus. U.S. Patent 3013467, 19 December 1961. p. 5. [Google Scholar]
- Hickey, S.M.; Ung, B.; Bader, C.; Brooks, R.; Lazniewska, J.; Johnson, I.R.D.; Sorvina, A.; Logan, J.; Martini, C.; Moore, C.R.; et al. Fluorescence Microscopy-An Outline of Hardware, Biological Handling, and Fluorophore Considerations. Cells 2021, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Pawley, J.B.; Masters, B.R. Handbook of Biological Confocal Microscopy, Third Edition. J. Biomed. Opt. 2008, 13, 029902. [Google Scholar] [CrossRef]
- Sivaguru, M.; Fried, G.; Sivaguru, B.S.; Sivaguru, V.A.; Lu, X.; Choi, K.H.; Saif, M.T.A.; Lin, B.; Sadayappan, S. Cardiac muscle organization revealed in 3-D by imaging whole-mount mouse hearts using twophoton fluorescence and confocal microscopy. BioTechniques 2015, 59, 295–308. [Google Scholar] [CrossRef]
- Niggli, E.; Lederer, W.J. Real-time confocal microscopy and calcium measurements in heart muscle cells: Towards the development of a fluorescence microscope with high temporal and spatial resolution. Cell Calcium 1990, 11, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Tyser, R.C.; Miranda, A.M.; Chen, C.M.; Davidson, S.M.; Srinivas, S.; Riley, P.R. Calcium handling precedes cardiac differentiation to initiate the first heartbeat. eLife 2016, 5, e17113. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.; Nesbitt, T.; Yost, M.; Goodwin, R. Using Confocal Microscopy to Explore Cardiac Development and Disease. Microsc. Microanal. 2007, 13, 182–183. [Google Scholar] [CrossRef]
- Ocaña, O.H.; Coskun, H.; Minguillón, C.; Murawala, P.; Tanaka, E.M.; Galcerán, J.; Muñoz-Chápuli, R.; Nieto, M.A. A righthanded signalling pathway drives heart looping in vertebrates. Nature 2017, 549, 86–90. [Google Scholar] [CrossRef]
- Huisken, J.; Swoger, J.; Del Bene, F.; Wittbrodt, J.; Stelzer, E.H. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 2004, 305, 1007–1009. [Google Scholar] [CrossRef]
- Huang, Z.; Gu, P.; Kuang, D.; Mi, P.; Feng, X. Dynamic imaging of zebrafish heart with multi-planar light sheet microscopy. J. Biophotonics 2021, 14, e202000466. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Fujita, K.; Tanaka, H.; Oyamada, M.; Nakamura, O.; Kawata, S.; Takamatsu, T. Real-Time Two-Photon Microscopy and Its Application for In Situ Imaging. Acta Histochem. Cytochem. 2001, 34, 399–403. [Google Scholar] [CrossRef]
- Truong, T.V.; Supatto, W.; Koos, D.S.; Choi, J.M.; Fraser, S.E. Deep and fast live imaging with two-photon scanned light-sheet microscopy. Nat. Methods 2011, 8, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Mahou, P.; Vermot, J.; Beaurepaire, E.; Supatto, W. Multicolor two-photon light-sheet microscopy. Nat. Methods 2014, 11, 600–601. [Google Scholar] [CrossRef]
- de Boer, B.A.; van den Berg, G.; de Boer, P.A.; Moorman, A.F.; Ruijter, J.M. Growth of the developing mouse heart: An interactive qualitative and quantitative 3D atlas. Dev. Biol. 2012, 368, 203–213. [Google Scholar] [CrossRef]
- Li-Villarreal, N.; Rasmussen, T.L.; Christiansen, A.E.; Dickinson, M.E.; Hsu, C.W. Three-dimensional microCT imaging of mouse heart development from early post-implantation to late fetal stages. Mamm. Genome 2023. [Google Scholar] [CrossRef]
- Wang, S.; Lopez, A.L.; Larin, K.V.; Overbeek, P.A.; Larina, I.V. Live four-dimensional optical coherence tomography reveals embryonic cardiac phenotype in mouse mutant. J. Biomed. Opt. 2015, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Dalmasso, G.; Musy, M.; Niksic, M.; Robert-Moreno, A.; Badía-Careaga, C.; Sanz-Ezquerro, J.J.; Sharpe, J. 4D reconstruction of murine developmental trajectories using spherical harmonics. Dev. Cell 2022, 57, 2140–2150.e5. [Google Scholar] [CrossRef] [PubMed]
- De Bakker, B.S.; De Jong, K.H.; Hagoort, J.; De Bree, K.; Besselink, C.T.; De Kanter, F.E.; Veldhuis, T.; Bais, B.; Schildmeijer, R.; Ruijter, J.M.; et al. An interactive three-dimensional digital atlas and quantitative database of human development. Science 2016, 354, aag0053. [Google Scholar] [CrossRef]
- Soufan, A.T.; Van Den Berg, G.; Moerland, P.D.; Massink, M.M.; Van Den Hoff, M.J.; Moorman, A.F.; Ruijter, J.M. Three-dimensional measurement and visualization of morphogenesis applied to cardiac embryology. J. Microsc. 2007, 225, 269–274. [Google Scholar] [CrossRef]
- Weninger, W.J.; Mohun, T. Phenotyping transgenic embryos: A rapid 3-D screening method based on episcopic fluorescence image capturing. Nat. Genet. 2002, 30, 59–65. [Google Scholar] [CrossRef]
- Weninger, W.J.; Meng, S.; Streicher, J.; Müller, G.B. A new episcopic method for rapid 3-D reconstruction: Applications in anatomy and embryology. Anat. Embryol. 1998, 197, 341–348. [Google Scholar] [CrossRef]
- Dunlevy, L.; Bennett, M.; Slender, A.; Lana-Elola, E.; Tybulewicz, V.L.; Fisher, E.M.; Mohun, T. Down’s syndrome-like cardiac developmental defects in embryos of the transchromosomic Tc1 mouse. Cardiovasc. Res. 2010, 88, 287–295. [Google Scholar] [CrossRef]
- Zietkiewicz, E.; Bukowy-Bieryllo, Z.; Rabiasz, A.; Daca-Roszak, P.; Wojda, A.; Voelkel, K.; Rutkiewicz, E.; Pogorzelski, A.; Rasteiro, M.; Witt, M. CFAP300: Mutations in slavic patients with primary ciliary dyskinesia and a role in ciliary dynein arms trafficking. Am. J. Respir. Cell Mol. Biol. 2019, 61, 400–449. [Google Scholar] [CrossRef]
- Larina, I.V.; Larin, K.V.; Justice, M.J.; Dickinson, M.E. Optical Coherence Tomography for live imaging of mammalian development. Curr. Opin. Genet. Dev. 2011, 21, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.L.; Larina, I.V. Dynamic Imaging of Mouse Embryos and Cardiac Development in Static Culture. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 2206, pp. 129–141. [Google Scholar] [CrossRef]
- Wang, V.Y.; Hussan, J.R.; Yousefi, H.; Bradley, C.P.; Hunter, P.J.; Nash, M.P. Modelling Cardiac Tissue Growth and Remodelling. J. Elast. 2017, 129, 283–305. [Google Scholar] [CrossRef]
- Männer, J.; Thrane, L.; Norozi, K.; Yelbuz, T.M. High-resolution in vivo imaging of the cross-sectional deformations of contracting embryonic heart loops using optical coherence tomography. Dev. Dyn. 2008, 237, 953–961. [Google Scholar] [CrossRef]
- Jenkins, M.W.; Rothenberg, F.; Roy, D.; Nikolski, V.P.; Hu, Z.; Watanabe, M.; Wilson, D.L.; Efimov, I.R.; Rollins, A.M. 4D embryonic cardiography using gated optical coherence tomography. Opt. Express 2006, 14, 736. [Google Scholar] [CrossRef] [PubMed]
- Rugonyi, S.; Shaut, C.; Liu, A.; Thornburg, K.; Wang, R.K. Changes in wall motion and blood flow in the outflow tract of chick embryonic hearts observed with optical coherence tomography after outflow tract banding and vitelline-vein ligation. Phys. Med. Biol. 2008, 53, 5077–5091. [Google Scholar] [CrossRef]
- Yelbuz, T.M.; Choma, M.A.; Thrane, L.; Kirby, M.L.; Izatt, J.A. Optical coherence tomography: A new high-resolution imaging technology to study cardiac development in chick embryos. Circulation 2002, 106, 2771–2774. [Google Scholar] [CrossRef]
- Wang, S.; Lakomy, D.S.; Garcia, M.D.; Lopez, A.L.; Larin, K.V.; Larina, I.V. Four-dimensional live imaging of hemodynamics in mammalian embryonic heart with Doppler optical coherence tomography. J. Biophotonics 2016, 9, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Paun, B.; Bijnens, B.; Cook, A.C.; Mohun, T.J.; Butakoff, C. Quantification of the detailed cardiac left ventricular trabecular morphogenesis in the mouse embryo. Med. Image Anal. 2018, 49, 89–104. [Google Scholar] [CrossRef]
- Le Garrec, J.F.; Ragni, C.V.; Pop, S.; Dufour, A.; Olivo-Marin, J.C.; Buckingham, M.E.; Meilhac, S.M. Quantitative analysis of polarity in 3D reveals local cell coordination in the embryonic mouse heart. Development 2013, 140, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Francou, A.; Saint-Michel, E.; Mesbah, K.; Kelly, R.G. TBX1 regulates epithelial polarity and dynamic basal filopodia in the second heart field. Development 2014, 141, 4320–4331. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Osanlouy, M.; Bradley, C.P.; Kubke, M.F.; Gerneke, D.A.; Hunter, P.J. A method for investigating spatiotemporal growth patterns at cell and tissue levels during C-looping in the embryonic chick heart. iScience 2022, 25, 104600. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.; Bowery, A.; Britten, R.; Budelmann, V.; Camara, O.; Christie, R.; Cookson, A.; Frangi, A.F.; Gamage, T.B.; Heidlauf, T.; et al. OpenCMISS: A multi-physics & multi-scale computational infrastructure for the VPH/Physiome project. Prog. Biophys. Mol. Biol. 2011, 107, 32–47. [Google Scholar] [CrossRef]
- Heemskerk, I.; Streichan, S.J. Tissue cartography: Compressing bio-image data by dimensional reduction. Nat. Methods 2015, 12, 1139–1142. [Google Scholar] [CrossRef]
- Lee, J.; Fei, P.; Packard, R.R.; Kang, H.; Xu, H.; Baek, K.I.; Jen, N.; Chen, J.; Yen, H.; Kuo, C.C.; et al. 4-Dimensional light-sheet microscopy to elucidate shear stress modulation of cardiac trabeculation. J. Clin. Investig. 2016, 126, 1679–1690. [Google Scholar] [CrossRef]
- Stalling, D.; Westerhoff, M.; Hege, H.C. Amira: A highly interactive system for visual data analysis. Vis. Handb. 2005, 38, 749–767. [Google Scholar] [CrossRef]
- Kawahira, N.; Ohtsuka, D.; Kida, N.; ichi Hironaka, K.; Morishita, Y. Quantitative Analysis of 3D Tissue Deformation Reveals Key Cellular Mechanism Associated with Initial Heart Looping. Cell Rep. 2020, 30, 3889–3903.e5. [Google Scholar] [CrossRef]
- Francou, A.; De Bono, C.; Kelly, R.G. Epithelial tension in the second heart field promotes mouse heart tube elongation. Nat. Commun. 2017, 8, 14770. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.; Sedaei, Z.; Ferdousi, R. Computerized cell tracking: Current methods, tools and challenges. Vis. Inform. 2021, 5, 1–13. [Google Scholar] [CrossRef]
- Ramasubramanian, A.; Latacha, K.S.; Benjamin, J.M.; Voronov, D.A.; Ravi, A.; Taber, L.A. Computational model for early cardiac looping. Ann. Biomed. Eng. 2006, 34, 1355–1369. [Google Scholar] [CrossRef]
- Shi, Y.; Yao, J.; Xu, G.; Taber, L.A. Bending of the looping heart: Differential growth revisited. J. Biomech. Eng. 2014, 136, 081002. [Google Scholar] [CrossRef]
- Stalsberg, H.; DeHaan, R.L. The precardiac areas and formation of the tubular heart in the chick embryo. Dev. Biol. 1969, 19, 128–159. [Google Scholar] [CrossRef]
- Latacha, K.S.; Rémond, M.C.; Ramasubramanian, A.; Chen, A.Y.; Elson, E.L.; Taber, L.A. Role of actin polymerization in bending of the early heart tube. Dev. Dyn. 2005, 233, 1272–1286. [Google Scholar] [CrossRef] [PubMed]
- Manasek, F.J.; Burnside, M.B.; Waterman, R.E. Myocardial cell shape change as a mechanism of embryonic heart looping. Dev. Biol. 1972, 29, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Taber, L.A. Theoretical study of Beloussov’s hyper-restoration hypothesis for mechanical regulation of morphogenesis. Biomech. Model. Mechanobiol. 2008, 7, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Itasaki, N.; Nakamura, H.; Sumida, H.; Yasuda, M. Actin bundles on the right side in the caudal part of the heart tube play a role in dextro-looping in the embryonic chick heart. Anat. Embryol. 1991, 183, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Desgrange, A.; Garrec, J.F.L.; Bernheim, S.; Bønnelykke, T.H.; Meilhac, S.M. Transient Nodal Signaling in Left Precursors Coordinates Opposed Asymmetries Shaping the Heart Loop. Dev. Cell 2020, 55, 413–431.e6. [Google Scholar] [CrossRef]
- Honda, H. Left-handed cardiac looping by cell chirality is mediated by position-specific convergent extensions. Biophys. J. 2021, 120, 5371–5383. [Google Scholar] [CrossRef]
- Honda, H.; Abe, T.; Fujimori, T. The Chiral Looping of the Embryonic Heart Is Formed by the Combination of Three Axial Asymmetries. Biophys. J. 2020, 118, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Courchaine, K.; Rykiel, G.; Rugonyi, S. Influence of blood flow on cardiac development. Prog. Biophys. Mol. Biol. 2018, 137, 95–110. [Google Scholar] [CrossRef]
- Courchaine, K.; Rugonyi, S. Quantifying blood flow dynamics during cardiac development: Demystifying computational methods. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170330. [Google Scholar] [CrossRef]
- Santhanakrishnan, A.; Miller, L.A. Fluid Dynamics of Heart Development. Cell Biochem. Biophys. 2011, 61, 1–22. [Google Scholar] [CrossRef]
- DeAlmeida, A.; McQuinn, T.; Sedmera, D. Increased ventricular preload is compensated by myocyte proliferation in normal and hypoplastic fetal chick left ventricle. Circ. Res. 2007, 100, 1363–1370. [Google Scholar] [CrossRef]
- Groenendijk, B.C.; Hierck, B.P.; Vrolijk, J.; Baiker, M.; Pourquie, M.J.; Gittenberger-De Groot, A.C.; Poelmann, R.E. Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ. Res. 2005, 96, 1291–1298. [Google Scholar] [CrossRef]
- Salman, H.E.; Yalcin, H.C. Computational modeling of blood flow hemodynamics for biomechanical investigation of cardiac development and disease. J. Cardiovasc. Dev. Dis. 2021, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Vedula, V.; Lee, J.; Xu, H.; Kuo, C.C.; Hsiai, T.K.; Marsden, A.L. A method to quantify mechanobiologic forces during zebrafish cardiac development using 4-D light sheet imaging and computational modeling. PLoS Comput. Biol. 2017, 13, e1005828. [Google Scholar] [CrossRef]
- Handler, C.; Scarcelli, G.; Zhang, J. Time-lapse mechanical imaging of neural tube closure in live embryo using Brillouin microscopy. Sci. Rep. 2023, 13, 263. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raiola, M.; Sendra, M.; Torres, M. Imaging Approaches and the Quantitative Analysis of Heart Development. J. Cardiovasc. Dev. Dis. 2023, 10, 145. https://doi.org/10.3390/jcdd10040145
Raiola M, Sendra M, Torres M. Imaging Approaches and the Quantitative Analysis of Heart Development. Journal of Cardiovascular Development and Disease. 2023; 10(4):145. https://doi.org/10.3390/jcdd10040145
Chicago/Turabian StyleRaiola, Morena, Miquel Sendra, and Miguel Torres. 2023. "Imaging Approaches and the Quantitative Analysis of Heart Development" Journal of Cardiovascular Development and Disease 10, no. 4: 145. https://doi.org/10.3390/jcdd10040145
APA StyleRaiola, M., Sendra, M., & Torres, M. (2023). Imaging Approaches and the Quantitative Analysis of Heart Development. Journal of Cardiovascular Development and Disease, 10(4), 145. https://doi.org/10.3390/jcdd10040145





